Unraveling Carbon and Nitrogen Dynamics in Cattle Manure: New Insights from Litterbag Incubation
Abstract
1. Introduction
2. Materials and Methods
2.1. Farmyard Cattle Manure Sampling
2.2. Analysis of the Composition of Farmyard Cattle Manure
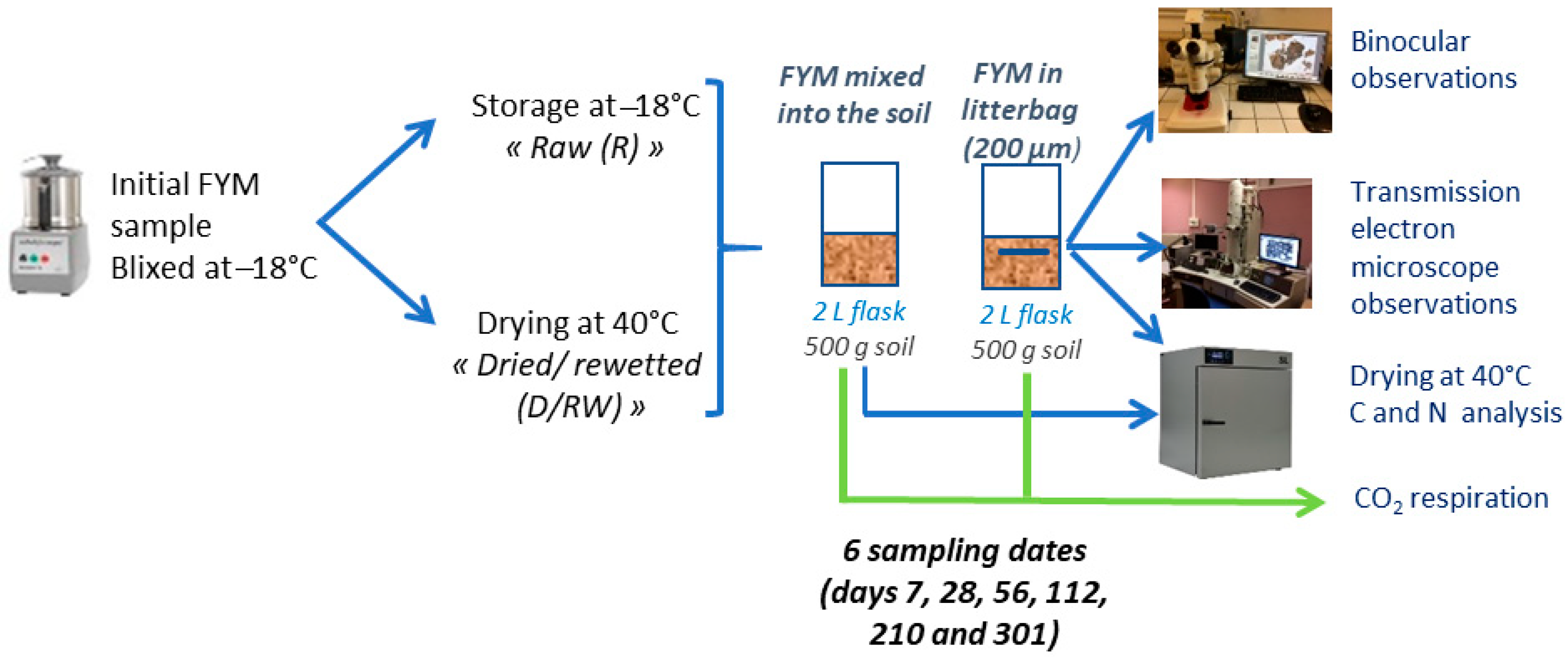
2.3. Design of the Incubation Experiment
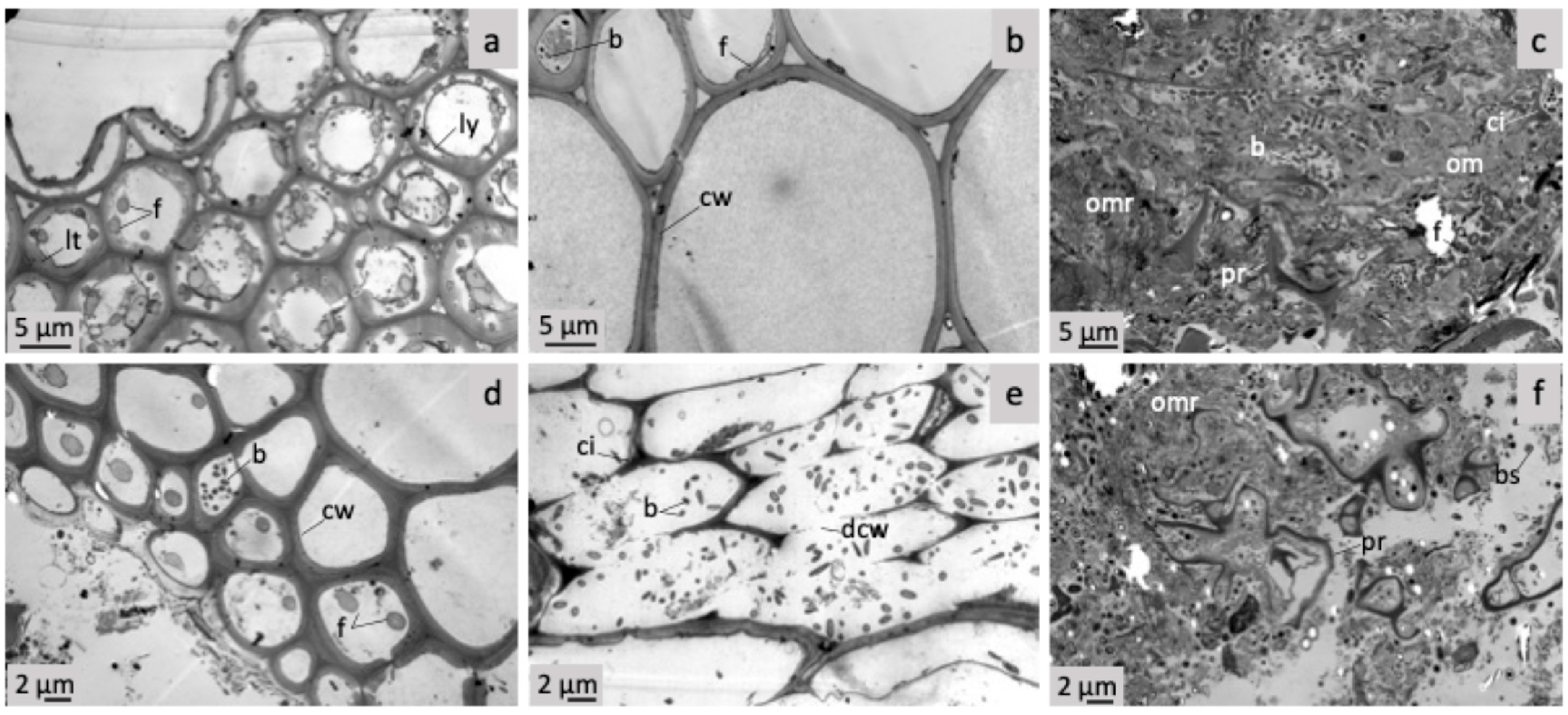
2.4. Transmission Electron Microscopy
3. Results
3.1. Farmyard Cattle Manure Organic Matter Composition
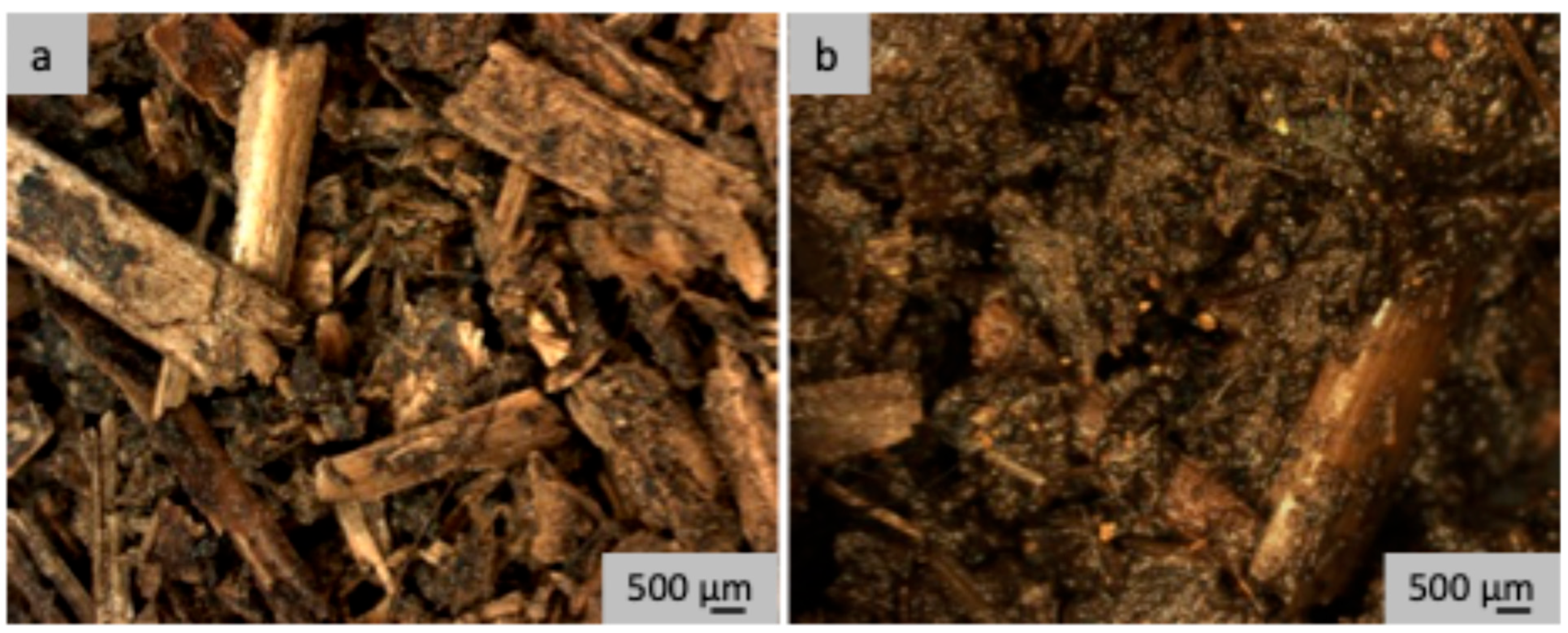
3.2. Microscopic Comparison of Raw and Dried Farmyard Cattle Manure
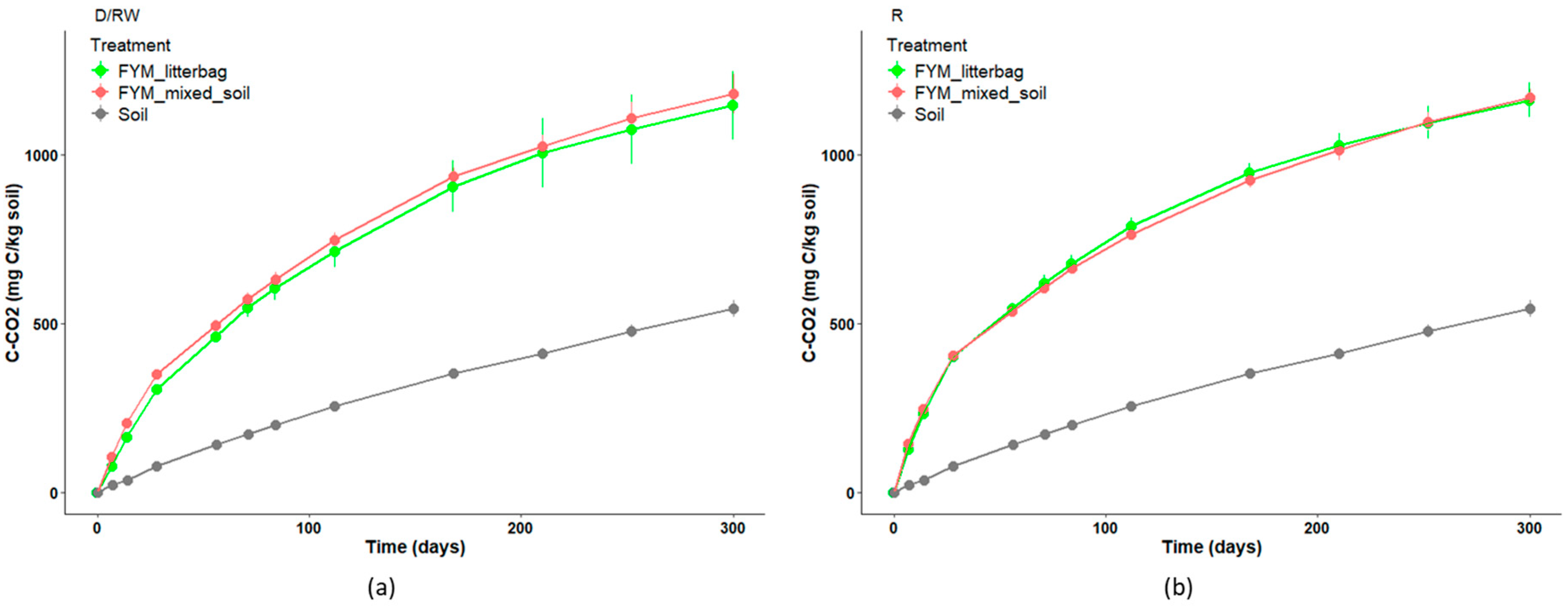
3.3. Carbon Mineralization Dynamics in Soil Versus Litterbags
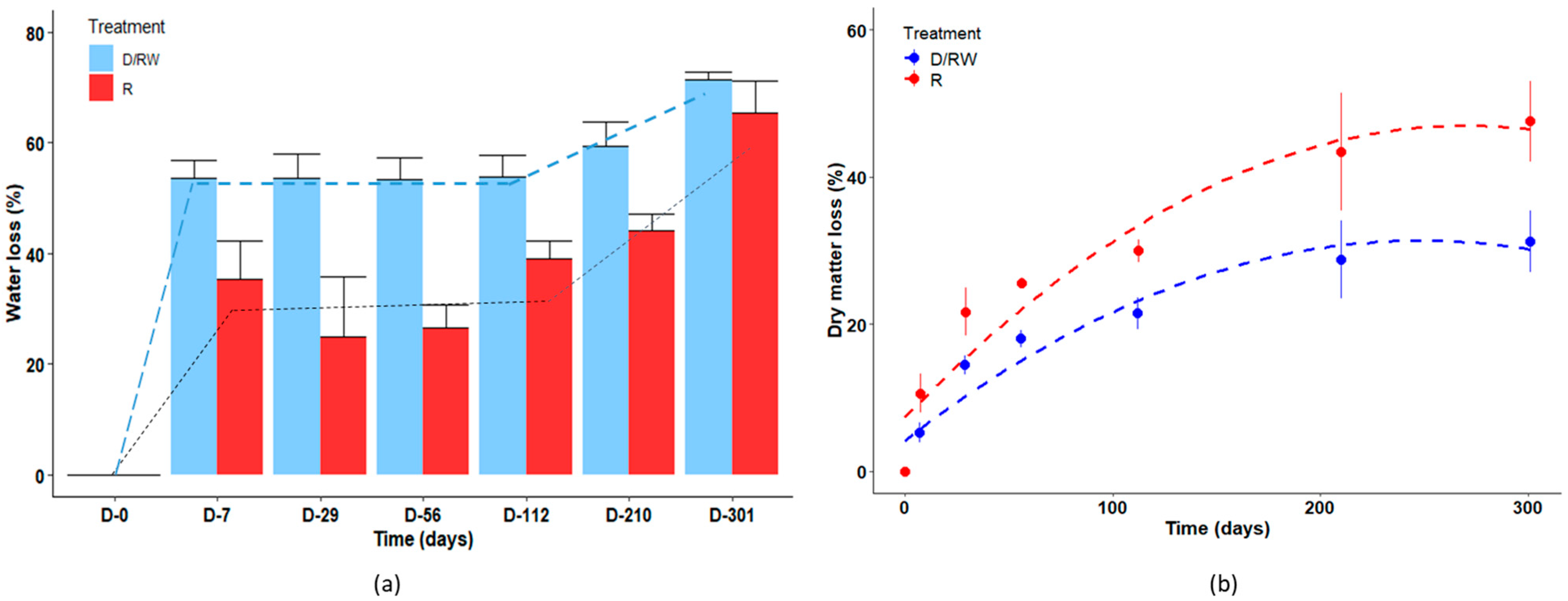
3.4. Water and Dry Matter Dynamics in the Litterbags
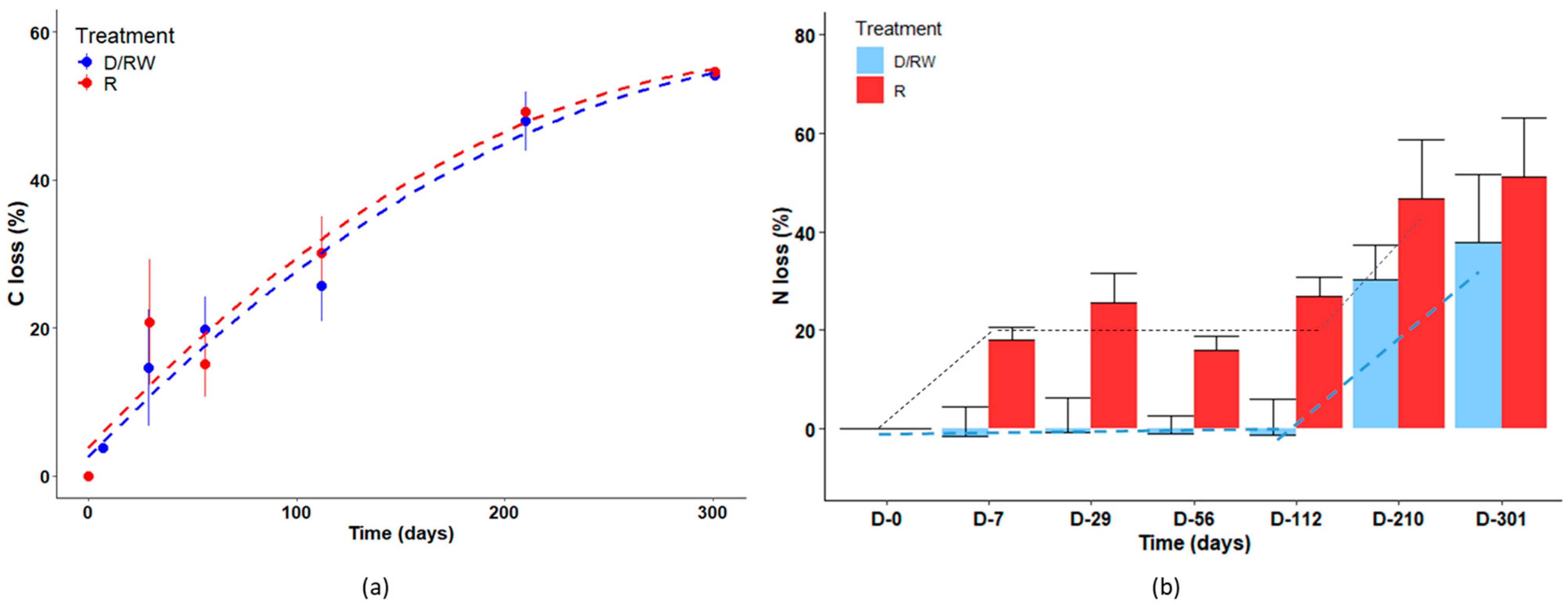
3.5. Carbon and Nitrogen Dynamics
