2.1. CerawaveTM Plasma Source
The torch used in this work leverages patented Cerawave™ technology to sustain near-atmospheric-pressure plasma using microwave energy from a 100 kW industrial magnetron operating in the 915 MHz industrial–scientific–medical frequency band. Cerawave™ replaces the traditional copper conductor in an inductive coil with rings composed of high-purity advanced technical ceramics carrying large alternating dielectric polarization currents, on the order of thousands of Amperes, with losses that are one hundred times lower than those in a traditional copper conductor. This improved efficiency enables plasma torch operation with dramatically reduced antenna cooling requirements.
In the plasma torch presented here, we use high-purity, high-density alumina rings with a relative dielectric constant of 10.0 and microwave loss tangent of less than 10−5. The rings have dimensions of 5.200 in OD × 2.125 in ID × 2.000 in H and are separated axially by a gap of 1.250 in. The two-ring design allows for an increased residence time for the plasma gas inside the high-field region. The rings are cooled by forced air.
The torch cavity is designed to resonate at a frequency of 911 MHz—the frequency of the incident microwaves. The alternating dielectric polarization current in the Cerawave™ rings drives strong, purely inductive electric fields interior to a 40 mm ID quartz torch, enabling the formation and sustainment of plasma. Plasma homogeneity is also improved given the lack of parasitic capacitively coupled electric fields.
At the maximum magnetron power of 100 kW employed in this work, the temperature of the alumina rings does not exceed 70 °C.
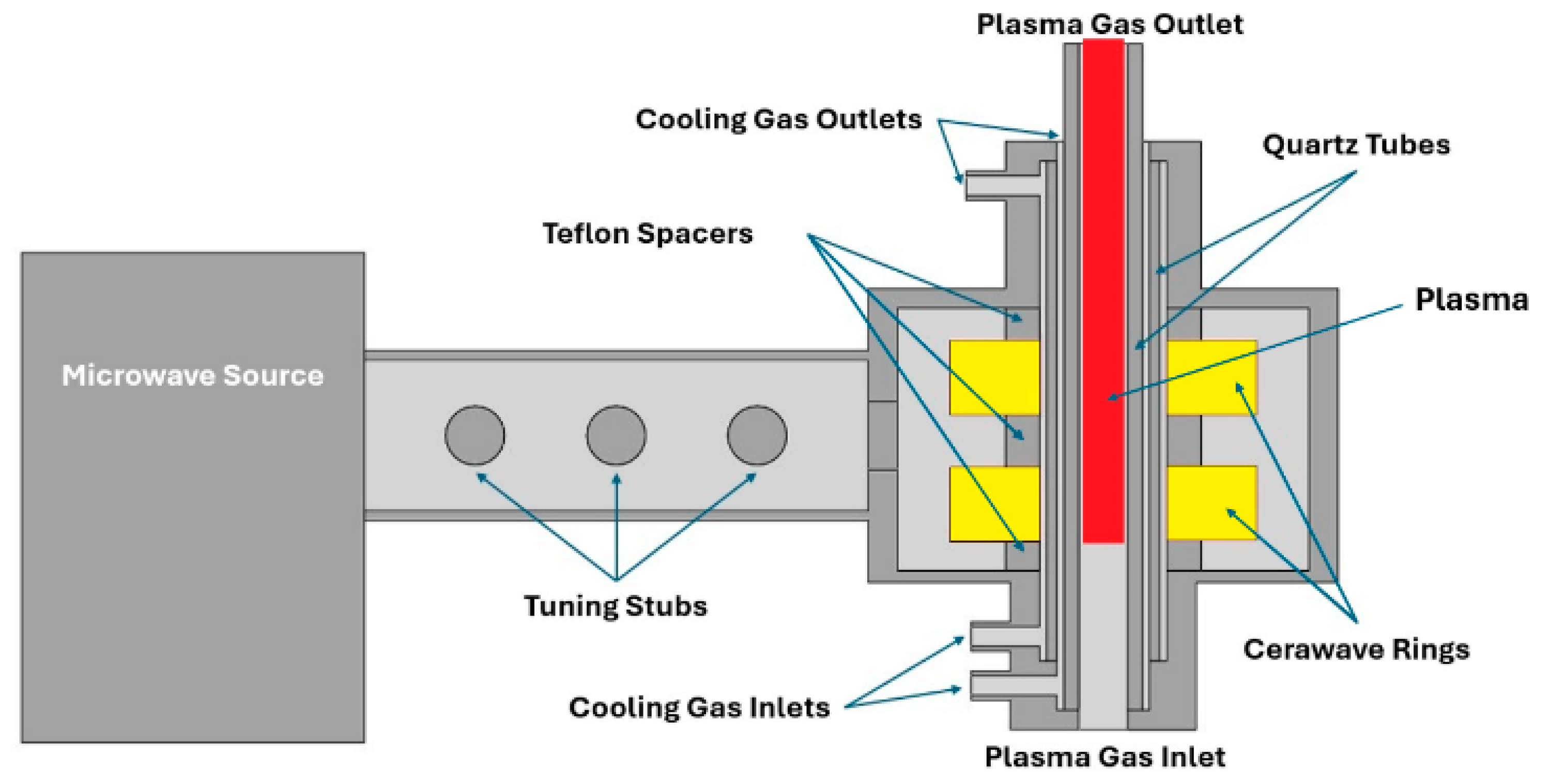
Figure 2 shows a cross-section of the geometry of the 100 kW plasma torch, where the Cerawave™ rings are shown in yellow.
The tuning stubs mitigate the power reflected by the plasma to the microwave generator, optimizing the net power received by the plasma. There are two cooling channels where air passes to keep the quartz tubes, the Cerawave™ rings, and the PTFE spacers cool during operation. These channels are supplied with air by two blowers.
The plasma torch presented here can safely operate continuously at power levels between 50 kW and 100 kW, but 50 kW is used for steady-state operation in this work unless stated otherwise. Note that 50 kW is the power absorbed by the plasma, corresponding to microwave generator input power of 60.5 kW, which implies grid-to-plasma power efficiency of 82.6%. Additionally, the reflected power remains small (less than 500 W) during operation due to the microwave autotuner.
