Differential N2O-Producing Activity of Soil Fungi Across Agricultural Systems: High in Vegetable Fields and Vineyards, Low in Paddies
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection
2.2. N2O Production Potential Measurement Based on Isotopomers Analysis
2.3. The Effect of Streptomycin and Formate on Fungi Denitrification
2.4. Statistical Analyses
3. Results and Discussion
3.1. The Fungal Role in N2O Emission Based on Isotopomers Analysis
3.2. The Fungal Role in N2O Emission Based on the SIRIN Method
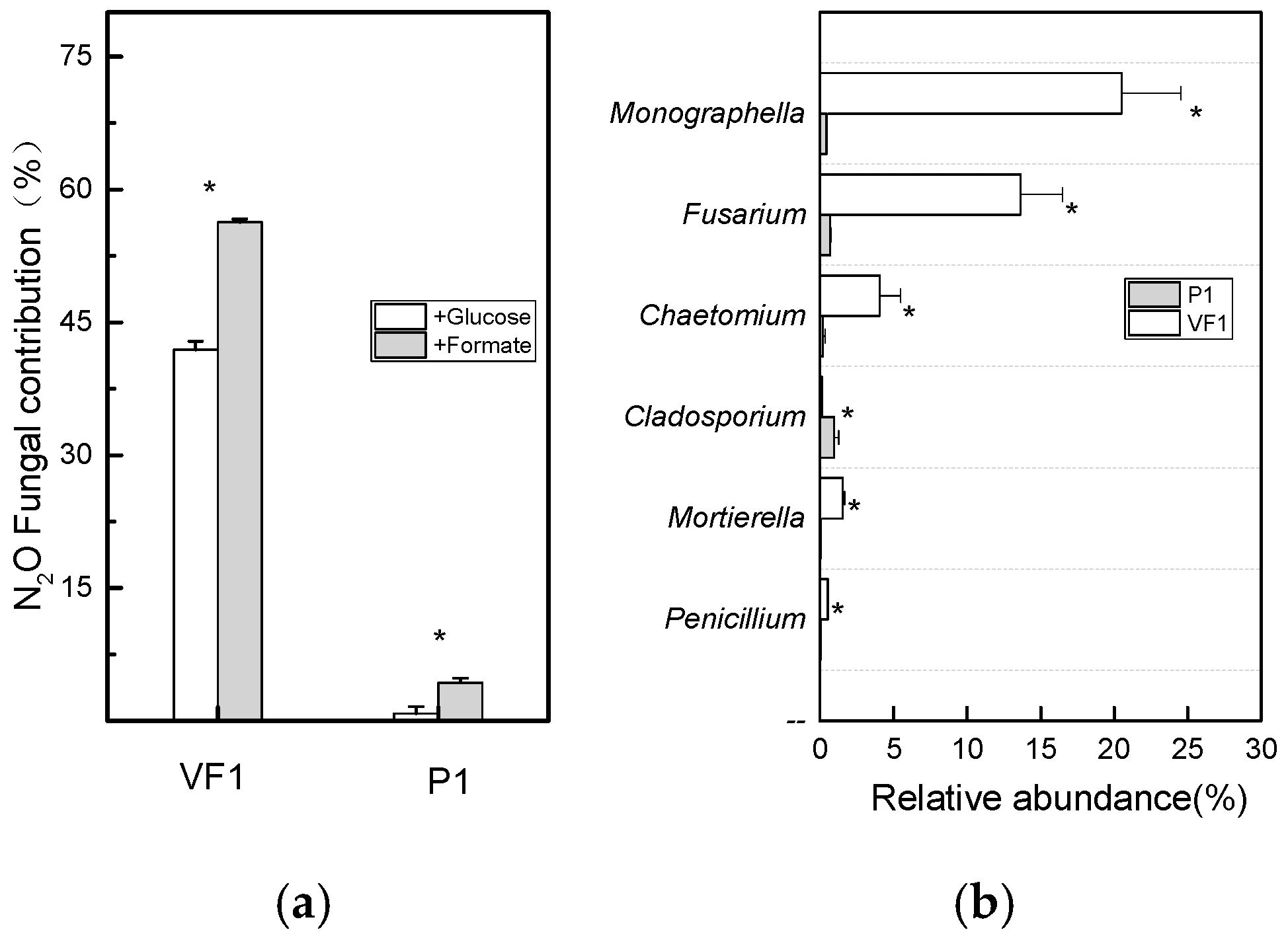
3.3. The Actual N2O-Producing Activity of Fungi in Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change (2013): The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovermental Panel on Climate Change; IPCC: Geneva, Switzerland, 2013. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Chen, H.; Mothapo, N.V.; Shi, W. The significant contribution of fungi to soil N2O production across diverse ecosystems. Appl. Soil Ecol. 2014, 73, 70–77. [Google Scholar] [CrossRef]
- Mothapo, N.; Chen, H.H.; Cubeta, M.A.; Grossman, J.M.; Fuller, F.; Shi, W. Phylogenetic, taxonomic and functional diversity of fungal denitrifiers and associated N2O production efficacy. Soil Biol. Biochem. 2015, 83, 160–175. [Google Scholar] [CrossRef]
- Ma, S.; Shan, J.; Yan, X. N2O emissions dominated by fungi in an intensively managed vegetable field converted from wheat-rice rotation. Appl. Soil Ecol. 2017, 116, 23–29. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, L.; Liu, M.; Qiu, H.; Zhou, S. Fungi dominate denitrification when Chinese milk vetch green manure is used in paddy soil. Soil Ecol. Lett. 2022, 4, 155–163. [Google Scholar] [CrossRef]
- Lu, Y.X.; Yin, B.F.; Li, Y.G.; Zang, Y.X.; Zhou, X.B.; Zhang, Y.M. Snow and nitrogen manipulation do not alter the dominant role of fungi in the N2O production of biocrusts in a temperate desert. Appl. Soil Ecol. 2025, 205, 105766. [Google Scholar] [CrossRef]
- Shoun, H.; Kim, D.-H.; Uchiyama, H.; Sugiyama, J. Denitrification by fungi. FEMS Microbiol. Lett. 1992, 94, 277–281. [Google Scholar] [CrossRef]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Shen, W.S.; Hu, M.C.; Qian, D.; Xue, H.W.; Gao, N.; Lin, X.G. Microbial deterioration and restoration in greenhouse-based intensive vegetable production systems. Plant Soil 2021, 463, 1–18. [Google Scholar] [CrossRef]