3.6. Dynamics of D/RW and R FYM Microstructures Throughout Incubation
4. Discussion
4.1. Drying Influences FYM Biochemical Composition by Inducing Biological Activity
4.2. Similar C Mineralization Dynamics for Products Mixed with Soil or Placed in Litterbags
4.3. Drying Impacts on FYM Biochemical Composition and Microbial Activity During Incubation
4.4. Mass Balance: Evidence of Mass Transfer from Litterbags to the Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loyon, L. Overview of Animal Manure Management for Beef, Pig, and Poultry Farms in France. Front. Sustain. Food Syst. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Houot, S.; Pons, M.-N.; Pradel, M.; Savini, I.; Tibi, A. Valorisation Des Matières Fertilisantes d’origine Résiduaire Sur Les Sols à Usage Agricole Ou Forestier. 2014. Available online: https://www.inrae.fr/sites/default/files/pdf/mafor-synthese-vf-oct2014.pdf (accessed on 15 October 2014).
- Menzi, H.; Oenema, O.; Burton, C.H.; Shipin, O.; Gerber, P. Impacts of Intensive Livestock Production and Manure Management on the Environment (Chapter 9). In Livestock in a Changing Landscape: Drivers, Consequences and Responses; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure Management: Implications for Greenhouse Gas Emissions. Anim. Feed Sci. Technol. 2011, 166–167, 514–531. [Google Scholar] [CrossRef]
- Heitkamp, F.; Raupp, J.; Ludwig, B. Impact of Fertilizer Type and Rate on Carbon and Nitrogen Pools in a Sandy Cambisol. Plant Soil 2009, 319, 259–275. [Google Scholar] [CrossRef]
- Lashermes, G.; Nicolardot, B.; Parnaudeau, V.; Thuriès, L.; Chaussod, R.; Guillotin, M.L.; Linères, M.; Mary, B.; Metzger, L.; Morvan, T.; et al. Indicator of Potential Residual Carbon in Soils after Exogenous Organic Matter Application. Eur. J. Soil Sci. 2009, 60, 297–310. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. Assoc. Off. Anal. Chem. 1990, 73, 491–497. [Google Scholar]
- Jimenez, J.; Lei, H.; Steyer, J.-P.; Houot, S.; Patureau, D. Methane Production and Fertilizing Value of Organic Waste: Organic Matter Characterization for a Better Prediction of Valorization Pathways. Bioresour. Technol. 2017, 241, 1012–1021. [Google Scholar] [CrossRef]
- Parnaudeau, V.; Nicolardot, B.; Pagès, J. Relevance of Organic Matter Fractions as Predictors of Wastewater Sludge Mineralization in Soil. J. Environ. Qual. 2004, 33, 1885–1894. [Google Scholar] [CrossRef]
- Lashermes, G.; Nicolardot, B.; Parnaudeau, V.; Thuries, L.; Chaussod, R.; Guillotin, M.L.; Lineres, M.; Mary, B.; Metzger, L.; Morvan, T.; et al. Typology of Exogenous Organic Matters Based on Chemical and Biochemical Composition to Predict Potential Nitrogen Mineralization. Bioresour. Technol. 2010, 101, 157–164. [Google Scholar] [CrossRef]
- Morvan, T.; Nicolardot, B.; Pean, L. Biochemical Composition and Kinetics of C and N Mineralization of Animal Wastes: A Typological Approach. Biol. Fertil. Soils 2006, 42, 513–522. [Google Scholar] [CrossRef]
- Peltre, C.; Christensen, B.T.; Dragon, S.; Icard, C.; Katterer, T.; Houot, S. RothC Simulation of Carbon Accumulation in Soil after Repeated Application of Widely Different Organic Amendments. Soil Biol. Biochem. 2012, 52, 49–60. [Google Scholar] [CrossRef]
- FD U44-163; Amendements Organiques et Supports de Culture-Caractérisation de La Matière Organique Par La Minéralisation Potentielle Du Carbone et de l’azote. Qualité du sol. AFNOR: Paris, France, 2018.