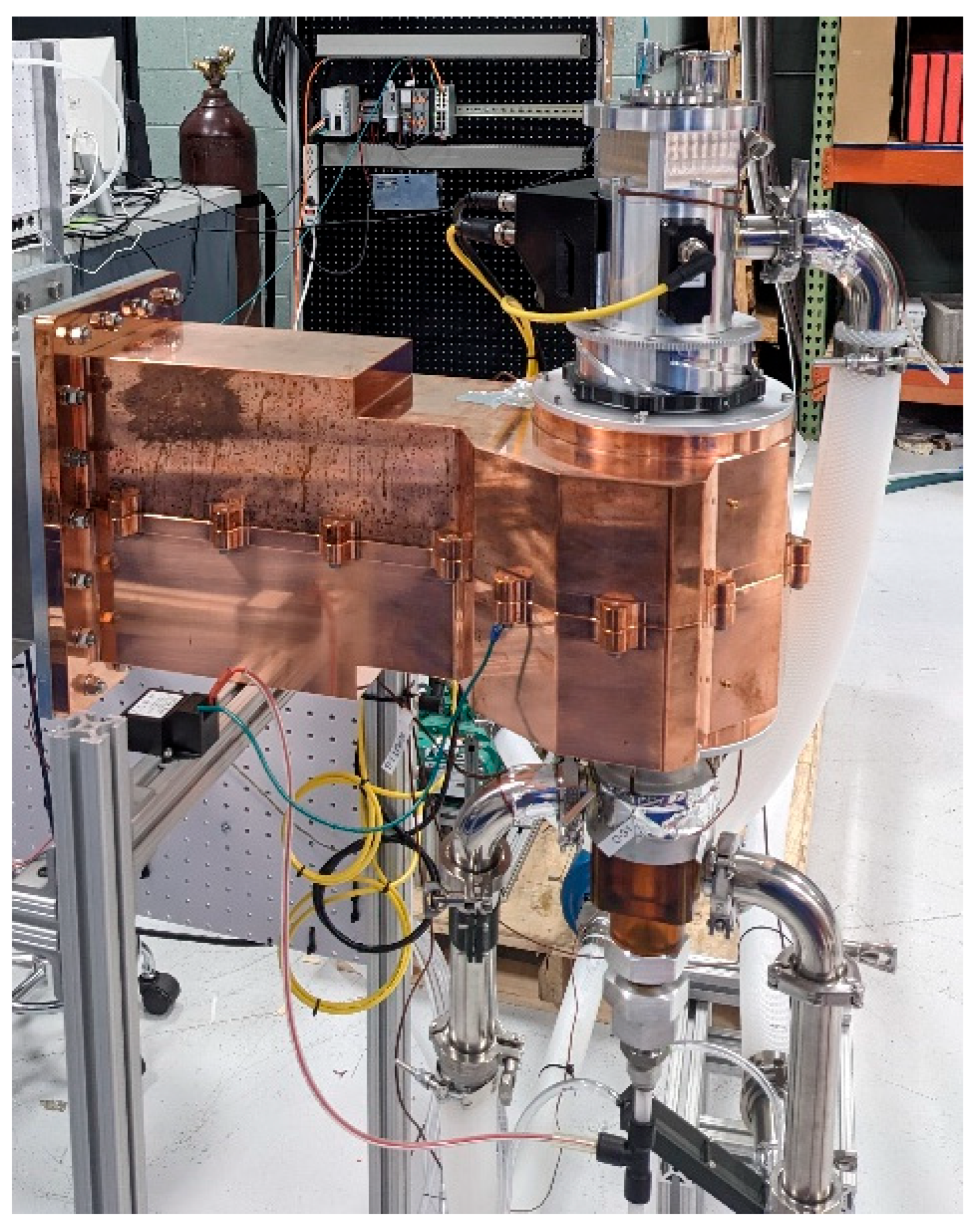
Figure 3 presents an image of the plasma torch used in this work, dismounted from the primary heat exchanger.
2.2. Plant Description
The plant consumes air, water, a small amount of argon (for plasma ignition), and electricity to produce nitric acid and heat. The plant is a closed-loop system consisting of the following components: a plasma torch, a primary heat exchanger, a secondary heat exchanger, four gas blowers (two for process gas and two for cooling gas), a tertiary heat exchanger, an oxidation chamber, two absorption columns, a condenser, a liquid reclamation system, a microwave generator, a microwave autotuner, a pressure swing adsorption (PSA) oxygen generator [
26], liquid pumps, sensors, and interlocks [
27]. Additional pieces of equipment include filters, mass flow controllers (MFCs), gas analyzers, and a programmable logic controller. This subsection describes each process-related component in detail.