- Wei, W.; Isobe, K.; Shiratori, Y.; Nishizawa, T.; Ohte, N.; Ise, Y.; Otsuka, S.; Senoo, K. Development of PCR primers targeting fungal nirK to study fungal denitrification in the environment. Soil Biol. Biochem. 2015, 81, 282–286. [Google Scholar] [CrossRef]
- Chen, H.H.; Mothapo, N.V.; Shi, W. Soil Moisture and pH Control Relative Contributions of Fungi and Bacteria to N2O Production. Microb. Ecol. 2015, 69, 180–191. [Google Scholar] [CrossRef]
- Ma, S.; Wang, J.; Yan, X. Is Nitrous Oxide Reduction Primarily Regulated by the Fungi-to-Bacteria Abundance Ratio in Fertilized Soils? Pedosphere 2019, 29, 569–576. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Agriculture. In Climate Change 2007: Mitigation; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Decock, C.; Six, J. How reliable is the intramolecular distribution of 15N in N2O to source partition N2O emitted from soil? Soil Biol. Biochem. 2013, 65, 114–127. [Google Scholar] [CrossRef]
- Groffman, P.M.; Holland, E.A.; Myrold, D.D.; Robertson, G.P.; Zou, X. Denitrification. In Standard Soil Methods for Long-Term Ecological Research; Oxford University Press, Inc.: New York, NY, USA, 1999; pp. 272–288. [Google Scholar]
- Rohe, L.; Anderson, T.-H.; Flessa, H.; Goeske, A.; Lewicka-Szczebak, D.; Wrage-Moennig, N.; Well, R. Comparing modified substrate-induced respiration with selective inhibition (SIRIN) and N2O isotope approaches to estimate fungal contribution to denitrification in three arable soils under anoxic conditions. Biogeosciences 2021, 18, 4629–4650. [Google Scholar] [CrossRef]
- Uchimura, H.; Enjoji, H.; Seki, T.; Taguchi, A.; Tsakaya, N.; Shoun+, H. Nitrate reductase-formate dehydrogenase couple involved in the fungal denitrification by Fusarium oxysporum. J. Biochem. 2002, 131, 579–586. [Google Scholar] [CrossRef]
- Cheung, M.K.; Au, C.H.; Chu, K.H.; Kwan, H.S.; Wong, C.K. Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. ISME J. 2010, 4, 1053–1059. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Wei, W.; Isobe, K.; Shiratori, Y.; Nishizawa, T.; Ohte, N.; Otsuka, S.; Senoo, K. N2O emission from cropland field soil through fungal denitrification after surface applications of organic fertilizer. Soil Biol. Biochem. 2014, 69, 157–167. [Google Scholar] [CrossRef]