- Kaiser, M.; Kleber, M.; Berhe, A.A. How Air-Drying and Rewetting Modify Soil Organic Matter Characteristics: An Assessment to Improve Data Interpretation and Inference. Soil Biol. Biochem. 2015, 80, 324–340. [Google Scholar] [CrossRef]
- Sparling, G.P.; Ross, D.J. Microbial Contributions to the Increased Nitrogen Mineralization after Air-Drying of Soils. Plant Soil 1988, 105, 163–167. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial Stress-Response Physiology and Its Implications for Ecosystem Function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Bocock, K.L.; Gilbert, O.J.W. The Disappearance of Leaf Litter under Different Woodland Conditions. Plant Soil 1957, 9, 179–185. [Google Scholar] [CrossRef]
- Kampichler, C.; Bruckner, A. The Role of Microarthropods in Terrestrial Decomposition: A Meta-Analysis of 40 Years of Litterbag Studies. Biol. Rev. 2009, 84, 375–389. [Google Scholar] [CrossRef]
- Vreeken-Buijs, M.J.; Brussaard, L. Soil Mesofauna Dynamics, Wheat Residue Decomposition and Nitrogen Mineralization in Buried Litterbags. Biol. Fertil. Soils 1996, 23, 374–381. [Google Scholar] [CrossRef]
- Bokhorst, S.; Wardle, D.A. Microclimate within Litter Bags of Different Mesh Size: Implications for the `arthropod Effect’ on Litter Decomposition. Soil Biol. Biochem. 2013, 58, 147–152. [Google Scholar] [CrossRef]
- Hanisch, J.; Engell, I.; Linsler, D.; Scheu, S.; Potthoff, M. The Role of Collembola for Litter Decomposition under Minimum and Conventional Tillage. J. Plant Nutr. Soil Sci. 2022, 185, 529–538. [Google Scholar] [CrossRef]
- Chassain, J.; Gonod, L.V.; Chenu, C.; Joimel, S. Role of Different Size Classes of Organisms in Cropped Soils: What Do Litterbag Experiments Tell Us? A Meta-Analysis. Soil Biol. Biochem. 2021, 162, 108394. [Google Scholar] [CrossRef]
- Potthoff, M.; Loftfield, N. How to Quantify Contamination of Organic Litter Bag Material with Soil? Pedobiologia 1998, 42, 147–153. [Google Scholar] [CrossRef]
- Frouz, J.; Roubíčková, A.; Heděnec, P.; Tajovský, K. Do Soil Fauna Really Hasten Litter Decomposition? A Meta-Analysis of Enclosure Studies. Eur. J. Soil Biol. 2015, 68, 18–24. [Google Scholar] [CrossRef]
- Jacobs, A.; Ludwig, B.; Schmidt, J.H.; Bergstermann, A.; Rauber, R.; Joergensen, R.G. Influence of Tillage on Degradation Kinetics Using the Litterbag Method. Eur. J. Soil Biol. 2011, 47, 198–204. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Olsson, S. Fungal Translocation-Creating and Responding to Environmental Heterogeneity. Mycologist 2004, 18, 79–88. [Google Scholar] [CrossRef]
- Peña-Peña, K.; Irmler, U. Nitrogen and Carbon Losses from Decomposing Litter in Natural and Agroecosystems of Two Different Climate Regions of Brazil. Eur. J. Soil Biol. 2018, 86, 26–33. [Google Scholar] [CrossRef]
- Taylor, B.R.; Bärlocher, F. Variable Effects of Air-Drying on Leaching Losses from Tree Leaf Litter. Hydrobiologia 1996, 325, 173–182. [Google Scholar] [CrossRef]
- NF ISO 11465; Qualité du sol-Détermination de La Teneur Pondérale En Matière Sèche et En Eau-Méthode Gravimétrique. AFNOR: Paris, France, 1994.