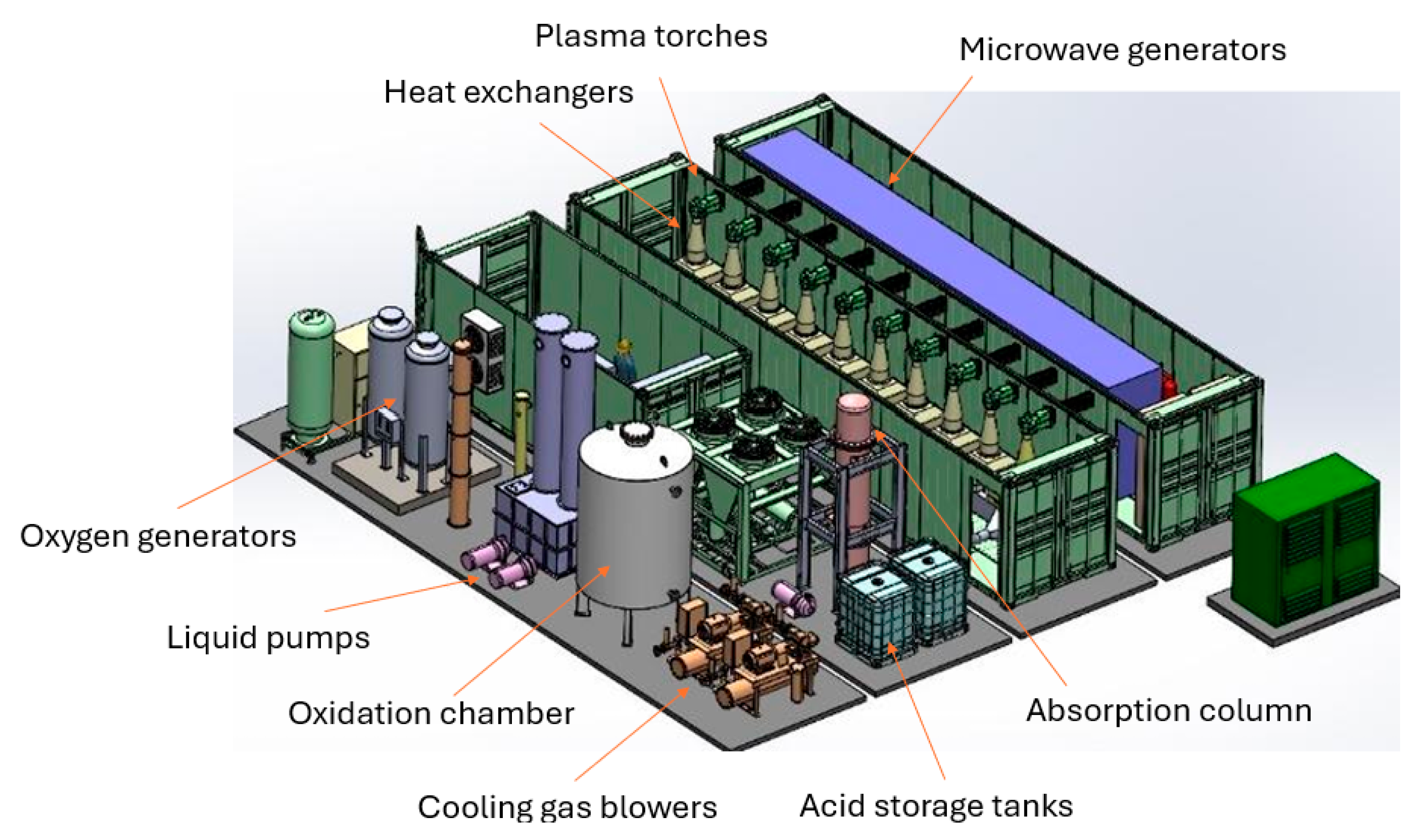
Figure 4 shows a 3D rendering of the plant, highlighting the key components.
Figure 5 shows the constructed nitrogen fixation plant, in which one can see the absorption columns, oxidation chamber, primary heat exchanger, microwave generator, and plasma torch.
Figure 6 shows a block diagram of the closed-loop gas circuit of the nitrogen fixation plant. The loop gas is processed by the plasma torch, converting a fraction of molecular oxygen and molecular nitrogen into nitrogen monoxide (NO). NO is further oxidized in the oxidation chamber, becoming primarily nitrogen dioxide, before the process gas is introduced to water-filled bubble absorption columns to form concentrated nitric acid. A demister and a condenser are used to limit liquid accumulation anywhere in the system except in the absorption columns. The loop gas is then returned to the plasma torch after being regenerated with the appropriate quantities of N
2 and O
2 from an air compressor and an oxygen PSA unit.
Stainless-steel components and piping are used to ensure resistance to corrosive gases and liquids. Where necessary, PTFE and acid-resistant flexible tubing are employed as alternatives to stainless steel. The plasma torch heats the loop gas from ambient temperature to a bulk temperature of 1500–3000 °C, depending on the input power. The plasma torch head consists of a copper-plated aluminum body, a pair of Cerawave™ rings, PTFE ring holders, and three quartz tubes. During batch operation, plasma gas, at 1,300 slpm, passes between the auxiliary and inner gas tubes. Auxiliary gas, controlled by MFC at 40 slpm, flows interior to the auxiliary tube. For cooling purposes, air, which does not mix with the loop gas, passes between the inner and outer quartz tubes and between the Cerawave™ rings and the outer tube.
The closed-loop system is equipped with three heat exchangers. A primary heat exchanger, HEX #1, is directly mounted to the top of the plasma torch outlet and features a conical diffuser with a top diameter of approximately 60 cm. The surface of the diffuser cone is currently not part of the heat recovery system and is simply cooled by forced air; its temperature typically ranges from 300 to 450 °C. The primary heat exchanger operates at approximately 100 L of water per minute and recovers approximately 60% of the plasma energy to heat a 7500 L water tank to a temperature of 60 °C. The 7500 L tank is meant to simulate an ammonia stripper in an agricultural setting. A secondary heat exchanger immediately follows the primary heat exchanger to further reduce the loop gas temperature from approximately 100 °C to room temperature. This is necessary to protect the process gas blowers. A tertiary heat exchanger is used to cool the loop gas after compression to improve both the oxidation and absorption efficiencies in the oxidation chamber and absorption columns, respectively.
