Microbial Biotechnologies for Salt Tolerance in Alfalfa: Agro-Nutritional Comparison Between Local and Imported Varieties
Abstract
1. Introduction
2. Materials and Methods
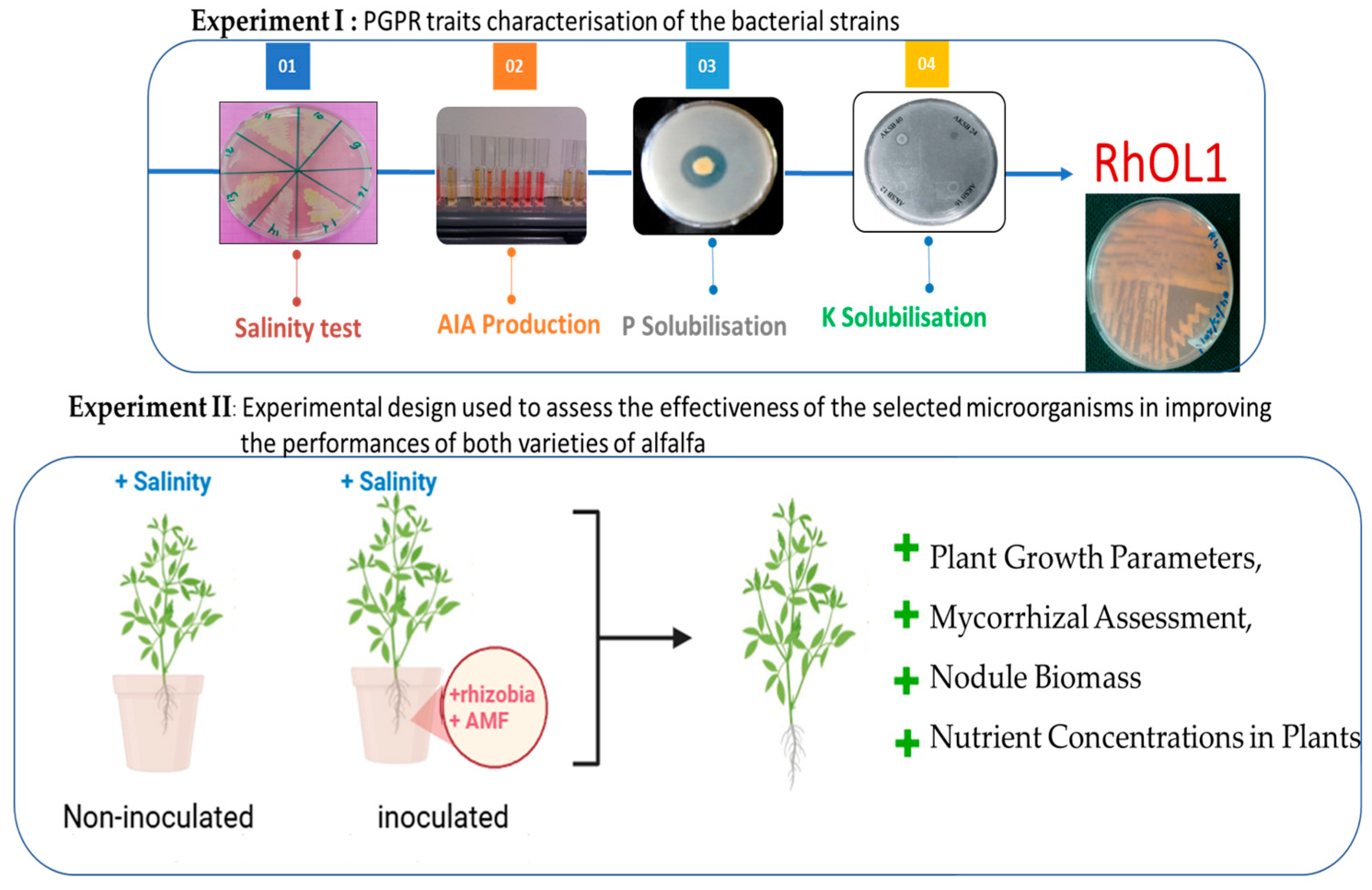
2.1. Experiment I
2.2. Experiment II
2.2.1. Plant and Biofertilizer Materials
2.2.2. Plant Growth Conditions
2.2.3. Experimental Design and Treatments
2.2.4. Plant Growth Parameters, Mycorrhizal Assessment, and Nodule Biomass
2.2.5. Determination of Nutrient Concentrations in Plants
2.2.6. Statistical Analysis
3. Results
3.1. Physiological Characterization of Rhizobia Strains
3.1.1. Assessment of Strain Tolerance to Salinity
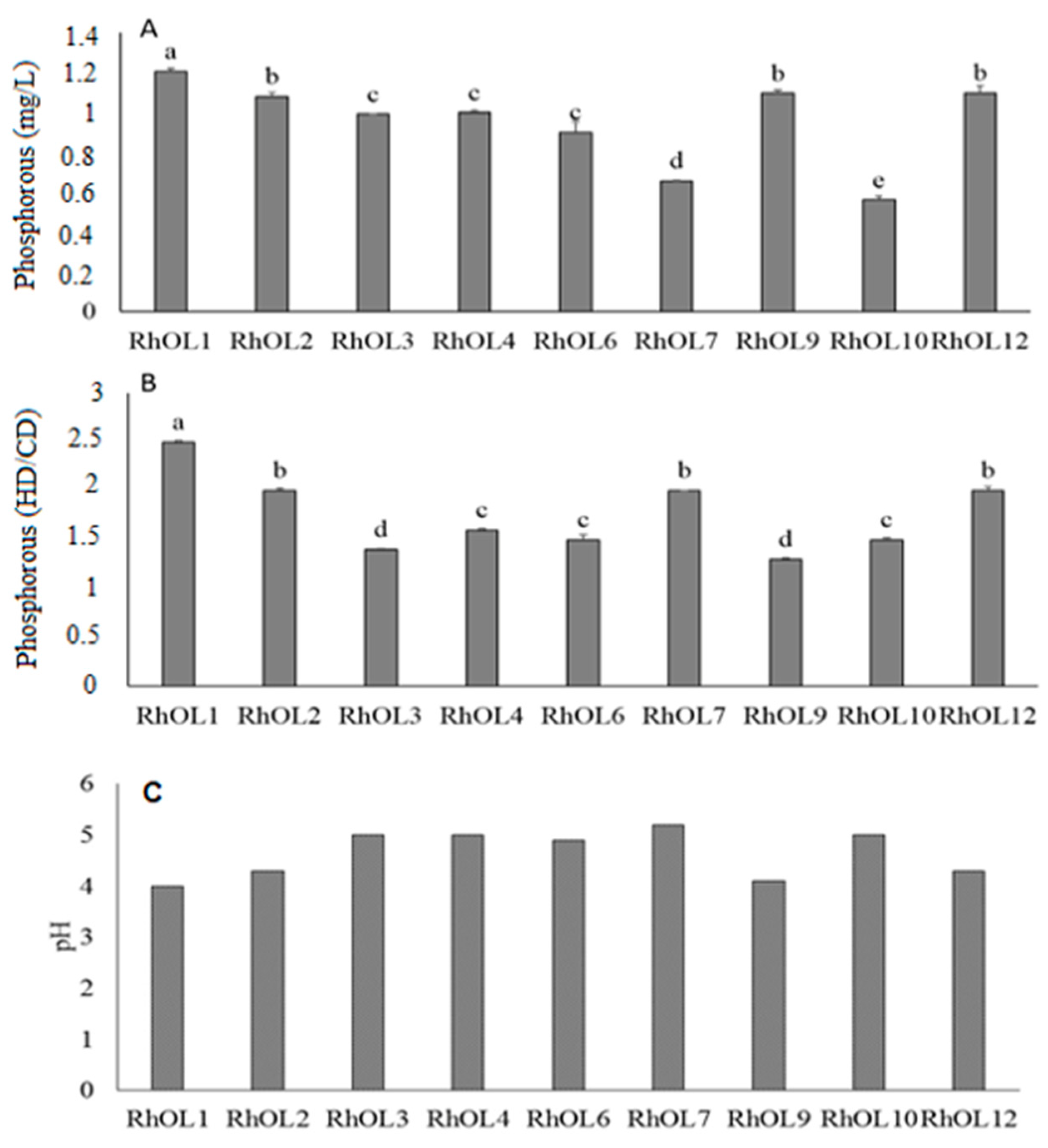
3.1.2. Evaluation of Solubilization Capacity of Insoluble Tricalcium Phosphate
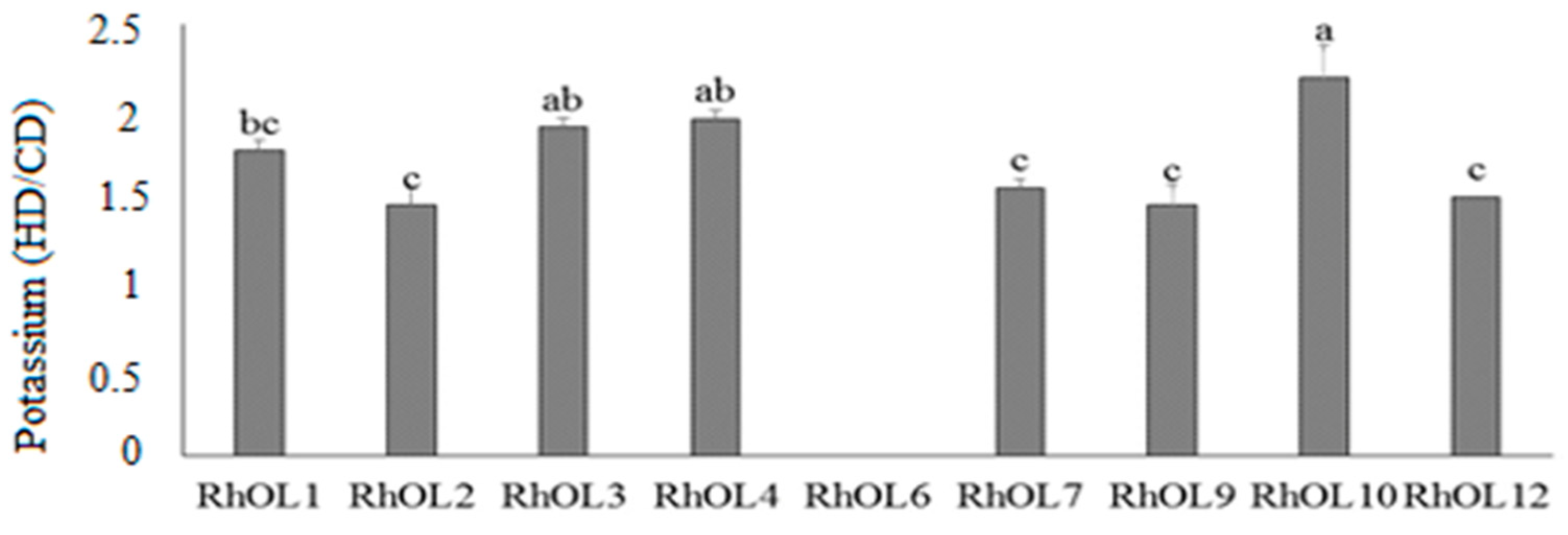
3.1.3. Evaluation of Potassium Solubilization Capacity
3.1.4. Assessment of IAA Production
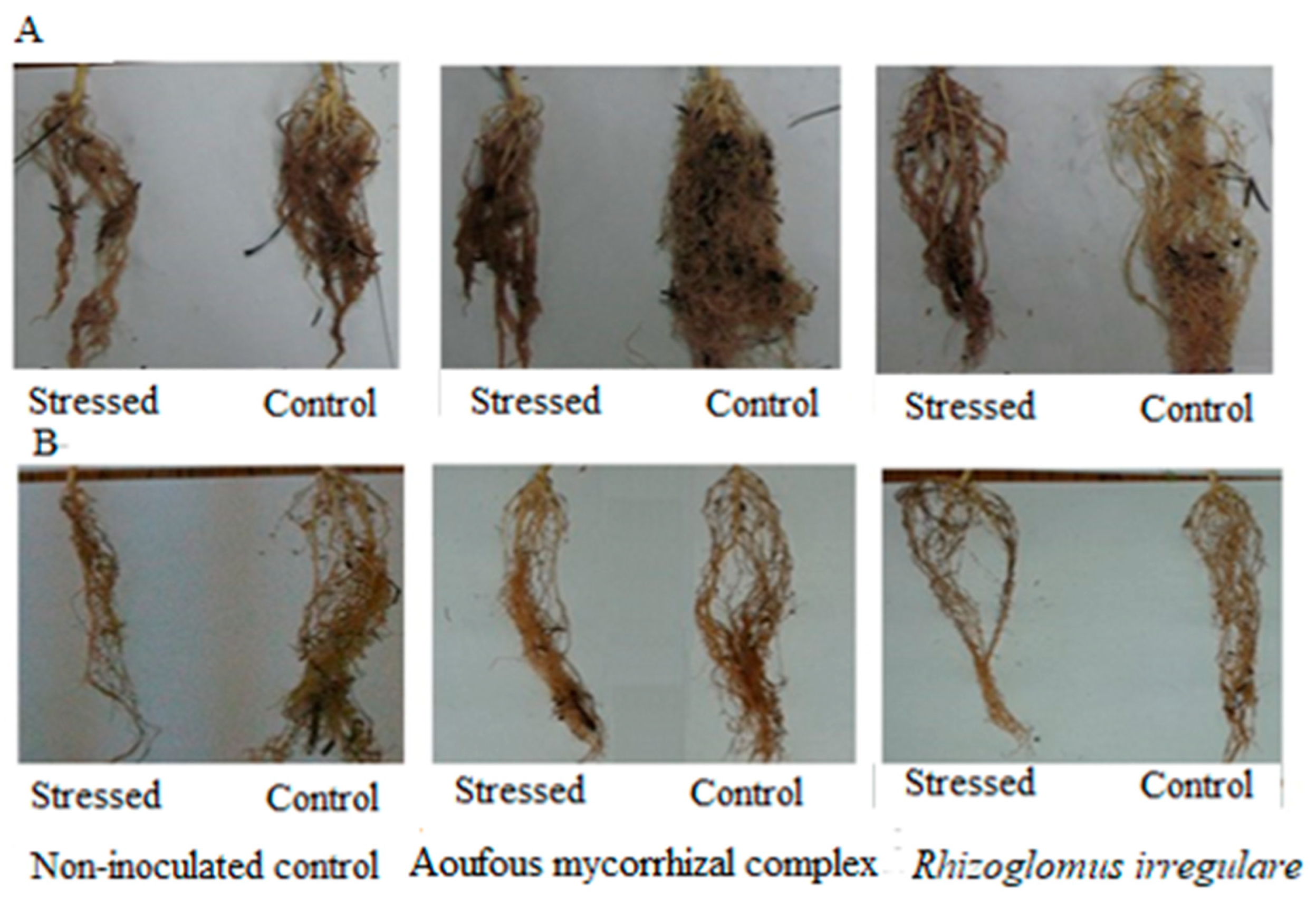
3.2. Effect of Salinity and Biofertilizers on Symbiotic Development
3.3. Effect of Salinity and Biofertilizers on Growth Parameters
3.4. Effect of Salinity and Biofertilizers on Plant Ion Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Fa | AMF infection frequency |
| IAA | Indole acetic acid |
| AMF | Arbuscular Mycorrhizal Fungi |
| Fv/Fm | chlorophyll fluorescence |
References
- Stavi, I.; Thevs, N.; Priori, S. Soil Salinity and Sodicity in Drylands: A Review of Causes, Effects, Monitoring, and Restoration Measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- Liang, C.; Yang, B.; Cao, Y.; Liu, K.; Wu, J.; Hao, F.; Han, Y.; Han, W. Salinization mechanism of lakes and controls on organic matter enrichment: From present to deep-time records. Earth-Sci. Rev. 2024, 251, 104720. [Google Scholar] [CrossRef]
- Cunillera-Montcusí, D.; Beklioğlu, M.; Cañedo-Argüelles, M.; Jeppesen, E.; Ptacnik, R.; Amorim, C.A.; Arnott, S.E.; Berger, S.A.; Brucet, S.; Dugan, H.A.; et al. Freshwater salinisation: A research agenda for a saltier world. Trends Ecol. Evol. 2022, 37, 440–453. [Google Scholar] [CrossRef]
- Liu, M.; Paredes, P.; Shi, H.; Ramos, T.B.; Dou, X.; Dai, L.; Pereira, L.S. Impacts of a shallow saline water table on maize evapotranspiration and groundwater contribution using static water table lysimeters and the dual Kc water balance model SIMDualKc. Agric. Water Manag. 2022, 273, 107887. [Google Scholar] [CrossRef]
- Prusty, P.; Farooq, S.H. Seawater intrusion in the coastal aquifers of India—A review. HydroResearch 2020, 3, 61–74. [Google Scholar] [CrossRef]
- Sp, C.; Latine, R. Perspectives Agricoles de l’OCDE et de la FAO 2019–2028; FAO and OECD: Rome, Italy, 2020; ISBN 9789264312715. [Google Scholar]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Pecetti, L.; Franguelli, N. Agronomic value of alfalfa semi-hybrids across contrasting Italian environments. Crop Sci. 2025, 65, e21425. [Google Scholar] [CrossRef]
- Raza, A.; Zahra, N.; Hafeez, M.B.; Ahmad, M.; Iqbal, S.; Shaukat, K.; Ahmad, G. Nitrogen Fixation of Legumes: Biology and Physiology. In The Plant Family Fabaceae; Springer: Singapore, 2020; pp. 43–74. [Google Scholar]
- Munns, R.; Gilliham, M.; Munns, R.; Gilliham, M. Tansley insight Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Babalola, O.O. The rhizosphere microbial complex in plant health: A review of interaction dynamics. J. Integr. Agric. 2022, 21, 2168–2182. [Google Scholar] [CrossRef]
