Wastewater Denitrification with Solid-Phase Carbon: A Sustainable Alternative to Conventional Electron Donors
Abstract
1. Introduction
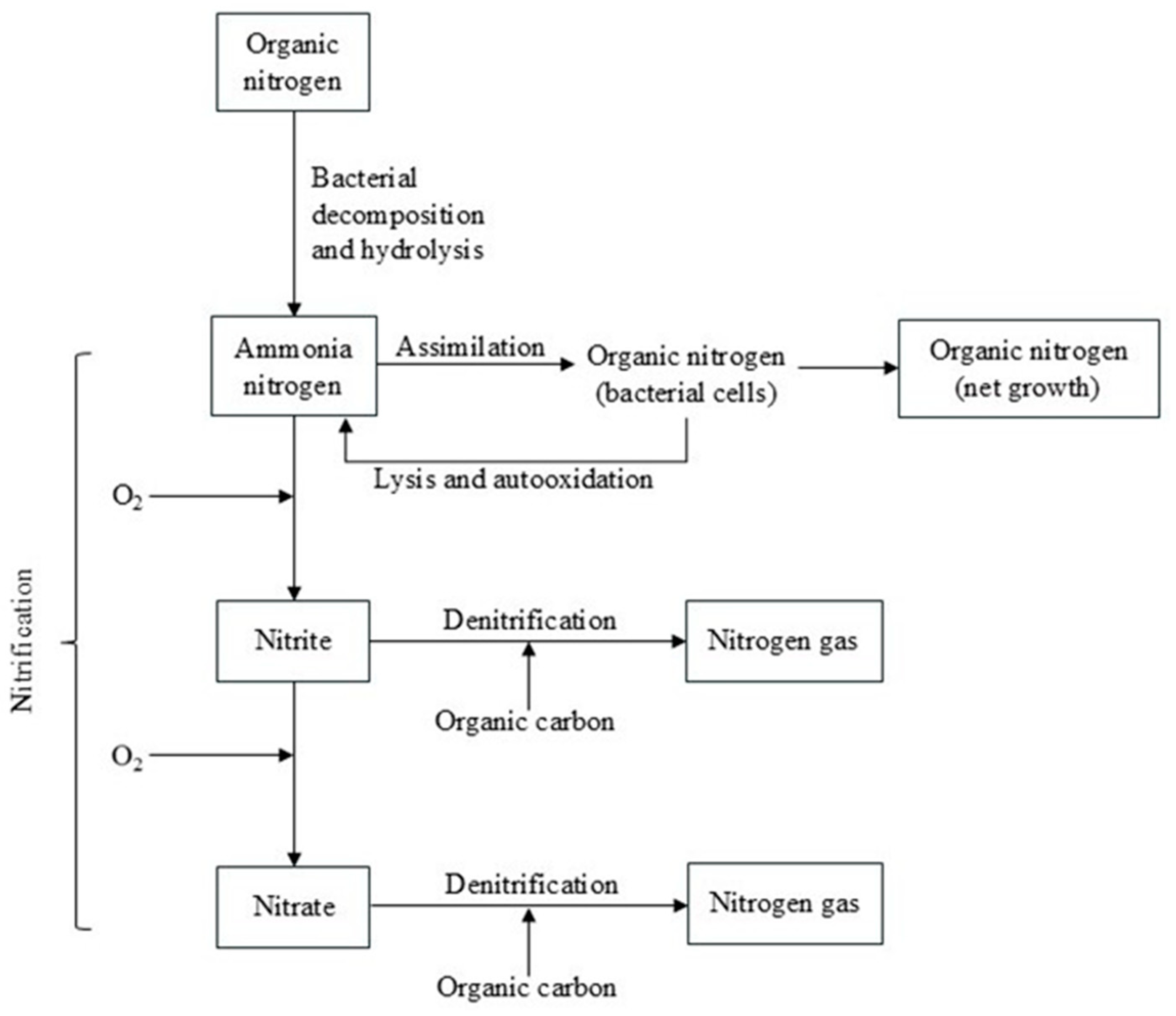
2. Denitrification
2.1. Overview of Biological Denitrification
2.2. Heterotrophic Denitrification Processes
2.3. Autotrophic Denitrification Processes
2.3.1. Hydrogen-Driven Denitrification Process
2.3.2. Anaerobic Ammonium Oxidation (ANAMMOX) Process
2.3.3. Sulfur-Based Denitrification SPD Process
3. Conventional Carbon Sources for Denitrification
3.1. Overview of the Conventional Carbon Sources
3.2. Process Control Strategies
4. Comparative Cost Analysis of Common Carbon Sources
5. The Environmental Impact of Various Carbon Sources
6. Emerging Alternatives: Solid-Phase Carbon Sources
6.1. Solid-Phase Denitrification: Principles and Applications
6.2. Factors Affecting Denitrification Efficiency
6.2.1. Concentration of Nitrate in the Influent
6.2.2. Temperature
6.2.3. pH
6.2.4. Hydraulic Retention Time
6.2.5. Surface Area
6.2.6. Trace Metal Elements
6.2.7. Dissolved Oxygen
6.2.8. Salinity
6.2.9. Type of Carbon Source
6.2.10. Yield
6.2.11. Maximum Specific Growth Rate (µmax)
6.3. Types of Biodegradable Plastics
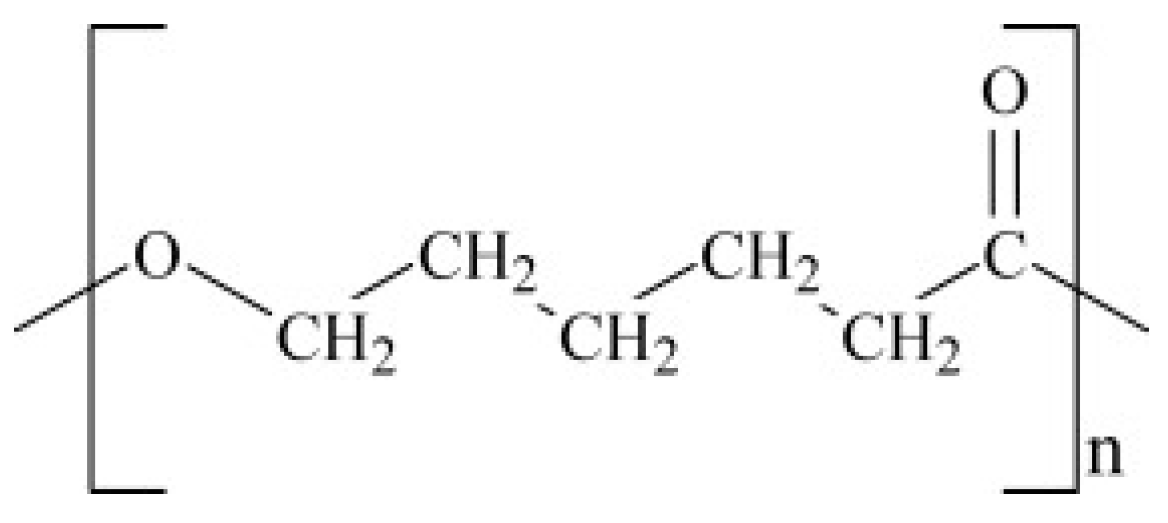
6.3.1. Polycaprolactone (PCL)
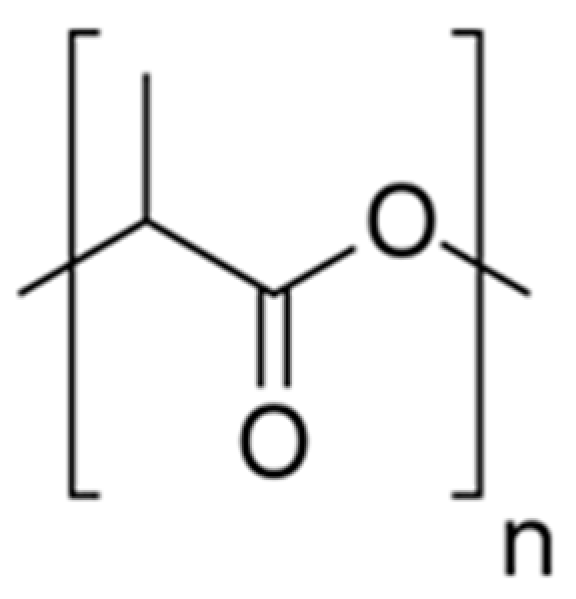
6.3.2. Polylactic Acid (PLA)
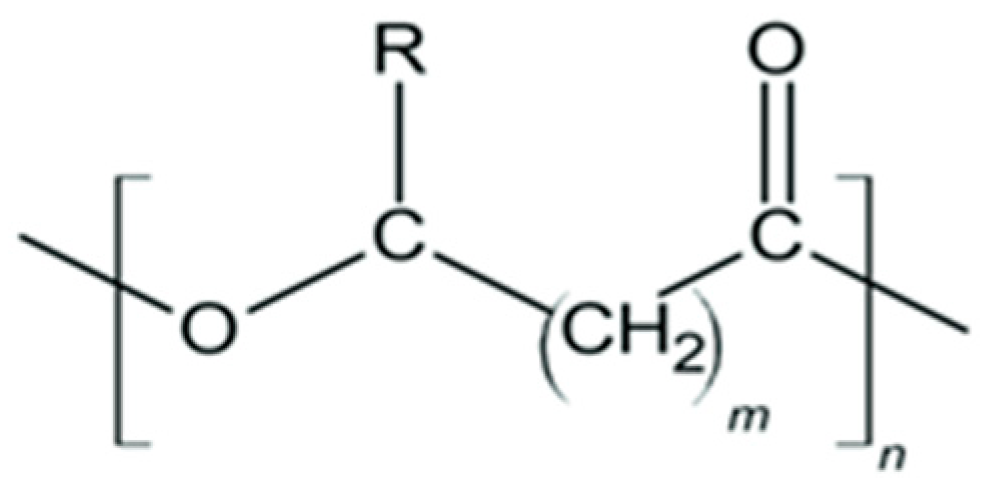
6.3.3. Polyhydroxyalkanoates (PHA)
7. Research Gaps
7.1. Mass Reduction and Post-Treatment Challenges
7.2. Post-Treatment Challenges
7.3. Biofilm Characteristics on Biodegradable Carriers
7.4. PCL Modification and Cost Reduction
7.5. Machine Learning for Operational Optimization
7.6. Nitrogen Recovery for Enhanced Nitrogen Removal
7.7. Environmental Impact Assessments for Bioplastics
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, L.; Wang, J. Denitrification performance and biofilm characteristics using biodegradable polymers PCL as carriers and carbon source. Chemosphere 2013, 91, 1310–1316. [Google Scholar] [CrossRef]
- Cunningham, W.P.; Cunningham, M.A.; O’Reilly, C.M.; Winsett, K.E. Environmental Science: A Global Concern, 6th ed.; McGraw Hill: New York, NY, USA, 2024; ISBN 978-1-264-64784-2. [Google Scholar]
- Strebel, O.; Duynisveld, W.H.M.; Böttcher, J. Nitrate pollution of groundwater in western Europe. Agric. Ecosyst. Environ. 1989, 26, 189–214. [Google Scholar] [CrossRef]
- Yoshino, H.; Tokumura, M.; Kawase, Y. Simultaneous removal of nitrate, hydrogen peroxide and phosphate in semiconductor acidic wastewater by zero-valent iron. J. Environ. Sci. Health Part A 2014, 49, 998–1006. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality. 2011. Available online: https://iris.who.int/handle/10665/4458 (accessed on 19 March 2025).