Two process gas blowers, which are controlled by variable-frequency drives, move gas around the closed-loop system. The plant can be operated in either quench or bypass mode, where
Figure 6 shows quench mode. In bypass mode, the output of blower #2 is routed to HEX #3 instead of HEX #1, where it combines with the output from blower #1 before passing through the remainder of the closed loop. Additionally, in bypass mode, a portion of the process gas, after being replenished with air and O
2, bypasses the plasma torch and is routed directly to HEX #1 to facilitate the quenching of the plasma gas. Quench mode is utilized primarily in this work because of the improved NO
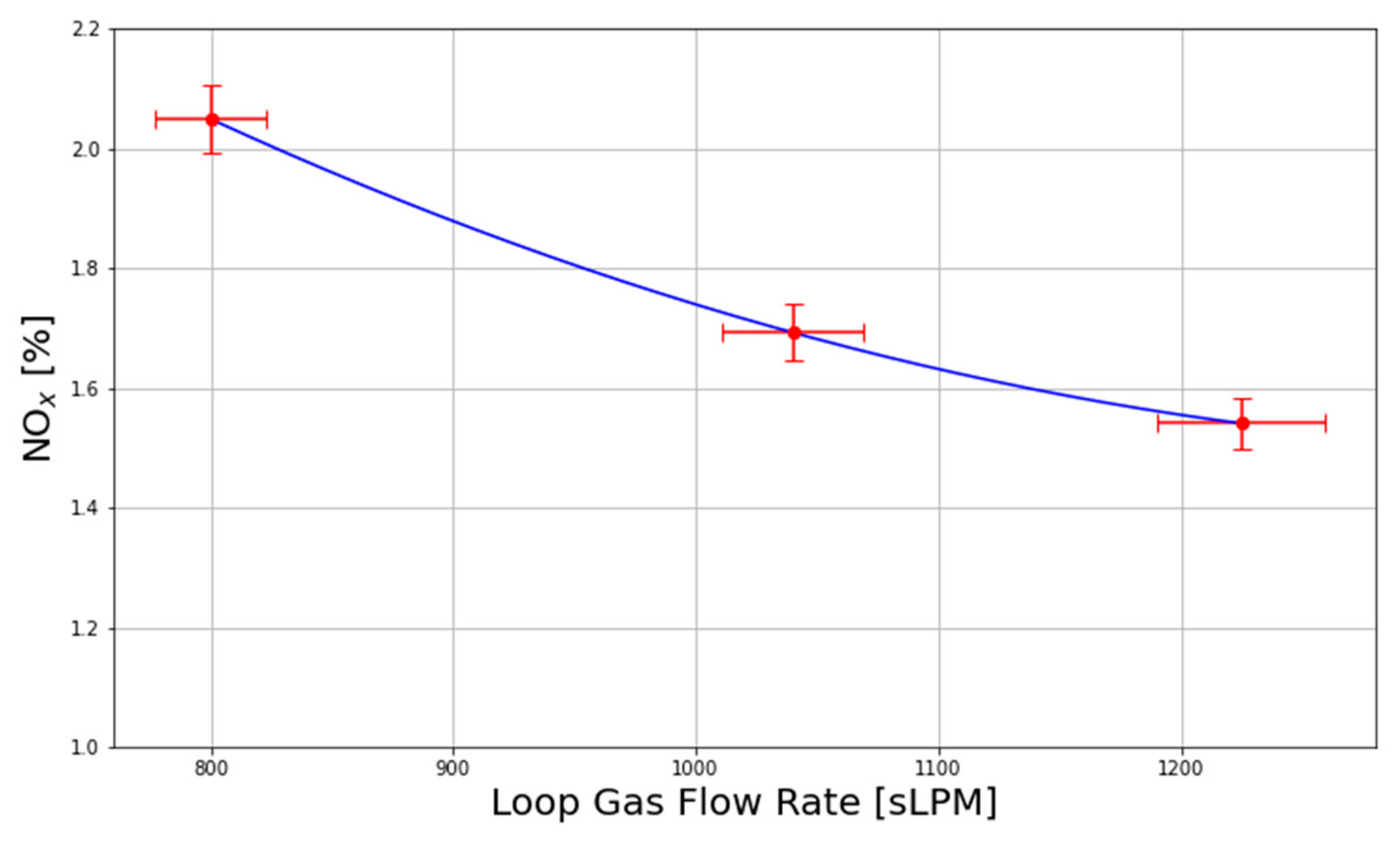
x absorption efficiency of the bubble columns associated with the greater loop gas residence time. Quench mode exhibits a greater loop gas residence time due to the lower loop gas flow rate because only one blower is forcing air around the closed loop, rather than two, as in bypass mode.
The oxidation chamber has a diameter and a height of 90 cm and 1.5 m, respectively, for a total volume of approximately one cubic meter. This implies a loop gas residence time of approximately 45 s for batch operation conditions. The gas pressure in the oxidation chamber is kept at 13.5 psig, enhancing the reaction rate of NO oxidation by approximately a factor of eight relative to ambient pressure. This reduces the necessary volume of the oxidation chamber by the same factor.
There are two bubble absorption columns connected in parallel. The choice of using two absorption columns was made to facilitate improved absorption efficiency given the gas flow requirements of the system set by the torch. The diameter and height of each column are 30 cm and 3 m, respectively. Each column contains 19 sintered stainless-steel gas diffusers, model SS6-7, from Oxidation Technologies. Approximately 100 L of water is added to each column prior to batch operation.