- NF ISO 10694; Qualité du sol-Dosage Du Carbone Organique et Du Carbone Total Après Combustion Sèche. Normes nationales et documents normatifs nationaux. AFNOR: Paris, France, 1995.
- Soest, P.J.V.; Wine, R.H. Use of Detergents in the Analysis of Fibrous Feeds. IV. Determination of Plant Cell-Wall Constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- FAO. Soils of the World; Food and Agriculture Organization and United Nations Educational, Scientific and Cultural Organization; Elsevier Science: New York, NY, USA, 1987. [Google Scholar]
- SOERE PRO Network. Available online: https://Valor-pro.Hub.Inrae.Fr/ (accessed on 26 February 2019).
- Zibilske, L.M. Carbon Mineralization. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1994; pp. 835–863. ISBN 978-0-89118-865-0. [Google Scholar]
- Motte, J.-C.; Watteau, F.; Escudié, R.; Steyer, J.-P.; Bernet, N.; Delgenes, J.-P.; Dumas, C. Dynamic Observation of the Biodegradation of Lignocellulosic Tissue under Solid-State Anaerobic Conditions. Bioresour. Technol. 2015, 191, 322–326. [Google Scholar] [CrossRef]
- Thuriès, L.; Pansu, M.; Feller, C.; Herrmann, P.; Rémy, J. Kinetics of Added Organic Matter Decomposition in a Mediterranean Sandy Soil. SOIL Biol. Biochem. 2001, 33, 997–1010. [Google Scholar] [CrossRef]
- Morvan, T.; Gogé, F.; Oboyet, T.; Carel, O.; Fouad, Y. A Dataset of the Chemical Composition and Near-Infrared Spectroscopy Measurements of Raw Cattle, Poultry and Pig Manure. Data Brief 2021, 39, 107475. [Google Scholar] [CrossRef]
- Gogé, F.; Thuriès, L.; Fouad, Y.; Damay, N.; Davrieux, F.; Moussard, G.; Roux, C.L.; Trupin-Maudemain, S.; Valé, M.; Morvan, T. Dataset of Chemical and Near-Infrared Spectroscopy Measurements of Fresh and Dried Poultry and Cattle Manure. Data Brief 2021, 34, 106647. [Google Scholar] [CrossRef]
- Bewley, J.D. Physiological Aspects of Desiccation Tolerance. Annu. Rev. Plant Biol. 1979, 30, 195–238. [Google Scholar] [CrossRef]
- Watteau, F.; Villemin, G.; Mansot, J.L.; Ghanbaja, J.; Toutain, F. Localization and Characterization by Electron Energy Loss Spectroscopy (EELS) of the Brown Cellular Substances of Beech Roots. Soil Biol. Biochem. 1996, 28, 1327–1332. [Google Scholar] [CrossRef]
- Castellanos, J.Z.; Pratt, P.F. Mineralization of Manure Nitrogen—Correlation with Laboratory Indexes. Soil Sci. Soc. Am. J. 1981, 45, 354–357. [Google Scholar] [CrossRef]
- Pansu, M.; Thuriès, L. Kinetics of C and N Mineralization, N Immobilization and N Volatilization of Organic Inputs in Soil. Soil Biol. Biochem. 2003, 35, 37–48. [Google Scholar] [CrossRef]
- Faust, S.; Koch, H.-J.; Dyckmans, J.; Joergensen, R.G. Response of Maize Leaf Decomposition in Litterbags and Soil Bags to Different Tillage Intensities in a Long-Term Field Trial. Appl. Soil Ecol. 2019, 141, 38–44. [Google Scholar] [CrossRef]
- Potthoff, M.; Dyckmans, J.; Flessa, H.; Beese, F.; Joergensen, R.G. Decomposition of Maize Residues after Manipulation of Colonization and Its Contribution to the Soil Microbial Biomass. Biol. Fertil. Soils 2008, 44, 891–895. [Google Scholar] [CrossRef]
- Dittmer, S.; Schrader, S. Longterm Effects of Soil Compaction and Tillage on Collembola and Straw Decomposition in Arable Soil. Pedobiologia 2000, 44, 527–538. [Google Scholar] [CrossRef]