- Ashok, V.; Hait, S. Remediation of nitrate-contaminated water by solid-phase denitrification process—A review. Environ. Sci. Pollut. Res. 2015, 22, 8075–8093. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Knobeloch, L.; Salna, B.; Hogan, A.; Postle, J.; Anderson, H. Blue babies and nitrate-contaminated well water. Environ. Health Perspect. 2000, 108, 675–678. [Google Scholar] [CrossRef]
- World Health Organization; United Nations Children’s Fund (UNICEF). Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151289-3. Available online: https://iris.who.int/handle/10665/258617 (accessed on 16 October 2024).
- Nuhoglu, A.; Pekdemir, T.; Yildiz, E.; Keskinler, B.; Akay, G. Drinking water denitrification by a membrane bio-reactor. Water Res. 2002, 36, 1155–1166. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, Y.; Wang, S. Denitrification potential enhancement by addition of external carbon sources in a pre-denitrification process. J. Environ. Sci. 2007, 19, 284–289. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Smith, R.J.; Bryant, R.G. Metal substitutions incarbonic anhydrase: A halide ion probe study. Biochem. Biophys Res. Commun. 1975, 66, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.R.L.; Moraes, J.S. Removal of organic pollutants from wastewater using chitosan: A literature review. Int. J. Environ. Sci. Technol. 2019, 16, 1741–1754. [Google Scholar] [CrossRef]
- Aryee, A.A.; Mpatani, F.M.; Du, Y.; Kani, A.N.; Dovi, E.; Han, R.; Li, Z.; Qu, L. Fe3O4 and iminodiacetic acid modified peanut husk as a novel adsorbent for the uptake of Cu (II) and Pb (II) in aqueous solution: Characterization, equilibrium and kinetic study. Environ. Pollut. 2021, 268, 115729. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Luch, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. ISBN 978-3-7643-8340-4. [Google Scholar]
- Vijay, A.; Sonawane, J.M.; Chhabra, M. Denitrification process in microbial fuel cell: A comprehensive review. Bioresour. Technol. Rep. 2022, 17, 100991. [Google Scholar] [CrossRef]
- Constantin, H.; Fick, M. Influence of C-sources on the denitrification rate of a high-nitrate concentrated industrial wastewater. Water Res. 1997, 31, 583–589. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Du, W.; Zhang, Q.; Shang, Y.; Wang, W.; Li, Q.; Yue, Q.; Gao, B.; Xu, X. Sulfate saturated biosorbent-derived Co-S@NC nanoarchitecture as an efficient catalyst for peroxymonosulfate activation. Appl. Catal. B Environ. 2020, 262, 118302. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Matějů, V.; Čižinská, S.; Krejčí, J.; Janoch, T. Biological water denitrification—A review. Enzym. Microb. Technol. 1992, 14, 170–183. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.; Yue, W.; Yue, Q. Preparation and utilization of wheat straw anionic sorbent for the removal of nitrate from aqueous solution. J. Environ. Sci. 2007, 19, 1305–1310. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Wang, J.; Xu, S.; Yu, L.; Philippe, C.; Wintgens, T. Nitrate removal from water by new polymeric adsorbent modified with amino and quaternary ammonium groups: Batch and column adsorption study. J. Taiwan Inst. Chem. Eng. 2016, 66, 191–199. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F.L.; Abu-Orf, M.; Bowden, G.; Pfrang, W.; Metcalf & Eddy (Eds.) Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2014; ISBN 978-0-07-340118-8. [Google Scholar]
- El Mrabet, H.; Kitanou, S.; Tahaikt, M.; Elazhar, F.; El Haloui, N.; Taky, M.; Elmidaoui, A. Biological denitrification of underground brackish water using methanol and sugar cane as a carbon source. Desalination Water Treat. 2024, 320, 100639. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, J. Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, Y.-G.; Liu, J.; Chen, Y.; Gao, M.; Guo, L.; Mupindu, P. Rapid conversion of heterotrophic denitrification to autotrophic denitrification in mariculture wastewater treatment: Denitrification performance and microbial communities under antibiotic stress. J. Water Process Eng. 2024, 62, 105391. [Google Scholar] [CrossRef]
- Sevda, S.; Sreekishnan, T.R.; Pous, N.; Puig, S.; Pant, D. Bioelectroremediation of perchlorate and nitrate contaminated water: A review. Bioresour. Technol. 2018, 255, 331–339. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, Y.; Wu, B.; Zhang, Y.; Huang, M.; Miyanaga, T.; Zhang, Z. Autotrophic denitrification for nitrate and nitrite removal using sulfur-limestone. J. Environ. Sci. 2011, 23, 1761–1769. [Google Scholar] [CrossRef]
- Wenk, C.B.; Blees, J.; Zopfi, J.; Veronesi, M.; Bourbonnais, A.; Schubert, C.J.; Niemann, H.; Lehmann, M.F. Anaerobic ammonium oxidation (anammox) bacteria and sulfide-dependent denitrifiers coexist in the water column of a meromictic south-alpine lake. Limnol. Oceanogr. 2013, 58, 1–12. [Google Scholar] [CrossRef]
- Arumugham, T.; Khudzari, J.; Abdullah, N.; Yuzir, A.; Iwamoto, K.; Homma, K. Research trends and future directions on nitrification and denitrification processes in biological nitrogen removal. J. Environ. Chem. Eng. 2024, 12, 111897. [Google Scholar] [CrossRef]
- Vázquez-Padín, J.; Fernádez, I.; Figueroa, M.; Mosquera-Corral, A.; Campos, J.-L.; Méndez, R. Applications of Anammox based processes to treat anaerobic digester supernatant at room temperature. Bioresour. Technol. 2009, 100, 2988–2994. [Google Scholar] [CrossRef]
- Sierra-Alvarez, R.; Beristain-Cardoso, R.; Salazar, M.; Gómez, J.; Razo-Flores, E.; Field, J.A. Chemolithotrophic denitrification with elemental sulfur for groundwater treatment. Water Res. 2007, 41, 1253–1262. [Google Scholar] [CrossRef]