- Han, S.; Yoshikuni, Y. Microbiome engineering for sustainable agriculture: Using synthetic biology to enhance nitrogen metabolism in plant-associated microbes. Curr. Opin. Microbiol. 2022, 68, 102172. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Asaf, S.; Kamran, M.; Shahzad, R.; Bilal, S.; Khan, M.A.; Kang, S.M.; Kim, Y.H.; Yun, B.W.; et al. Plant growth-promoting endophyte Sphingomonas sp. LK11 alleviates salinity stress in Solanum pimpinellifolium. Environ. Exp. Bot. 2017, 133, 58–69. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Acharya, B.R.; Gill, S.P.; Kaundal, A.; Sandhu, D. Strategies for combating plant salinity stress: The potential of plant growth-promoting microorganisms. Front. Plant Sci. 2024, 15, 1406913. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Ech-chatir, L.; Anli, M.; Ben-Laouane, R.; Boutasknit, A.; Meddich, A. Secondary Metabolites, Osmolytes and Antioxidant Activity as the Main Attributes Enhanced by Biostimulants for Growth and Resilience of Lettuce to Drought Stress. Gesunde Pflanz. 2023, 75, 1737–1753. [Google Scholar] [CrossRef]
- Ben-Laouane, R.; Baslam, M.; Ait-El-mokhtar, M.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Toubali, S.; Mitsui, T.; Oufdou, K.; Wahbi, S.; et al. Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms 2020, 8, 1695. [Google Scholar] [CrossRef]
- Gorgia, P.; Tsikou, D. Tripartite Symbiosis Between Legumes, Arbuscular Mycorrhizal Fungi and Nitrogen Fixing Rhizobia: Interactions and Regulation. Plant. Cell Environ. 2025. [Google Scholar] [CrossRef]
- El-Khalloufi, F. Hytotoxicité Induite par les Cyanotoxines: Effets des Microcystines sur la Croissance de Solanum Lycopersicum et sur Medicago sativa et sa Microflore Rhizosphérique. Ph.D. Thesis, Cadi Ayyad University, Marrakesh, Morocco, 2012. [Google Scholar]
- Olsen, S.R.; Dean, L.A. Phosphorus: Methods of soil analysis. Am. Soc. Agron. 1965, 9, 920–926. [Google Scholar]
- Alikhani, H.A.; Saleh-Rastin, N.; Antoun, H. Phosphate solubilization activity of rhizobia native to Iranian soils. Plant Soil 2006, 287, 35–41. [Google Scholar] [CrossRef]
- Parmar, P.; Sindhu, S. Potassium solubilization by rhizosphere bacteria: Influence of nutritional and environmental conditions. J. Microbiol. Res. 2013, 3, 25–31. [Google Scholar]
- Bano, N.; Musarrat, J. Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr. Microbiol. 2003, 46, 324–328. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst. 2020, 4, 131. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Ben-Laouane, R.; Lahlali, R.; Anli, M.; Ikan, C.; Boutasknit, A.; Slimani, A.; Oufdou, K.; Baslam, M.; Ait Barka, E.; et al. Application of Indigenous Rhizospheric Microorganisms and Local Compost as Enhancers of Lettuce Growth, Development, and Salt Stress Tolerance. Microorganisms 2022, 10, 1625. [Google Scholar] [CrossRef]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ben Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef]
- Meddich, A.; Jaiti, F.; Bourzik, W.; Asli, A.E.; Hafidi, M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Farissi, M.; Bouizgaren, A.; Faghire, M.; Bargaz, A.; Ghoulam, C. Agro-physiological responses of Moroccan alfalfa (Medicago sativa L.) populations to salt stress during germination and early seedling stages. Seed Sci. Technol. 2011, 39, 389–401. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Ventorino, V.; Caputo, R.; De Pascale, S.; Fagnano, M.; Pepe, O.; Moschetti, G. Response to salinity stress of Rhizobium leguminosarum bv. viciae strains in the presence of different legume host plants. Ann. Microbiol. 2012, 62, 811–823. [Google Scholar] [CrossRef]
- Muntyan, V.S.; Roumiantseva, M.L. Molecular Phylogenetic Analysis of Salt-Tolerance-Related Genes in Root-Nodule Bacteria Species Sinorhizobium meliloti. Agronomy 2022, 12, 1968. [Google Scholar] [CrossRef]