- Liu, J.; Hou, H.; Zhang, W. Fungi contribute more to N2O emissions than bacteria in two paddy soils with different textures. Eur. J. Soil Biol. 2023, 115, 103476. [Google Scholar] [CrossRef]
- Laughlin, R.J.; Stevens, R.J. Evidence for fungal dominance of denitrification and codenitrification in a grassland soil. Soil Sci. Soc. Am. J. 2002, 66, 1540–1548. [Google Scholar] [CrossRef]
- Seo, D.C.; DeLaune, R.D. Fungal and bacterial mediated denitrification in wetlands: Influence of sediment redox condition. Water Res. 2010, 44, 2441–2450. [Google Scholar] [CrossRef]
- Ma, W.; Farrell, R.; Siciliano, S. Soil formate regulates the fungal nitrous oxide emission pathway. Appl. Environ. Microbiol. 2008, 74, 6690–6696. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhao, M.; Yuan, B.; Yao, J.; Chen, J.; Hrynshpan, D.; Savitskaya, T. A fungus–bacterium co-culture synergistically promoted nitrogen removal by enhancing enzyme activity and electron transfer. Sci. Total Environ. 2021, 754, 142109. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Zhang, J.; Wu, T.; Miao, Y.; Fang, H.; Wang, H.; Niu, H.; Ma, L. Differential N2O-Producing Activity of Soil Fungi Across Agricultural Systems: High in Vegetable Fields and Vineyards, Low in Paddies. Nitrogen 2025, 6, 57. https://doi.org/10.3390/nitrogen6030057
Ma S, Zhang J, Wu T, Miao Y, Fang H, Wang H, Niu H, Ma L. Differential N2O-Producing Activity of Soil Fungi Across Agricultural Systems: High in Vegetable Fields and Vineyards, Low in Paddies. Nitrogen. 2025; 6(3):57. https://doi.org/10.3390/nitrogen6030057
Chicago/Turabian StyleMa, Shutan, Jintao Zhang, Ting Wu, Yuqing Miao, Hua Fang, Haitao Wang, Huayuan Niu, and Lan Ma. 2025. "Differential N2O-Producing Activity of Soil Fungi Across Agricultural Systems: High in Vegetable Fields and Vineyards, Low in Paddies" Nitrogen 6, no. 3: 57. https://doi.org/10.3390/nitrogen6030057
APA StyleMa, S., Zhang, J., Wu, T., Miao, Y., Fang, H., Wang, H., Niu, H., & Ma, L. (2025). Differential N2O-Producing Activity of Soil Fungi Across Agricultural Systems: High in Vegetable Fields and Vineyards, Low in Paddies. Nitrogen, 6(3), 57. https://doi.org/10.3390/nitrogen6030057