- Soares, M.I.M. Denitrification of groundwater with elemental sulfur. Water Res. 2002, 36, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Sahinkaya, E.; Dursun, N.; Kilic, A.; Demirel, S.; Uyanik, S.; Cinar, O. Simultaneous heterotrophic and sulfur-oxidizing autotrophic denitrification process for drinking water treatment: Control of sulfate production. Water Res. 2011, 45, 6661–6667. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef]
- Bill, K.A.; Bott, C.B.; Murthy, S.N. Evaluation of alternative electron donors for denitrifying moving bed biofilm reactors (MBBRs). Water Sci. Technol. 2009, 60, 2647–2657. [Google Scholar] [CrossRef]
- Gómez, M.A.; González-López, J.; Hontoria-García, E. Influence of carbon source on nitrate removal of contaminated groundwater in a denitrifying submerged filter. J. Hazard. Mater. 2000, 80, 69–80. [Google Scholar] [CrossRef]
- Ginige, M.P.; Hugenholtz, P.; Daims, H.; Wagner, M.; Keller, J.; Blackall, L.L. Use of Stable-Isotope Probing, Full-Cycle rRNA Analysis, and Fluorescence In Situ Hybridization-Microautoradiography To Study a Methanol-Fed Denitrifying Microbial Community. Appl. Environ. Microbiol. 2004, 70, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Hallin, S.; Pell, M. Metabolic Properties of Denitrifying Bacteria Adapting to Methanol and Ethanol in Activated Sludge. Water Res. 1998, 32, 13–18. [Google Scholar] [CrossRef]
- Bhuvanesh, S.; Maneesh, N.; Sreekrishnan, T.R. Start-up and performance of a hybrid anoxic reactor for biological denitrification. Bioresour. Technol. 2013, 129, 78–84. [Google Scholar] [CrossRef]
- Zheng, X.; Su, Y.; Chen, Y.; Huang, H.; Shen, Q. Global transcriptional responses of denitrifying bacteria to functionalized single-walled carbon nanotubes revealed by weighted gene-coexpression network analysis. Sci. Total Environ. 2018, 613–614, 1240–1249. [Google Scholar] [CrossRef]
- Aslan, S.; Turkman, A. Nitrate and pesticides removal from contaminated water using biodenitrification reactor. Process Biochem. 2006, 41, 882–886. [Google Scholar] [CrossRef]
- Akunna, J.C.; Bizeau, C.; Moletta, R. Nitrate and nitrite reductions with anaerobic sludge using various carbon sources: Glucose, glycerol, acetic acid, lactic acid and methanol. Water Res. 1993, 27, 1303–1312. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Wastewater Treatment Fact Sheet: External Carbon Sources for Nitrogen Removal; U.S. Environmental Protection Agency. Fact Sheet. 2013. Available online: https://www.epa.gov/sites/default/files/2019-08/documents/external_carbon_surces_for_nitrogen_removal_fact_sheet_p100il8f.pdf (accessed on 10 December 2024).