- Pagare, K.A.; Navale, A.M.; Naik, R.M.; Durgude, A.G. Effect of inoculation of salt tolerant rhizobium on nodulation and leghaemoglobin content of soybean. Biosci. Biotechnol. Res. Commun. 2019, 12, 377–383. [Google Scholar] [CrossRef]
- Kearl, J.; McNary, C.; Lowman, J.S.; Mei, C.; Aanderud, Z.T.; Smith, S.T.; West, J.; Colton, E.; Hamson, M.; Nielsen, B.L. Salt-Tolerant Halophyte Rhizosphere Bacteria Stimulate Growth of Alfalfa in Salty Soil. Front. Microbiol. 2019, 10, 1849. [Google Scholar] [CrossRef]
- Wekesa, C.; Asudi, G.O.; Okoth, P.; Reichelt, M.; Muoma, J.O.; Furch, A.C.U.; Oelmüller, R. Rhizobia Contribute to Salinity Tolerance in Common Beans (Phaseolus vulgaris L.). Cells 2022, 11, 3628. [Google Scholar] [CrossRef]
- Domínguez-Ferreras, A.; Pérez-Arnedo, R.; Becker, A.; Olivares, J.; Soto, M.J.; Sanjuán, J. Transcriptome profiling reveals the importance of plasmid pSymB for osmoadaptation of Sinorhizobium meliloti. J. Bacteriol. 2006, 188, 7617–7625. [Google Scholar] [CrossRef]
- Pérez-Montaño, F.; del Cerro, P.; Jiménez-Guerrero, I.; López-Baena, F.J.; Cubo, M.T.; Hungria, M.; Megías, M.; Ollero, F.J. RNA-seq analysis of the Rhizobium tropici CIAT 899 transcriptome shows similarities in the activation patterns of symbiotic genes in the presence of apigenin and salt. BMC Genom. 2016, 17, 198. [Google Scholar] [CrossRef]
- Moussaid, S.; Domínguez-Ferreras, A.; Muñoz, S.; Aurag, J.; Berraho, E.B.; Sanjuán, J. Increased trehalose biosynthesis improves Mesorhizobium ciceri growth and symbiosis establishment in saline conditions. Symbiosis 2015, 67, 103–111. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 46–49. [Google Scholar] [CrossRef]
- Bechtaoui, N.; Raklami, A.; Tahiri, A.; Benidire, L.; El Alaoui, A.; Meddich, A.; Göttfert, M.; Oufdou, K. Characterization of plant growth promoting rhizobacteria and their benefits on growth and phosphate nutrition of faba bean and wheat. Biol. Open 2019, 53, bio043968. [Google Scholar] [CrossRef]
- Bechtaoui, N.; El Alaoui, A.; Raklami, A.; Benidire, L.; Tahiri, A.I.; Oufdou, K. Impact of intercropping and co-inoculation with strains of plant growth-promoting rhizobacteria on phosphorus and nitrogen concentrations and yield of durum wheat (Triticum durum) and faba bean (Vicia faba). Crop Pasture Sci. 2019, 70, 649–658. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from. Biology 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Bianco, C.; Defez, R. Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Appl. Environ. Microbiol. 2010, 76, 4626–4632. [Google Scholar] [CrossRef]
- Cafiero, J.H.; Salvetti Casasco, M.; Lozano, M.J.; Vacca, C.; López García, S.L.; Draghi, W.O.; Lagares, A.; Del Papa, M.F. Genomic analysis of Sinorhizobium meliloti LPU63, an acid-tolerant and symbiotically efficient alfalfa-nodulating rhizobia. Front. Agron. 2023, 5, 1175524. [Google Scholar] [CrossRef]
- Satyaprakash, M.; Nikitha, T.; Reddi, E.U.B.; Sadhana, B.; Vani, S.S. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2133–2144. [Google Scholar] [CrossRef]
- Shome, S.; Barman, A.; Solaiman, Z.M. Rhizobium and Phosphate Solubilizing Bacteria Influence the Soil Nutrient Availability, Growth, Yield, and Quality of Soybean. Agriculture 2022, 12, 1136. [Google Scholar] [CrossRef]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef]
- Singh, G.; Biswas, D.R.; Marwaha, T.S. Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.): A hydroponics study under phytotron growth chamber. J. Plant Nutr. 2010, 33, 1236–1251. [Google Scholar] [CrossRef]