- Yuste, J.C.; Peñuelas, J.; Estiarte, M.; Garcia-Mas, J.; Mattana, S.; Ogaya, R.; Pujol, M.; Sardans, J. Drought-Resistant Fungi Control Soil Organic Matter Decomposition and Its Response to Temperature. Glob. Change Biol. 2011, 17, 1475–1486. [Google Scholar] [CrossRef]
- Treseder, K.K.; Berlemont, R.; Allison, S.D.; Martiny, A.C. Drought Increases the Frequencies of Fungal Functional Genes Related to Carbon and Nitrogen Acquisition. PLoS ONE 2018, 13, e0206441. [Google Scholar] [CrossRef]
- Herzog, C.; Hartmann, M.; Frey, B.; Stierli, B.; Rumpel, C.; Buchmann, N.; Brunner, I. Microbial Succession on Decomposing Root Litter in a Drought-Prone Scots Pine Forest. ISME J. 2019, 13, 2346–2362. [Google Scholar] [CrossRef]
- Buresova, A.; Kopecky, J.; Hrdinkova, V.; Kamenik, Z.; Omelka, M.; Sagova-Mareckova, M. Succession of Microbial Decomposers Is Determined by Litter Type, but Site Conditions Drive Decomposition Rates. Appl. Environ. Microbiol. 2019, 85, e01760-19. [Google Scholar] [CrossRef] [PubMed]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in Leaf Litter: Novel Insights into Community Dynamics of Bacteria and Fungi during Litter Decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yuan, S.; Hu, N.; Lou, Y.; Wang, S. Predicting Soil Fauna Effect on Plant Litter Decomposition by Using Boosted Regression Trees. SOIL Biol. Biochem. 2015, 82, 81–86. [Google Scholar] [CrossRef]
- Lummer, D.; Scheu, S.; Butenschoen, O. Connecting Litter Quality, Microbial Community and Nitrogen Transfer Mechanisms in Decomposing Litter Mixtures. Oikos 2012, 121, 1649–1655. [Google Scholar] [CrossRef]

| Content | R | D/RW |
|---|---|---|
| Organic C | 282.2 ± 7.3 | 266 ± 7.4 |
| Organic N | 13.6 ± 1.1 | 15.0 ± 0.7 |
| N-NH4 | 6.56 ± 0.2 | 1.70 ± 0.05 |
| R | D/RW | |||||
|---|---|---|---|---|---|---|
| Day | C_loss | C-CO2 | C loss–DM loss | C_loss | C-CO2 | C loss–DM loss |
| 7 | −0.03 (7.3) | 4.9 (0.5) | −3.7 (22.1) | 3.8 (10.4) | 3.0 (0.1) | 12.0 (44.9) |
| 28 | 20.7 (8.5) | 15.4 (1.3) | 26.3 (7.6) | 14.6 (7.9) | 12.4 (0.8) | 28.0 (17.8) |
| 56 | 15.0 (4.4) | 19.1 (2.0) | 16.7 (4.6) | 19.8 (4.4) | 17.0 (1.1) | 29.7 (8.0) |
| 112 | 30.1 (4.91) | 25.0 (3.1) | 28.3 (3.1) | 25.7 (4.9) | 24.5 (1.9) | 32.0 (4.0) |
| 210 | 49.2 (11.3) | 29.0 (3.9) | 32.0 (1.7) | 47.9 (4.0) | 31.3 (3.6) | 45.0 (5.6) |
| 301 | 54.6 (10.5) | 29.0 (4.3) | 32.3 (3.5) | 54.1 (9.0) | 31.6 (3.5) | 46.0 (1.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morvan, T.; Watteau, F.; Robin, P. Unraveling Carbon and Nitrogen Dynamics in Cattle Manure: New Insights from Litterbag Incubation. Nitrogen 2025, 6, 56. https://doi.org/10.3390/nitrogen6030056
Morvan T, Watteau F, Robin P. Unraveling Carbon and Nitrogen Dynamics in Cattle Manure: New Insights from Litterbag Incubation. Nitrogen. 2025; 6(3):56. https://doi.org/10.3390/nitrogen6030056
Chicago/Turabian StyleMorvan, Thierry, Françoise Watteau, and Paul Robin. 2025. "Unraveling Carbon and Nitrogen Dynamics in Cattle Manure: New Insights from Litterbag Incubation" Nitrogen 6, no. 3: 56. https://doi.org/10.3390/nitrogen6030056
APA StyleMorvan, T., Watteau, F., & Robin, P. (2025). Unraveling Carbon and Nitrogen Dynamics in Cattle Manure: New Insights from Litterbag Incubation. Nitrogen, 6(3), 56. https://doi.org/10.3390/nitrogen6030056