- Exponent; Theis, T.L.; Hicks, A. Methanol Use in Wastewater Denitrification; Methanol Institute, 2012. White Paper. 2012. Available online: https://www.methanol.org/wp-content/uploads/2016/06/Exponent-Methanol-Denitrification-Report-July-2012-1.pdf (accessed on 11 December 2024).
- Wu, W.; Yang, L.; Wang, J. Denitrification performance and microbial diversity in a packed-bed bioreactor using PCL as carbon source and biofilm carrier. Appl. Microbiol. Biotechnol. 2013, 97, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Puznava, N.; Zeghal, S.; Reddet, E. Simple control strategies of methanol dosing for post-denitrification. Water Sci. Technol. 1998, 38, 291–297. [Google Scholar] [CrossRef]
- Boley, A.; Müller, W.-R.; Haider, G. Biodegradable polymers as solid substrate and biofilm carrier for denitrification in recirculated aquaculture systems. Aquac. Eng. 2000, 22, 75–85. [Google Scholar] [CrossRef]
- Li, P.; Zuo, J.; Wang, Y.; Zhao, J.; Tang, L.; Li, Z. Tertiary nitrogen removal for municipal wastewater using a solid-phase denitrifying biofilter with polycaprolactone as the carbon source and filtration medium. Water Res. 2016, 93, 74–83. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental impact of bioplastic use: A review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Buis, A. The Atmosphere: Getting a Handle on Carbon Dioxide. 2019. Available online: https://science.nasa.gov/earth/climate-change/greenhouse-gases/the-atmosphere-getting-a-handle-on-carbon-dioxide/ (accessed on 14 January 2025).
- Armand, M. The history of polymer electrolytes. Solid State Ion. 1994, 69, 309–319. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterial and Polymer Membranes: Synthesis, Characterization, and Applications; Elsevier: San Diego, CA, USA, 2016; ISBN 978-0-12-801440-0. [Google Scholar]
- Walker, S.; Rothman, R. Life cycle assessment of bio-based and fossil-based plastic: A review. J. Clean. Prod. 2020, 261, 121158. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Muhammad Shamsuddin, I. Bioplastics as Better Alternative to Petroplastics and Their Role in National Sustainability: A Review. Adv. Biosci. Bioeng. 2017, 5, 63. [Google Scholar] [CrossRef]
- Shen, Z.; Zhou, Y.; Hu, J.; Wang, J. Denitrification performance and microbial diversity in a packed-bed bioreactor using biodegradable polymer as carbon source and biofilm support. J. Hazard. Mater. 2013, 250–251, 431–438. [Google Scholar] [CrossRef]
- Bodík, I.; Blšťáková, A.; Sedláček, S.; Hutňan, M. Biodiesel waste as source of organic carbon for municipal WWTP denitrification. Bioresour. Technol. 2009, 100, 2452–2456. [Google Scholar] [CrossRef]
- Lee, D.-U.; Lee, I.-S.; Choi, Y.-D.; Bae, J.-H. Effects of external carbon source and empty bed contact time on simultaneous heterotrophic and sulfur-utilizing autotrophic denitrification. Process Biochem. 2001, 36, 1215–1224. [Google Scholar] [CrossRef]
- Gutierrez-Wing, M.T.; Malone, R.F.; Rusch, K.A. Evaluation of polyhydroxybutyrate as a carbon source for recirculating aquaculture water denitrification. Aquac. Eng. 2012, 51, 36–43. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, X.; Chai, X. Effect of influent pH on biological denitrification using biodegradable PHBV/PLA blends as electron donor. Biochem. Eng. J. 2018, 131, 24–30. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, X.; Chai, X. Biological denitrification using PHBV polymer as solid carbon source and biofilm carrier. Biochem. Eng. J. 2019, 146, 186–193. [Google Scholar] [CrossRef]
- Yang, X.-L.; Jiang, Q.; Song, H.-L.; Gu, T.-T.; Xia, M.-Q. Selection and application of agricultural wastes as solid carbon sources and biofilm carriers in MBR. J. Hazard. Mater. 2015, 283, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zuo, Q.; Liu, C.; Li, L.; Deliz Quiñones, K.Y.; He, Q. Insights into solid phase denitrification in wastewater tertiary treatment: The role of solid carbon source in carbon biodegradation and heterotrophic denitrification. Bioresour. Technol. 2023, 376, 128838. [Google Scholar] [CrossRef]
- Honda, Y.; Osawa, Z. Microbial denitrification of wastewater using biodegradable polycaprolactone. Polym. Degrad. Stab. 2002, 76, 321–327. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, T.-L.; Han, M.-L.; Li, J.; Wang, X.-M. Heterotrophic Denitrification of Nitrate-Contaminated Water Using Different Solid Carbon Sources. Procedia Environ. Sci. 2011, 10, 72–77. [Google Scholar] [CrossRef]