Figure 7 shows the NO
x concentration before and after the absorption columns, implying that the absorption efficiency of the columns employed in this work is only 65% near batch operation conditions, offering a significant opportunity for improvement. Note that the data presented in
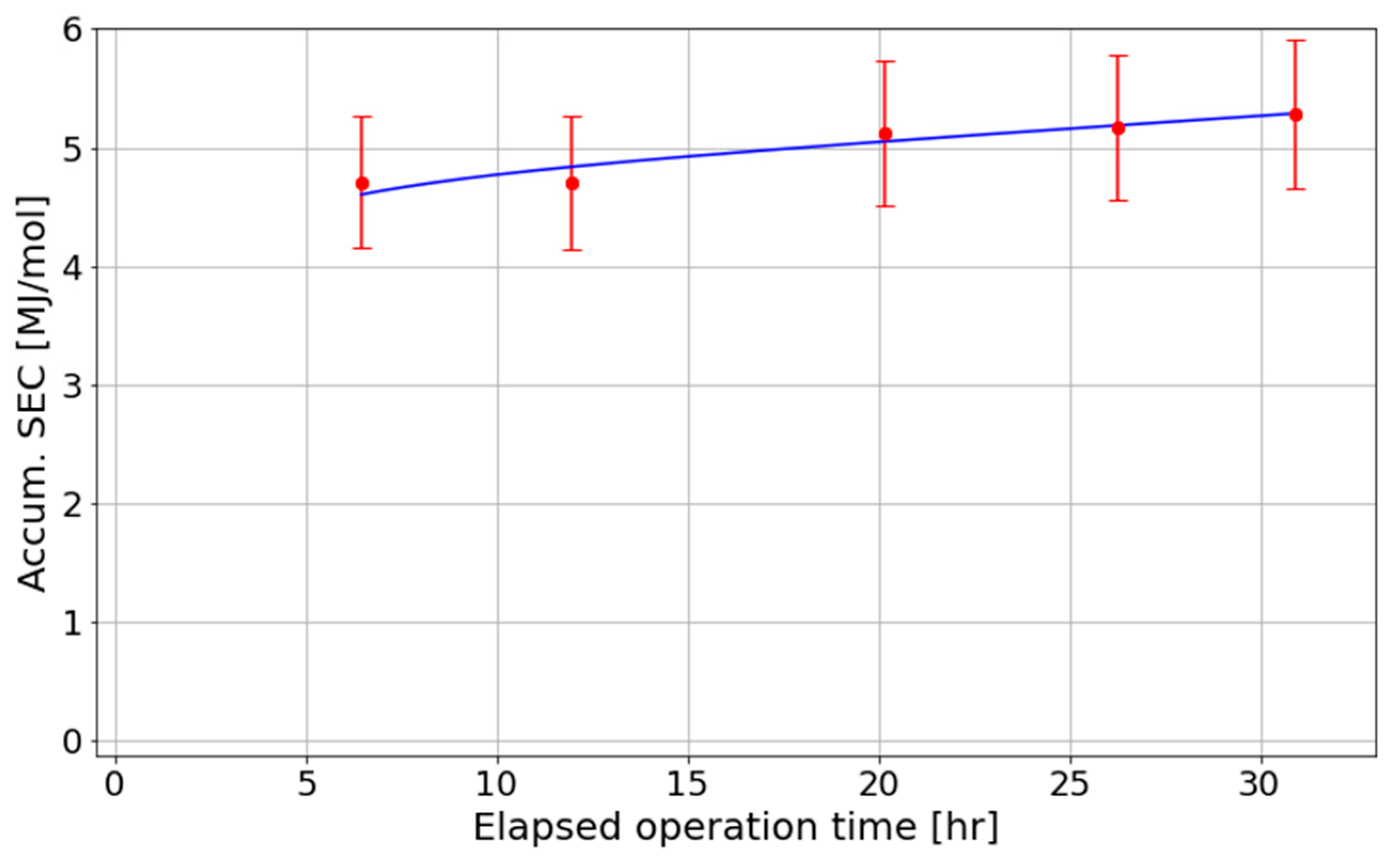
Figure 7 correspond to the absorption columns containing low-concentration nitric acid (<5%). As the concentration of nitric acid increases, the absorption efficiency decreases, as evidenced by the worsening accumulated SEC of the system presented in
Section 3.
Water and acid are introduced, extracted, and recirculated through the absorption columns by an acid-resistant liquid pump to facilitate mixing and safe removal. Air is supplied to the system by an air compressor and routed to the PSA oxygen generator, the air resupply MFC, and the auxiliary gas MFC. Note that, since NOx does not pass through any of the MFCs in the system, gas calibration with respect to NOx is unnecessary. During the oxidation and absorption processes, NO is converted to NO2 before being absorbed by water to form HNO3, effectively removing a portion of N and O from the process gas. Therefore, it is necessary to resupply nitrogen and oxygen to the system to maintain steady-state conditions. To maintain an oxidation chamber pressure of 13.5 psig and an O2 concentration of 20%, the equilibrium air resupply rate is about 50 slpm (40 slpm for auxiliary flow and 10 slpm for the resupply MFC), and the equilibrium oxygen (95% O2, 5% Ar) resupply rate is approximately 18 slpm. An additional challenge within the closed-loop system is the accumulation of argon, an inert gas that remains unaffected by the chemical process and can accumulate to a concentration of 5% or more in the loop. Therefore, purging of the gas is necessary to prevent Ar from concentrating and to maintain system efficiency. The matter of Ar accumulation is addressed in this work by a small process gas leak near the process gas blowers, which is immediately diluted and removed via the exhaust system
Ideally, all liquid in the system remains in the absorption columns. However, droplets and acid vapor can travel throughout the system and condense, causing undesirable liquid accumulation outside the absorption columns. To prevent this, a demister and a condenser are installed directly after the absorption columns. An automated, pressure-based system is used to return liquid collected by the condenser back to the absorption columns after enough liquid is collected.
Given the chemical reactions taking place and the power involved, extensive safety measures are implemented to both mitigate risks and ensure safe operation of the system. The hazards include high-power radio-frequency (RF) emissions, corrosive gases, corrosive liquids, high temperatures, and high pressures. Regarding high-power RF, the plasma torch is equipped with electromagnetic radiation chokes that do not permit, as verified by electromagnetic emissions testing, radiation intensity outputs exceeding one milliwatt per square centimeter. Regarding corrosive gases, industrial-strength exhaust systems are installed above both gas blowers and both absorption columns to capture leaking gas and to refresh the air in the experiment hall. Two NOx gas sensors with sound and vibration alarms are installed on opposite ends of the plant to notify operators of significant gas leaks. Regarding corrosive liquids, appropriate PPE is worn during acid sample acquisition or any other activity that could lead to nitric acid exposure. Liquid and solid sodium bicarbonate are also readily available for acid neutralization. Lastly, communication between operators is facilitated via wireless headsets to minimize the potential for miscommunication in the noisy plant environment.
2.3. Instrumentation
Maintaining critical system parameters, such as oxygen concentration and pressure, at constant levels is essential for optimal performance. To achieve this, the pilot plant is equipped with an array of sensors, meters, gas analyzers, and feedback control loops. These instruments are designed to ensure the safe, reliable, and repeatable operation of the plant. Detailed information about the pressure, oxygen, temperature, NOx concentration, and other relevant measurements will be provided in the following subsection.