- Olaniyan, F.T.; Alori, E.T.; Adekiya, A.O.; Ayorinde, B.B.; Daramola, F.Y.; Osemwegie, O.O.; Babalola, O.O. The use of soil microbial potassium solubilizers in potassium nutrient availability in soil and its dynamics. Ann. Microbiol. 2022, 72, 45. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects—A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Bouizgarne, B.; Bakki, M.; Boutasknit, A.; Banane, B.; El Ouarrat, H.; Ait El Maalem, S.; Amenzou, A.; Ghousmi, A.; Meddich, A. Phosphate and potash solubilizing bacteria from Moroccan phosphate mine showing antagonism to bacterial canker agent and inducing effective tomato growth promotion. Front. Plant Sci. 2023, 14, 970382. [Google Scholar] [CrossRef] [PubMed]
- Yanni, Y.G.; Rizk, R.Y.; El-Fattah, F.K.A.; Squartini, A.; Corich, V.; Giacomini, A.; de Bruijn, F.; Rademaker, J.; Maya-Flores, J.; Ostrom, P.; et al. The beneficial plant growth-promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Aust. J. Plant Physiol. 2001, 28, 845–870. [Google Scholar] [CrossRef]
- Biswas, J.C.; Ladha, J.K.; Dazzo, F.B. Rhizobia inoculation improves nutrient uptake and growth of lowland rice. Soil Sci. Soc. Am. J. 2000, 64, 1644–1650. [Google Scholar] [CrossRef]
- Noor, A.; Ziaf, K.; Naveed, M.; Khan, K.S.; Ghani, M.A.; Ahmad, I.; Anwar, R.; Siddiqui, M.H.; Shakeel, A.; Khan, A.I. L-Tryptophan-Dependent Auxin-Producing Plant-Growth-Promoting Bacteria Improve Seed Yield and Quality of Carrot by Altering the Umbel Order. Horticulturae 2023, 9, 954. [Google Scholar] [CrossRef]
- Iyer, B.; Rajkumar, S. Rhizobia. Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128096338. [Google Scholar]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Mal, S.; Panchal, S. Drought and salt stress mitigation in crop plants using stress-tolerant auxin-producing endophytic bacteria: A futuristic approach towards sustainable agriculture. Front. Plant Sci. 2024, 15, 1422504. [Google Scholar] [CrossRef] [PubMed]
- Lebrazi, S.; Fadil, M.; Chraibi, M.; Fikri-Benbrahim, K. Screening and optimization of indole-3-acetic acid production by Rhizobium sp. strain using response surface methodology. J. Genet. Eng. Biotechnol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Bruning, B.; Rozema, J. Symbiotic nitrogen fixation in legumes: Perspectives for saline agriculture. Environ. Exp. Bot. 2013, 92, 134–143. [Google Scholar] [CrossRef]
- Duan, H.X.; Luo, C.L.; Wang, X.; Cheng, Y.S.; Abrar, M.; Batool, A. Responses of Legumes to Rhizobia and Arbuscular Mycorrhizal Fungi Under Abiotic Stresses: A Global Meta-Analysis. Agronomy 2024, 14, 2597. [Google Scholar] [CrossRef]
- Ben-Laouane, R.; Meddich, A.; Bechtaoui, N.; Oufdou, K.; Wahbi, S. Effects of Arbuscular Mycorrhizal Fungi and Rhizobia Symbiosis on the Tolerance of Medicago Sativa to Salt Stress. Gesunde Pflanz. 2019, 71, 135–146. [Google Scholar] [CrossRef]
- Namdari, A.; Baghbani Arani, A.; Moradi, A. Arbuscular mycorrhizal (Funneliformis mosseae) improves alfalfa (Medicago sativa L.) re-growth ability in saline soil through enhanced nitrogen remobilization and improved nutritional balance. J. Cent. Eur. Agric. 2018, 19, 166–183. [Google Scholar] [CrossRef]
- Liu, L.; Yahaya, B.S.; Li, J.; Wu, F. Enigmatic role of auxin response factors in plant growth and stress tolerance. Front. Plant Sci. 2024, 15, 1398818. [Google Scholar] [CrossRef]
- Haque, S.I.; Matsubara, Y.-I. Salinity tolerance and sodium localization in mycorrhizal strawberry plants. Commun. Soil Sci. Plant Anal. 2018, 49, 2782–2792. [Google Scholar] [CrossRef]