- Blackmer, A.M.; Bremner, J.M. Inhibitory effect of nitrate on reduction of N2O to N2 by soil microorganisms. Soil Biol. Biochem. 1978, 10, 187–191. [Google Scholar] [CrossRef]
- Canziani, R.; Vismara, R.; Basilico, D.; Zinni, L. Nitrogen Removal in Fixed-Bed Submerged Biofilters without Backwashing. Water Sci. Technol. 1999, 40, 142–152. [Google Scholar] [CrossRef]
- Shen, Z.; Hu, J.; Wang, J.; Zhou, Y. Biological denitrification using starch/polycaprolactone blends as carbon source and biofilm support. Desalination Water Treat. 2015, 54, 609–615. [Google Scholar] [CrossRef]
- Cameron, S.G.; Schipper, L.A. Hydraulic properties, hydraulic efficiency and nitrate removal of organic carbon media for use in denitrification beds. Ecol. Eng. 2012, 41, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J. Removal of nitrate from groundwater by heterotrophic denitrification using the solid carbon source. Sci. China Ser. B-Chem. 2009, 52, 236–240. [Google Scholar] [CrossRef]
- Dold, P.; Takács, I.; Mokhayeri, Y.; Nichols, A.; Hinojosa, J.; Riffat, R.; Bott, C.; Bailey, W.; Murthy, S. Denitrification with Carbon Addition—Kinetic Considerations. Water Environ. Res. 2008, 80, 417–427. [Google Scholar]
- Deng, Y.-L.; Ruan, Y.-J.; Zhu, S.-M.; Guo, X.-S.; Han, Z.-Y.; Ye, Z.-Y.; Liu, G.; Shi, M.-M. The impact of DO and salinity on microbial community in poly(butylene succinate) denitrification reactors for recirculating aquaculture system wastewater treatment. AMB Expr. 2017, 7, 113. [Google Scholar] [CrossRef]
- Song, H.; Feng, J.; Zhang, L.; Yin, H.; Pan, L.; Li, L.; Fan, C.; Wang, Z. Advanced treatment of low C/N ratio wastewater treatment plant effluent using a denitrification biological filter: Insight into the effect of medium particle size and hydraulic retention time. Environ. Technol. Innov. 2021, 24, 102044. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, J.L. Nitrate removal from groundwater using solid-phase denitrification process without inoculating with external microorganisms. Int. J. Environ. Sci. Technol. 2013, 10, 955–960. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J. Denitrification of groundwater using PHBV blends in packed bed reactors and the microbial diversity. Chemosphere 2016, 155, 463–470. [Google Scholar] [CrossRef]
- Jia, W.; Liang, S.; Zhang, J.; Ngo, H.H.; Guo, W.; Yan, Y.; Zou, Y. Nitrous oxide emission in low-oxygen simultaneous nitrification and denitrification process: Sources and mechanisms. Bioresour. Technol. 2013, 136, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P.J.F. Proton-linked sugar transport systems in bacteria. J. Bioenerg. Biomembr. 1990, 22, 525–569. [Google Scholar] [CrossRef]
- Huang, Y.; Lemieux, M.J.; Song, J.; Auer, M.; Wang, D.-N. Structure and Mechanism of the Glycerol-3-Phosphate Transporter from Escherichia coli. Science 2003, 301, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yu, Z.; Yuan, S.; Tawfik, A.; Meng, F. An ecological explanation for carbon source-associated denitrification performance in wastewater treatment plants. Water Res. 2023, 247, 120762. [Google Scholar] [CrossRef]
- Plattes, M.; Lahore, H.M.F. Perspectives on the Monod model in biological wastewater treatment. J. Chem. Tech. Amp. Biotech. 2023, 98, 833–837. [Google Scholar] [CrossRef]
- Mokhayeri, Y.; Nichols, A.; Murthy, S.; Riffat, R.; Dold, P.; Takacs, I. Examining the influence of substrates and temperature on maximum specific growth rate of denitrifiers. Water Sci. Technol. 2006, 54, 155–162. [Google Scholar] [CrossRef]
- Bhandari, S.; Gupta, P. Chemical Depolymerization of Polyurethane Foam via Ammonolysis and Aminolysis. In Recycling of Polyurethane Foams; Elsevier: Amsterdam, The Netherlands, 2018; pp. 77–87. ISBN 978-0-323-51133-9. [Google Scholar]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Mariya, D.; Usman, J.; Nimmy Mathew, E.; PH, H.; Azeez, A. Reverse Vending Machine for Plastic Bottle Recycling. Int. J. Comput. Sci. Technol. 2020, 8, 65–69. [Google Scholar]
- Mozaffari, N.; Kholdebarin, A. A Review: Investigation of Plastics Effect on the Environment, Bioplastic Global Market Share and Its Future Perspectives. Zenodo 2019, 5, 47–54. [Google Scholar] [CrossRef]