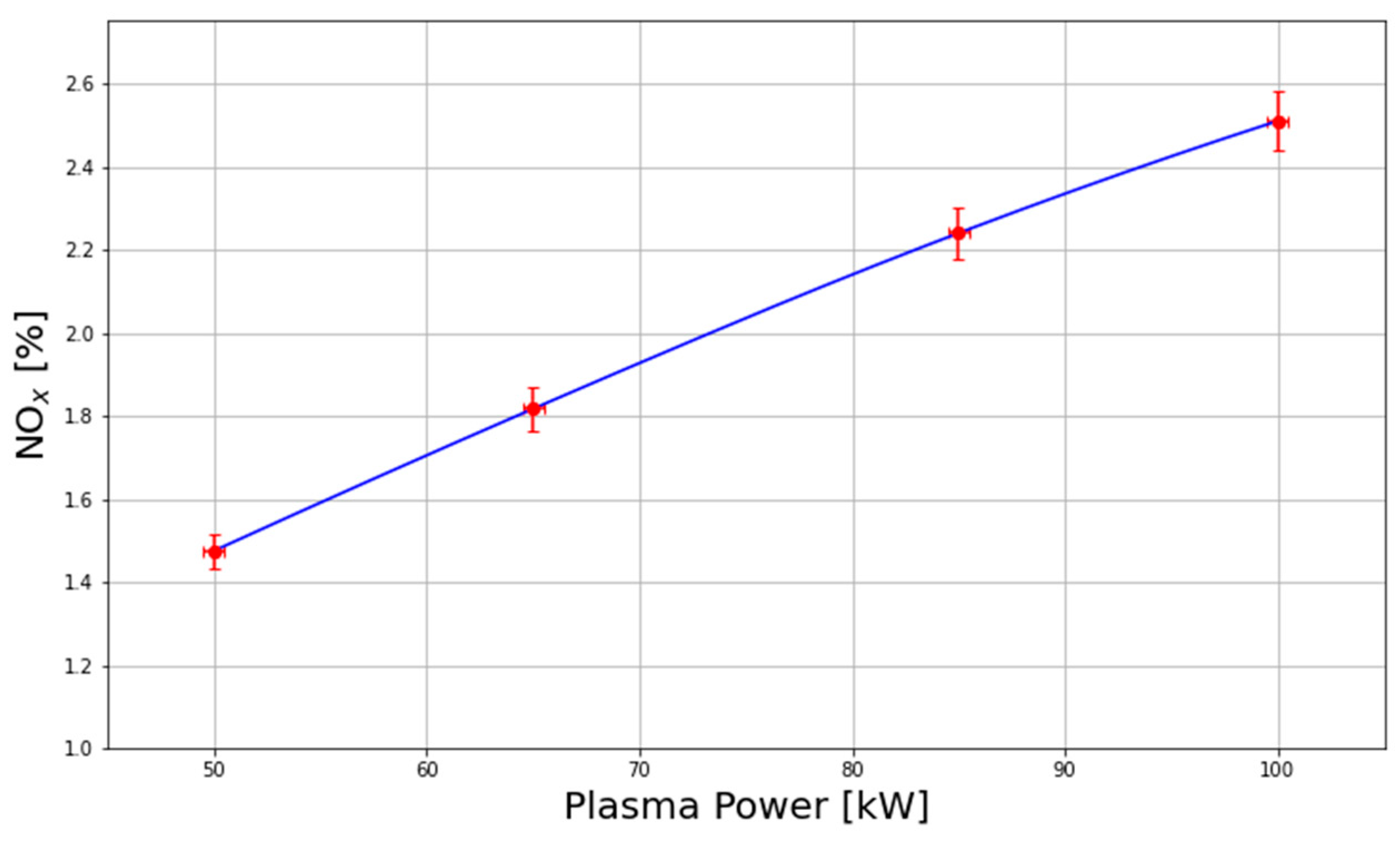
A Thermo Scientific 42iQHL pre-calibrated gas analyzer and an O2 analyzer from Hubei Cubic-Ruiyi Instrument Co., Ltd. were installed at the output of the oxidation chamber to determine the NO, NO2, and O2 gas concentrations. The gas sample was regulated via a needle valve and passed through a series of filtration stages, including water filter traps with charcoal and gravity traps, to protect the equipment from acid droplets and vapor. The NOx concentration was recorded in the internal database of the analyzer, while the O2 measurements from the oxygen analyzer were used as inputs for a proportional–integral–derivative (PID) control system. Due to the limited NOx measurement range (0–5000 ppm) of the gas analyzer, the sampling gas was diluted tenfold with nitrogen. The output gas from the analyzers was routed to the experimental hall exhaust, providing an extra purge for the system. The Thermo Scientific 42iQHL gas analyzer used in this work is used to quantify nitric oxide and nitrogen dioxide based on the principle of chemiluminescence. In this method, NO reacts with ozone to form electronically excited nitrogen dioxide, which subsequently decays to its ground state by emitting a photon in the ultraviolet–visible range. The intensity of the emitted radiation is directly proportional to the NO concentration in the sampled gas. This light is detected and amplified by a photomultiplier tube, which converts the optical signal into an electrical signal for quantitative analysis. Measurement of NO2 is performed indirectly by passing the sample gas through a heated molybdenum converter, where NO2 is reduced to NO. The resulting total NOx (i.e., NO and converted NO2) is then measured using the same chemiluminescence process. The concentration of NO2 is determined by subtracting the directly measured NO from the total NOx. The maximum NOx concentration achieved during plasma operation was approximately 3.0% using 100 kW of absorbed plasma power and low gas flow rates (< 900 slpm).
Gas flows through the absorption columns and torch were monitored using pre-calibrated ProSense FTS and Keyence MPF flow meters. The operational principle of these flow meters renders them insensitive to fluctuations in temperature and pressure. A liquid flow meter from Keyence was used to monitor the water circulation flow rate on the liquid side of the primary heat exchanger. An array of mass flow controllers, specifically the Sevenstar D07 models, was installed to regulate the resupply gas flow rates.