- Parvin, S.; Van Geel, M.; Yeasmin, T.; Verbruggen, E.; Honnay, O. Effects of single and multiple species inocula of arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 2020, 30, 431–444. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, H.; He, Y.; Jiang, C.; Yang, M.; Ye, S. Legume-bacteria-soil interaction networks linked to improved plant productivity and soil fertility in intercropping systems. Ind. Crops Prod. 2023, 196, 116504. [Google Scholar] [CrossRef]
- Wang, H.; An, T.; Huang, D.; Liu, R.; Xu, B.; Zhang, S.; Deng, X.; Siddique, K.H.M.; Chen, Y. Arbuscular mycorrhizal symbioses alleviating salt stress in maize is associated with a decline in root-to-leaf gradient of Na+/K+ ratio. BMC Plant Biol. 2021, 21, 457. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Glenn, E.P.; Volkov, V. Could vesicular transport of Na+ and Cl− Be a feature of salt tolerance in halophytes? Ann. Bot. 2019, 123, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010, 12, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.; Shagol, C.C.; Kim, K.; Han, S.; Sa, T. Spore associated bacteria regulates maize root K+/Na+ ion homeostasis to promote salinity tolerance during arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2018, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Tahjib-Ul-Arif, M.; Rhaman, M.S. Exogenous Auxin-Mediated Salt Stress Alleviation in Faba Bean (Vicia faba L.). Agronomy 2021, 11, 547. [Google Scholar] [CrossRef]
- Nasslahsen, B.; Prin, Y.; Ferhout, H.; Smouni, A.; Duponnois, R. Mycorrhizae helper bacteria for managing the mycorrhizal soil infectivity. Front. Soil Sci. 2022, 2, 979246. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.-A. Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef]
- Calderon, R.B.; Dangi, S.R. Arbuscular Mycorrhizal Fungi and Rhizobium Improve Nutrient Uptake and Microbial Diversity Relative to Dryland Site-Specific Soil Conditions. Microorganisms 2024, 12, 667. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; El-Enany, A.-W.E.; Nafady, N.A.; Khalaf, D.M.; Morsy, F.M. Synergistic interaction of Rhizobium leguminosarum bv. viciae and arbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiol. Res. 2014, 169, 49–58. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Duponnois, R.; Plenchette, C. A mycorrhiza helper bacterium enhances ectomycorrhizal and endomycorrhizal symbiosis of Australian Acacia species. Mycorrhiza 2003, 13, 85–91. [Google Scholar] [CrossRef]
- Püschel, D.; Janoušková, M.; Voříšková, A.; Gryndlerová, H.; Vosátka, M.; Jansa, J. Arbuscular mycorrhiza stimulates biological nitrogen fixation in two Medicago spp. through improved phosphorus acquisition. Front. Plant Sci. 2017, 8, 390. [Google Scholar] [CrossRef]
- Andrino, A.; Guggenberger, G.; Kernchen, S.; Mikutta, R.; Sauheitl, L.; Boy, J. Production of Organic Acids by Arbuscular Mycorrhizal Fungi and Their Contribution in the Mobilization of Phosphorus Bound to Iron Oxides. Front. Plant Sci. 2021, 12, 661842. [Google Scholar] [CrossRef]
- Xie, M.M.; Zou, Y.N.; Wu, Q.S.; Zhang, Z.Z.; Kuča, K. Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover. Plant Soil Environ. 2020, 66, 287–294. [Google Scholar] [CrossRef]
- Azcón, R.; Barea, J.-M. Mycorrhizosphere interactions for legume improvement. In Microbes for Legume Improvement; Springer: Cham, Switzerland, 2010; pp. 237–271. ISBN 9783211997536. [Google Scholar]
- Franzini, V.I.; Azcon, R.; Mendes, F.L.; Aroca, R. Interactions between Glomus species and Rhizobium strains affect the nutritional physiology of drought-stressed legume hosts. J. Plant Physiol. 2010, 167, 614–619. [Google Scholar] [CrossRef]
- Franzini, V.I.; Azcón, R.; Méndes, F.L.; Aroca, R. Different interaction among Glomus and Rhizobium species on Phaseolus vulgaris and Zea mays plant growth, physiology and symbiotic development under moderate drought stress conditions. Plant Growth Regul. 2013, 70, 265–273. [Google Scholar] [CrossRef]