- Kumar, K.; Umapathi, R.; Ghoreishian, S.M.; Tiwari, J.N.; Hwang, S.K.; Huh, Y.S.; Venkatesu, P.; Shetti, N.P.; Aminabhavi, T.M. Microplastics and biobased polymers to combat plastics waste. Chemosphere 2023, 341, 140000. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Removal of Pharmaceutical and Personal Care Products (PPCPs) under nitrifying and denitrifying conditions. Water Res. 2010, 44, 3214–3224. [Google Scholar] [CrossRef] [PubMed]
- Ganthavee, V.; Trzcinski, A.P. Artificial intelligence and machine learning for the optimization of pharmaceutical wastewater treatment systems: A review. Environ. Chem. Lett. 2024, 22, 2293–2318. [Google Scholar] [CrossRef]
- Ita-Nagy, D.; Vázquez-Rowe, I.; Kahhat, R.; Chinga-Carrasco, G.; Quispe, I. Reviewing environmental life cycle impacts of biobased polymers: Current trends and methodological challenges. Int. J. Life Cycle Assess. 2020, 25, 2169–2189. [Google Scholar] [CrossRef]
| Substrate | Stoichiometric Equation |
|---|---|
| Ethanol | 5C2H5OH + 12NO3− → 10HCO3− + 2OH− + 9H2O + 6N2 |
| 0.613C2H5OH + NO3− → 0.102C5H7NO2 + 0.714CO2 + 0.286OH− + 0.980H2O + 0.449N2 | |
| Acetic acid | 5CH3COOH + 8NO3− → 8HCO3− + 2CO2 + 6H2O + N2 |
| 0.819CH3COOH + NO3− → 0.068C5H7NO2 + HCO3− + 0.301CO2 + 0.902H2O + 0.466N2 | |
| Glucose | C6H12O6 + 2.8NO3− + 0.5NH4+ → 2.3H+ + 0.5C5H7NO2 + 1.4N2 + 3.5CO2 + 6.4H2O |
| Cellulose | 5(C6H10O5)n + 24n NO3− → 6n CO2 + 13n H2O + 12n N2 + 24n HCO3− |
| Carbon Source | Denitrification Rate (mg NO3-N/(g VSS/h)) | Sludge Yield (g MLSS/g COD) | Adaptation Time | Response Time |
|---|---|---|---|---|
| Methanol | 3.2 | 0.40 | Long (40 d) | Slow (several days) |
| Ethanol | 9.6 | 0.42 | Short | Fast (several hours) |
| Acetate | 12 | 0.65 | Short | Fast (several hours) |
| Denitrification Type | Carbon Source | Key Characteristics | Performance and Efficiency | Limitations |
|---|---|---|---|---|
| Heterotrophic Denitrification (Soluble Carbon Source) | External liquid carbon (e.g., methanol, ethanol, acetate) | Requires frequent dosing of organic carbon for microbial growth. Rapid nitrate removal under optimal conditions. | High denitrification efficiency (≥95%) when sufficient carbon is available. Acetate shows a higher removal rate than methanol, which requires a longer adaptation time. | Safety concerns due to flammability, risk of overdosing, and requires continuous carbon supply and monitoring. |
| Heterotrophic Denitrification (Solid-Phase Carbon Source) | Biodegradable polymers (e.g., PCL, PLA, PHBV) | Uses slow-release solid carbon sources, reducing the need for continuous dosing. Denitrification occurs as the solid material degrades. | Provides long-term denitrification with sustained carbon release. PCL and PHA blends achieve nitrate removal > 90%. | Higher initial material cost. Biofilm formation and degradation rate depend on microbial activity and environmental conditions. |
| Autotrophic Denitrification | Inorganic electron donors (e.g., sulfur, hydrogen, ammonia) | Suitable for wastewater with low organic carbon content. Bacteria oxidize inorganic compounds instead of using an organic carbon source. | Anammox can reduce aeration costs by 63%. Can be effective in treating nitrate-rich industrial wastewater. | Slow bacterial growth, long start-up time, and potential sulfate accumulation. |
| Reference | Carbon Source | Temperature (°C) | Price (CAD/kg Substrate) | Substance Utilization (kg/kg NO3−-N) | Cost (CAD/kg NO3−-N Removed) |
|---|---|---|---|---|---|
| [51] | PCL | 20–25 | 7.45 | 1.33–1.77 | 10–13.1 |
| Acetic acid | 3.6 | 3.5 | 12.6 | ||
| Methanol | 1.5 | 2.08–3.98 | 3.1–6 | ||
| Ethanol | 1.8 | 2.0 | 3.6 | ||
| [52] | PCL | 18–20 | 13.1 | 2.34 | 30.6 |
| Methanol | 25 | 0.5 | 2.46 * | 1.2 | |
| Sodium acetate | 20 | 0.87 | 5.78 | 5 |
| Reference | Carbon Source | Influent | Influent Nitrate Conc. (mg/L) | Temperature (°C) | Days of Operation (d) | Reactor Type | Denitrification Rate (gN/Ld) | Nitrate Removal (%) |
|---|---|---|---|---|---|---|---|---|
| [67] | PCL/PS (peanut shell) | synthetic water | 20 | 25 | 162 | Continuous up-flow SPD reactor | 87.60 ± 0.06 | |
| PCL/SB (sugarcane bagasse) | 87.93 ± 0.05 | |||||||
| PCL/TPS (thermal plastic starch) | 81.83 ± 0.05 | |||||||
| PCL | 83.28 ± 0.07 | |||||||