The pressure sensors utilized in the pilot plant were the ProSense EPS25-V145-1001 and the Keyence MP-F models. Pressure drops across various components in the system were continually monitored during steady-state operation, with the primary contributors to the pressure drop being the absorption columns (approximately 5 psi from the liquid height and gas diffusers combined at full flow) and the plasma torch (approximately 7 psi of pressure drop at full flow). Type K thermocouples were employed to monitor the temperatures of various torch components and provided real-time feedback to the PLC system for the activation of interlocks if the gas temperatures exceeded the safety thresholds. The absorption columns were welded to two load cells to measure changes in weight due to increased acid concentrations. As the concentration of the nitric acid increases, the density of the solution correspondingly increases, providing an additional way to monitor the progress of nitric acid production.
The concentration of nitric oxides was continuously monitored during plasma operation, as outlined previously. The efficiency of the absorption columns was also ascertained by sampling the process gas both upstream and downstream of the absorption columns, as shown in
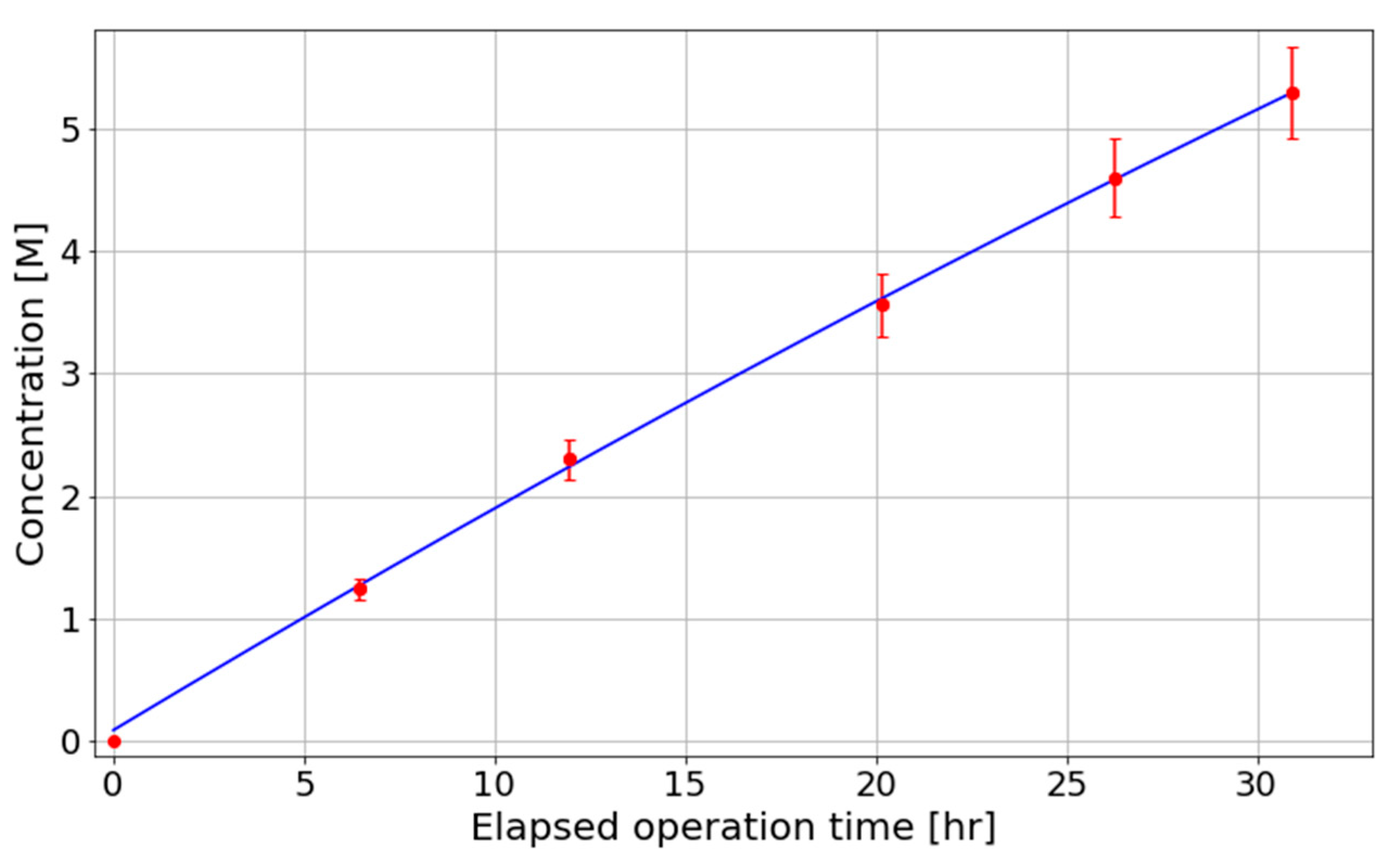
Figure 7. Additionally, after extended periods of plant operation, the concentration of nitric acid was determined through titrimetric analysis to determine the rate of acid accumulation. Titrimetric analysis was performed in triplicate for each sample using either 0.1 M NaOH or 2 M NaOH as the titrant, depending on the stage of plasma operation; specifically, 0.1 M NaOH was employed for samples collected at the beginning of the plasma process, while 2 M NaOH was used for samples obtained at later stages, where higher acid concentrations were expected. The concentration of HNO
3 in each sample was determined by averaging the results of the three titrations. The resulting data are presented in
Section 3. Prior to sampling, the liquid within the absorption columns was thoroughly homogenized by operating the liquid pump connected to the absorption columns and evacuating a substantial volume of liquid to ensure that the sample represented a pure, homogeneous liquid. The performance of the pilot plant was subsequently evaluated by means of two independent measurements: one based on the decrease in the NO
x concentration across the absorption column at a given gas flow rate and the other from the results of titrimetric tests. The efficiency of oxidation of NO could also be ascertained by observing the relative contributions of NO and NO
2 to the overall NO
x post-oxidation chamber. The findings from these gas composition analyses are detailed in the Results section.
2.4. Process Modeling
This section presents numerical models that were developed for the dynamical analysis of the plasma-based nitric acid production process operating in a closed-loop configuration. The analysis yields time-dependent concentrations of individual gas species, models pressure drops across loop components, and provides an estimate of the absorption efficiency for bubble columns. The model was found to be useful in plant design and in programming automated process controls, allowing for a knowledge-driven approach to optimizing the performance of the nitrogen fixation system.