- Van der Veken, L.; Cabasan, M.T.N.; Elsen, A.; Swennen, R.; De Waele, D. Effect of single or dual inoculation of the arbuscular mycorrhizal fungus Glomus mosseae and root-nodulating rhizobacteria on reproduction of the burrowing nematode Radopholus similis on non-leguminous and leguminous banana intercrops. J. Plant Dis. Prot. 2021, 128, 961–971. [Google Scholar] [CrossRef]
- Ben-Laouane, R.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Ait Rahou, Y.; Raklami, A.; Oufdou, K.; Wahbi, S.; Meddich, A. Green Compost Combined with Mycorrhizae and Rhizobia: A Strategy for Improving Alfalfa Growth and Yield Under Field Conditions. Gesunde Pflanz. 2021, 73, 193–207. [Google Scholar] [CrossRef]


| Strains | NaCl Concentration (mM) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 341 | 427 | 512 | 598 | 683 | 769 | 854 | |
| RhOL1 | + | + | + | + | ± | ± | ± | - |
| RhOL2 | + | + | + | ± | ± | - | - | - |
| RhOL3 | + | + | + | ± | ± | - | - | - |
| RhOL4 | + | + | + | ± | ± | - | - | - |
| RhOL6 | + | + | + | + | ± | - | - | - |
| RhOL7 | + | + | + | ± | ± | - | - | - |
| RhOL9 | + | + | + | ± | ± | - | - | - |
| RhOL10 | + | + | + | ± | ± | - | - | - |
| RhOL12 | + | + | + | ± | ± | - | - | - |
| AMF Infection Frequency (%) | AMF Infection Intensity (%) | Nodule Number | |||||
|---|---|---|---|---|---|---|---|
| Siriver | Demnate | Siriver | Demnate | Siriver | Demnate | ||
| C | - | - | - | - | - | - | |
| AM1 | 82.22 ± 2.22 bc | 77.78 ± 2.22 a | 41.83 ± 2.41 b | 30.37 ± 0.53 a | - | - | |
| AM2 | 77.78 ± 4.44 c | 73.33 ± 2.22 a | 38.03 ± 0.57 b | 21.43 ± 0.81 b | - | - | |
| 0 mM | RhOL1 | - | - | - | - | 73.33 ± 1.20 c | 78.33 ± 4.40 a |
| AM1 + RhOL1 | 97.77 ± 2.22 a | 75.56 ± 2.22 a | 63.50 ± 0.20 a | 29.23 ± 0.34 a | 108.66 ± 1.85 a | 89.33 ± 5.48 a | |
| AM2 + RhOL1 | 86.67 ± 3.84 b | 66.67 ± 2.22 b | 38.67 ± 2.82 b | 16.27 ± 0.67 c | 91.66 ± 6.00 b | 86.00 ± 4.16 a | |
| C | - | - | - | - | - | - | |
| AM1 | 37.78 ± 2.22 ef | 37.78 ± 3.84 c | 14.97 ± 0.53 d | 12.43 ± 1.09 d | - | - | |
| AM2 | 33.33 ± 0.00 f | 31.11 ± 2.22 cd | 12.03 ± 1.43 d | 8.10 ± 1.05 e | - | - | |
| 120 mM | RhOL1 | - | - | - | - | 17.66 ± 1.45 e | 21.33 ± 1.85 c |
| AM1 + RhOL1 | 53.33 ± 0.00 d | 37.78 ± 0.00 c | 21.27 ± 1.51 c | 11.73 ± 0.70 d | 30.66 ± 3.35 d | 26.00 ± 5.50 c | |
| AM2 + RhOL1 | 42.22 ± 2.22 e | 26.67 ± 2.22 d | 14.73 ± 1.23 d | 7.60 ± 0.21 e | 15.66 ± 2.96 e | 24.00 ± 3.05 c | |
| Stem Length (cm) | Number of Leaves/Plant | Total Biomass (g) | |||||
|---|---|---|---|---|---|---|---|
| Siriver | Demnate | Siriver | Demnate | Siriver | Demnate | ||
| C | 27.56 ± 3.10 d | 33.66 ± 0.33 bc | 18.00 ± 0.47 d | 15.66 ± 0.98 c | 1.13 ± 0.18 f | 1.34 ± 0.12 e | |
| AM1 | 45.33 ± 1.20 b | 42.00 ± 3.00 a | 21.33 ± 1.51 abcd | 24.00 ± 2.44 a | 2.24 ± 0.12 c | 1.92 ± 0.11 a | |
| AM2 | 34.33 ± 0.88 c | 42.65 ± 1.45 a | 21.66 ± 2.17 abcd | 22.33 ± 1.65 ab | 2.21 ± 0.11 c | 1.82 ± 0.11 ab | |
| 0 mM | RhOL1 | 42.00 ± 0.57 b | 40.66 ± 1.36 a | 24.33 ± 2.37 abc | 20.66 ± 0.98 abc | 2.97 ± 0.12 b | 1.65 ± 0.11 cd |
| AM1 + RhOL1 | 50.66 ± 0.88 a | 44.66 ± 0.88 a | 27.33 ± 2.68 a | 21.00 ± 1.24 abc | 3.73 ± 0.08 a | 1.77 ± 0.09 bc | |
| AM2 + RhOL1 | 45.00 ± 0.57 b | 38.66 ± 1.85 ab | 21.00 ± 0.47 bcd | 21.00 ± 0.47 abc | 2.76 ± 0.32 b | 1.31 ± 0.02 e | |
| C | 15.66 ± 0.33 e | 24.16 ± 3.83 e | 10.66 ± 0.98 e | 15.66 ± 1.44 c | 0.56 ± 0.03 g | 0.73 ± 0.73 g | |
| AM1 | 28.66 ± 0.33 d | 30.63 ± 0.18 cde | 19.00 ± 0.94 cd | 20.66 ± 1.08 abc | 1.55 ± 0.14 de | 1.61 ± 0.10 d | |
| AM2 | 25.00 ± 2.25 d | 31.00 ± 1.20 cde | 15.66 ± 1.65 de | 17.33 ± 1.51 bc | 1.39 ± 0.06 ef | 1.53 ± 0.03 d | |
| 120 mM | RhOL1 | 27.00 ± 1.15 d | 25.00 ± 4.17 de | 18.33 ± 0.98 cd | 17.33 ± 0.98 bc | 1.75 ± 0.02 d | 1.07 ± 0.07 f |
| AM1 + RhOL1 | 33.66 ± 2.18 c | 31.66 ± 0.57 cd | 25.33 ± 1.36 ab | 22.00 ± 0.81 ab | 2.32 ± 0.20 c | 1.63 ± 0.10 cd | |
| AM2 + RhOL1 | 26.66 ± 2.33 d | 28.66 ± 1.00 cde | 19.00 ± 0.47 cd | 17.00 ± 0.94 bc | 1.83 ± 0.29 d | 1.20 ± 0.13 ef | |
| A | Ca2+ | K+ | Na+ | P | N | Ca2+/Na+ | K+/Na+ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 11.38 ± 0.24 e | 26.70 ± 0.72 c | 19.61 ± 0.79 d | 0.32 ± 0.01 h | 2.25 ± 0.09 g | 0.58 | 1.36 | ||||||
| AM1 | 12.57 ± 0.82 e | 40.24 ± 4.24 a | 7.89 ± 0.55 f | 1.29 ± 0.01 d | 3.78 ± 0.01 c | 1.59 | 5.09 | ||||||
| 0 mM | AM2 | 12.57 ± 0.82 e | 41.44 ± 0.18 a | 8.78 ± 0.10 f | 1.29 ± 0.05 d | 3.00 ± 0.01 e | 1.43 | 4.71 | |||||
| RhOL1 | 13.81 ± 2.17 e | 32.04 ± 2.10 b | 13.29 ± 1.42 e | 0.80 ± 0.04 e | 5.34 ± 0.01 a | 1.03 | 2.41 | ||||||
| AM1 + RhOL1 | 12.53 ± 0.08 e | 37.89 ± 0.11 a | 5.48 ± 0.06 g | 1.61 ± 0.02 b | 4.28 ± 0.04 b | 2.28 | 6.91 | ||||||
| AM2 + RhOL1 | 10.80 ± 0.71 e | 40.74 ± 0.39 a | 4.70 ± 0.10 g | 1.81 ± 0.02 a | 3.83 ± 0.04 c | 2.29 | 8.65 | ||||||
| C | 21.20 ± 1.66 d | 18.20 ± 0.52 d | 49.36 ± 0.92 a | 0.09 ± 0.00 i | 1.20 ± 0.01 h | 0.42 | 0.36 | ||||||
| AM1 | 33.45 ± 0.64 b | 25.37 ± 1.45 c | 37.62 ± 0.52 c | 0.74 ± 0.03 e | 2.88 ± 0.01 f | 0.88 | 0.67 | ||||||
| 120 mM | AM2 | 33.52 ± 0.30 b | 25.64 ± 2.20 c | 41.25 ± 0.27 b | 0.47 ± 0.01 g | 2.94 ± 0.01 ef | 0.81 | 0.62 | |||||
| RhOL1 | 27.44 ± 0.14 c | 26.30 ± 0.24 c | 36.29 ± 0.08 c | 0.24 ± 0.01 h | 2.94 ± 0.01 ef | 0.75 | 0.72 | ||||||
| AM1 + RhOL1 | 40.86 ± 1.55 a | 32.88 ± 0.68 b | 38.02 ± 1.02 c | 1.00 ± 0.05 c | 3.72 ± 0.01 c | 1.07 | 0.86 | ||||||
| AM2 + RhOL1 | 39.00 ± 0.84 a | 25.38 ± 0.36 c | 41.73 ± 0.45 b | 0.64 ± 0.00 f | 3.58 ± 0.03 d | 0.93 | 0.60 | ||||||
| B | Ca2+ | K+ | Na+ | P | Ca2+/Na+ | K+/Na+ | |||||||
| C | 9.98 ± 0.90 ab | 15.42 ± 0.18 e | 12.88 ± 0.18 e | 0.16 ± 0.00 i | 0.77 | 1.19 | |||||||
| AM1 | 11.50 ± 0.67 a | 32.61 ± 0.72 b | 5.62 ± 0.16 g | 1.47 ± 0.02 e | 2.04 | 5.79 | |||||||
| 0 mM | AM2 | 9.94 ± 0.82 ab | 37.8 ± 0.16 a | 6.08 ± 0.04 g | 1.93 ± 0.01 b | 1.63 | 6.21 | ||||||
| RhOL1 | 8.64 ± 1.88 ab | 29.66 ± 0.26 c | 8.65 ± 0.13 f | 0.78 ± 0.01 g | 0.99 | 3.42 | |||||||
| AM1 + RhOL1 | 9.98 ± 1.22 ab | 32.17 ± 2.17 b | 8.48 ± 0.08 f | 2.13 ± 0.00 a | 1.17 | 3.79 | |||||||
| AM2 + RhOL1 | 9.50 ± 0.34 ab | 18.44 ± 1.48 d | 5.20 ± 0.38 g | 1.66 ± 0.06 d | 1.82 | 3.54 | |||||||
| C | 9.05 ± 1.90 ab | 5.05 ± 0.51 g | 24.02 ± 0.44 a | 0.16 ± 0.00 i | 0.37 | 0.21 | |||||||
| AM1 | 9.78 ± 1.37 ab | 6.57 ± 0.17 fg | 15.90 ± 0.40 d | 0.86 ± 0.02 f | 0.61 | 0.41 | |||||||
| 120 mM | AM2 | 11.22 ± 1.55 a | 7.12 ± 0.08 fg | 20.38 ± 0.29 b | 1.47 ± 0.02 e | 0.55 | 0.34 | ||||||
| RhOL1 | 8.18 ± 1.06 b | 7.38 ± 0.03 fg | 21.38 ± 0.05 b | 0.34 ± 0.00 h | 0.38 | 0.34 | |||||||
| AM1 + RhOL1 | 10.22 ± 0.35 ab | 8.20 ± 0.36 f | 17.13 ± 0.56 c | 1.75 ± 0.03 c | 0.59 | 0.47 | |||||||
| AM2 + RhOL1 | 10.01 ± 0.86 ab | 7.34 ± 0.49 fg | 18.05 ± 0.81 c | 0.84 ± 0.01 fg | 0.55 | 0.40 | |||||||
| A | Ca2+ | K+ | Na+ | P | N | Ca2+/Na+ | K+/Na+ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 16.13 ± 0.30 d | 17.32 ± 0.04 f | 19.72 ± 0.04 c | 0.30 ± 0.00 h | 3.77 ± 0.01 e | 0.81 | 0.87 | ||||||
| AM1 | 19.84 ± 0.62 bc | 23.82 ± 0.03 cd | 11.40 ± 0.17 e | 1.76 ± 0.09 b | 4.76 ± 0.14 b | 1.74 | 2.09 | ||||||