A simplified mass balance model of the closed-loop system was developed, as shown in
Figure 8. NO is generated in the plasma torch from N
2 and O
2. The gas exiting the torch is compressed by a blower and directed to an oxidation chamber, where it is assumed that all NO is converted to NO
2 via oxidation with O
2. Subsequently, the gas flows into an absorption column, where NO
2 is absorbed with specified absorption efficiency. To prevent the accumulation of argon in the loop from the PSA resupply gas, a portion of the loop gas is purged following the absorption column. Compressed air and oxygen are then resupplied to the process gas, which is returned to the torch.
The model considers five gas components: NO, NO2, N2, O2, and Ar. A steady-state condition is determined using mass balance equations. To describe the dynamic behavior of the process, both the pressure drop and equipment volume must be included. The oxidation chamber and absorption column, due to their significant volumes, contribute significantly to the dynamic capacitance of the system. Their sizes are incorporated into the dynamic model. Valves are included in the model between all components, except between the torch and oxidation chamber, to account for pressure variations. The pressure differential between the torch and oxidation chamber is managed by a compressor, whose flow rate–pressure relationship is determined experimentally. The equations for the mass balance and pressure drop of each unit are formulated as follows.
Resupply:
where
i,in and
i,out are the molar flow rates of component i at the inlet and outlet of each unit, respectively. P
in and P
out are the pressures at the inlet and outlet of each unit, respectively. f is the friction coefficient used to calculate the pressure drop from the molar flow rate for the valves. K
1 and K
2 are coefficients used to calculate the pressure drop of the compressor. x
NO is the mole fraction of NO in the torch outlet gas. η is the absorption efficiency. p is the purge fraction. x
i,air and x
i,PSA are the mole fractions of component i in the resupply air and the PSA gas, respectively. P
i,ox and P
i,ab are the partial pressures of component i in the oxidation chamber and the absorption column, respectively. V
ox and V
ab are the volumes of the oxidation chamber and the absorption column, respectively.
To ensure the smooth operation of the nitrogen fixation process, it is essential to maintain both the pressure and oxygen concentration within the oxidation chamber.
Figure 8 illustrates the fundamental control structure for this system (dashed lines). The pressure is regulated by adjusting the flow rate of compressed air, whereas the oxygen concentration is controlled by modulating the flow rate of oxygen produced by the PSA system. Two PID controllers are employed to manage these controls.
The absorption efficiency serves as an input for the closed-loop model and can be predicted using a separate model specifically for the absorption column. The absorption of NOx in the bubble columns is a crucial step in converting gaseous NOx, generated by plasma, into a liquid product of nitric acid at the desired concentration. To achieve a more comprehensive understanding of NOx absorption within a bubble column, we have developed a dedicated model to elucidate this process.
The absorption of NO
x in a bubble column is a complex process that encompasses multiphase fluid flow, mass transfer, and chemical reactions. The model considers several components, including nitric oxide, nitrogen dioxide (NO
2), dinitrogen trioxide (N
2O
3), dinitrogen tetroxide (N
2O
4), nitrous acid (HNO
2), nitric acid (HNO
3), oxygen (O
2), and water (H
2O). If ozone is present in the gas phase, it can react with NO
2 to form nitrogen trioxide radical (NO
3) and O
2. The NO
3 radical can subsequently react with additional NO
2 to produce dinitrogen pentoxide (N
2O
5) [
28]. However, due to the negligible concentration of ozone under the current conditions, the formation of both NO
3 and N
2O
5 is insignificant. Therefore, these species are not included in the present model.
The gas phase is governed by a set of complex equilibria, described by Equations (37)–(40). During the absorption process, NO
2, N
2O
4, and N
2O
3 are absorbed into the aqueous phase (Equations (41)–(43)), where they react with water to form HNO
2 and HNO
3 (Equations (44)–(46)). The generated HNO
2 subsequently decomposes in the liquid phase, releasing NO (Equation (47)), which then desorbs back into the gas phase (Equation (48)). The desorbed NO can react with O
2 to regenerate NO
2 (Equation (49)); however, this is a relatively slow reaction. The resulting change in the NO
2 concentration can shift the equilibrium of other gas-phase components.
The model is based on the following assumptions:
The gas phase exhibits plug flow behavior;
The liquid holdup is uniform throughout the column, leading to a consistent effective interfacial area and uniform mass transfer coefficients across the column;
The gases follow ideal gas behavior;
There are no radial concentration gradients in the gas phase;
There are no concentration gradients in the liquid phase in either the radial or axial directions;
The column operates under steady-state conditions;
The process is maintained under isothermal conditions.
The governing equations that describe the mass balance for the gases bubbling through the liquid in the bubble column are expressed as follows.
Divalent nitrogen balance:
Reactive nitrogen balance:
Oxygen balance:
where
YNO*,
YN*,
YH2O*, and
YO2 represent the concentrations (in kmol per kmol of inert) of divalent nitrogen species, reactive nitrogen species, water (including oxyacids and free water vapor), and oxygen, respectively.
h denotes the height from the bottom of the bubble column, and
S is the column’s cross-sectional area.
G represents the inert gas flow rate,
R is the universal gas constant, and
T is the temperature.
k1 is the reaction rate constant for the oxidation of NO, while
pNO and
pO2 are the partial pressures of NO and O
2, respectively. ε
G denotes the gas holdup. The terms Ra
NO,G, Ra
NO2,G, Ra
N2O3,G, Ra
N2O4,G, Ra
HNO2,G, Ra
HNO3,G, and Ra
H2O,G represent the gas-phase mass transfer rates of NO, NO
2, N
2O
3, N
2O
4, HNO
2, HNO
3, and H
2O, respectively. These equations are coupled linear differential equations. By solving them simultaneously, the absorption efficiency is determined based on the calculated outlet NO
x concentration and the predetermined inlet NO
x concentration. The method for the calculation of each term within the governing equations is comprehensively outlined in Suchak et al. [
29]. Additionally, the volumetric mass transfer coefficients for both gas and liquid phases in a bubble column are detailed in Liu et al. [
30].