| 0 mM | AM2 | 15.4 ± 0.06 d | 36.76 ± 0.16 a | 9.72 ± 0.06 e | 1.44 ± 0.01 cd | 4.53 ± 0.03 b | 1.58 | 3.78 | |||||
| RhOL1 | 22.50 ± 0.13 a | 22.48 ± 1.20 de | 14.04 ± 0.16 d | 1.05 ± 0.05 f | 5.48 ± 0.03 a | 1.60 | 1.60 | ||||||
| AM1 + RhOL1 | 20.44 ± 0.28 b | 24.01 ± 0.16 cd | 15.42 ± 0.26 d | 1.89 ± 0.05 a | 4.12 ± 0.01 c | 1.32 | 1.55 | ||||||
| AM2 + RhOL1 | 19.08 ± 0.17 c | 28.81 ± 0.08 b | 15.44 ± 0.03 d | 1.47 ± 0.01 cd | 4.10 ± 0.04 c | 1.23 | 1.86 | ||||||
| C | 9.96 ± 0.10 g | 7.4 ± 0.36 h | 37.85 ± 0.01 a | 0.20 ± 0.01 h | 2.00 ± 0.17 f | 0.26 | 0.19 | ||||||
| AM1 | 12.46 ± 0.29 f | 21.89 ± 0.32 e | 34.40 ± 0.67 b | 1.35 ± 0.02 d | 4.16 ± 0.06 c | 0.36 | 0.63 | ||||||
| 120 mM | AM2 | 15.76 ± 0.10 d | 25.42 ± 0.10 c | 34.80 ± 1.22 b | 1.41 ± 0.02 d | 4.05 ± 0.15 cd | 0.45 | 0.73 | |||||
| RhOL1 | 14.2 ± 0.56 e | 12.33 ± 1.16 g | 34.45 ± 1.15 b | 0.44 ± 0.04 g | 4.53 ± 0.03 b | 0.41 | 0.35 | ||||||
| AM1 + RhOL1 | 15.90 ± 0.05 d | 21.73 ± 0.12 e | 33.33 ± 1.19 b | 1.55 ± 0.00 c | 3.78 ± 0.01 de | 0.47 | 0.65 | ||||||
| AM2 + RhOL1 | 14.44 ± 0.50 e | 12.90 ± 0.05 g | 33.06 ± 1.29 b | 1.22 ± 0.03 e | 3.73 ± 0.01 e | 0.43 | 0.39 | ||||||
| B | Ca2+ | K+ | Na+ | P | Ca2+/Na+ | K+/Na+ | |||||||
| C | 3.86 ± 0.13 c | 8.42 ± 0.12 de | 7.14 ± 0.12 de | 0.75 ± 0.00 de | 0.54 | 1.17 | |||||||
| AM1 | 4.26 ± 0.26 c | 12.48 ± 0.14 b | 7.57 ± 0.08 d | 1.59 ± 0.01 b | 0.56 | 1.64 | |||||||
| 0 mM | AM2 | 4.13 ± 0.13 c | 17.80 ± 1.06 a | 6.52 ± 0.31 ef | 1.65 ± 0.01 b | 0.63 | 2.73 | ||||||
| RhOL1 | 5.92 ± 0.70 c | 8.30 ± 0.10 de | 6.26 ± 0.11 fg | 1.11 ± 0.01 c | 0.94 | 1.32 | |||||||
| AM1 + RhOL1 | 4.40 ± 0.40 c | 10.22 ± 0.13 c | 4.78 ± 0.03 h | 2.54 ± 0.07 a | 0.91 | 2.13 | |||||||
| AM2 + RhOL1 | 4.88 ± 0.63 c | 8.69 ± 0.09 d | 5.54 ± 0.23 gh | 0.77 ± 0.01 d | 0.87 | 1.56 | |||||||
| C | 12.12 ± 1.70 b | 2.21 ± 0.03 h | 30.26 ± 0.07 a | 0.48 ± 0.00 f | 0.40 | 0.07 | |||||||
| AM1 | 16.37 ± 0.99 a | 4.41 ± 0.02 g | 25.28 ± 0.02 b | 0.82 ± 0.01 d | 0.64 | 0.17 | |||||||
| 120 mM | AM2 | 10.54 ± 0.55 b | 5.94 ± 0.03 f | 24.54 ± 0.72 b | 0.67 ± 0.01 e | 0.42 | 0.24 | ||||||
| RhOL1 | 10.65 ± 0.65 b | 4.97 ± 0.66 fg | 22.80 ± 0.22 c | 0.46 ± 0.03 f | 0.46 | 0.21 | |||||||
| AM1 + RhOL1 | 11.81 ± 1.22 b | 7.46 ± 0.04 e | 23.14 ± 0.14 c | 1.06 ± 0.00 c | 0.51 | 0.32 | |||||||
| AM2 + RhOL1 | 12.06 ± 1.54 b | 4.37 ± 0.01 g | 24.64 ± 0.18 b | 0.49 ± 0.05 f | 0.48 | 0.17 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Laouane, R.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Oufdou, K.; Wahbi, S.; Meddich, A. Microbial Biotechnologies for Salt Tolerance in Alfalfa: Agro-Nutritional Comparison Between Local and Imported Varieties. Nitrogen 2025, 6, 27. https://doi.org/10.3390/nitrogen6020027
Ben-Laouane R, Ait-El-Mokhtar M, Anli M, Boutasknit A, Oufdou K, Wahbi S, Meddich A. Microbial Biotechnologies for Salt Tolerance in Alfalfa: Agro-Nutritional Comparison Between Local and Imported Varieties. Nitrogen. 2025; 6(2):27. https://doi.org/10.3390/nitrogen6020027
Chicago/Turabian StyleBen-Laouane, Raja, Mohamed Ait-El-Mokhtar, Mohamed Anli, Abderrahim Boutasknit, Khalid Oufdou, Said Wahbi, and Abdelilah Meddich. 2025. "Microbial Biotechnologies for Salt Tolerance in Alfalfa: Agro-Nutritional Comparison Between Local and Imported Varieties" Nitrogen 6, no. 2: 27. https://doi.org/10.3390/nitrogen6020027
APA StyleBen-Laouane, R., Ait-El-Mokhtar, M., Anli, M., Boutasknit, A., Oufdou, K., Wahbi, S., & Meddich, A. (2025). Microbial Biotechnologies for Salt Tolerance in Alfalfa: Agro-Nutritional Comparison Between Local and Imported Varieties. Nitrogen, 6(2), 27. https://doi.org/10.3390/nitrogen6020027