| [52] | PCL | Secondary effluent | 25–35 | 8 | 249 | SPD biofilter | 1.23–1.67 | 20–31 |
| 18 | 249 | SPD biofilter | 1.23–3.80 | 88–99 | ||||
| [68] | PCL | Synthetic water | 55 | 25 | 70 | Continuous-flow reactor | 0.64 | 70 |
| [1] | PCL | Groundwater | 60–80 | 20–30 | 561 | Fixed-bed bioreactor | 0.19–0.56 | 92–96 |
| [49] | PCL | Tap water with nitrate | 15–52 | 25 | 184 | Packed-bed bioreactor | 0.59–0.66 | 93 |
| [60] | PCL/Starch | Synthetic water | 15–50 | 15–25 | 280 | Packed-bed bioreactor | 0.54–0.64 | 90 |
| [69] | PCL, PLA and PHBV | Synthetic water | 3–4.2 | 30 | 72 | Batch | 0.16 | 82–95 |
| Term | Definition |
|---|---|
| Bioplastics | Plastics that (1) can be degraded naturally or (2) may or may not decompose but are made from biological substances or renewable resources. |
| Biodegradable plastics | Biodegradable materials can be broken down by microorganisms into monomeric or polymeric substances, such as biomass, water, and carbon dioxide or methane. In an industrial context, biodegradable materials are referred to as “compostable” and can be almost entirely converted into non-toxic waste within a few months when situated in a composter. |
| Bio-based plastics | Plastics classified as partially bio-based, or hybrid plastics, incorporate renewable carbon materials, such as plant matter. These materials possess a dual composition that includes both renewable resources and carbon derived from conventional fossil fuels. This combination allows for a more sustainable approach to plastic production, as it utilizes renewable inputs while still relying on established fossil fuel sources. |
| References | Aspect | Key Findings | Challenges and Research Gaps |
|---|---|---|---|
| [11,23,41] | Conventional Carbon Sources (Methanol, Ethanol, Acetate, etc.) | Effective electron donors for denitrification demonstrate high removal efficiencies. Ethanol and acetate outperform methanol in terms of adaptation time and denitrification rate. | Methanol is characterized by high costs, toxicity, flammability, and inconsistent residual organic loads. Additionally, it has a long adaptation time. |
| [1,49,60] | Solid-Phase Carbon Sources (PCL, PLA, PHA, etc.) | Facilitate ongoing carbon release to support long-term denitrification. PCL presents a promising, cost-effective option. | High production costs indicate a need for optimization in biofilm formation and degradation rate control. |
| [7,31,74] | Factors Affecting Denitrification Efficiency (Temperature, pH, HRT, etc.) | Higher temperatures improve enzyme activity and denitrification rates. HRT optimization is critical for efficiency. | Low temperatures significantly reduce performance. Short HRT can lead to nitrite accumulation. |
| [6,53,57] | Environmental Impact of Carbon Sources | Solid-phase carbon sources reduce flammability risks and offer controlled release, improving effluent quality. Bioplastics degrade with lower greenhouse gas emissions than traditional plastics. | Certain bioplastics have environmental impacts through land use changes and chemical processing. |
| [33,91] | Machine Learning and AI for Process Optimization | Potential to predict process efficiencies, optimize dosing, and improve decision-making in denitrification. | Limited real-world applications; high computational demands. |
| [7,49,92] | Research Gaps | There is a need to reduce costs in biodegradable plastics, optimize biofilm dynamics, and integrate machine learning. Further studies are required on nitrogen recovery and the removal of emerging contaminants. | Cost remains a significant barrier; further studies are needed on biofilm interactions and post-treatment challenges. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkhordari, D.; Mathew, J.; Haroun, B.; Rehmann, L.; Murthy, S.; Santoro, D. Wastewater Denitrification with Solid-Phase Carbon: A Sustainable Alternative to Conventional Electron Donors. Nitrogen 2025, 6, 22. https://doi.org/10.3390/nitrogen6020022
Barkhordari D, Mathew J, Haroun B, Rehmann L, Murthy S, Santoro D. Wastewater Denitrification with Solid-Phase Carbon: A Sustainable Alternative to Conventional Electron Donors. Nitrogen. 2025; 6(2):22. https://doi.org/10.3390/nitrogen6020022
Chicago/Turabian StyleBarkhordari, Dorsa, Jithin Mathew, Basem Haroun, Lars Rehmann, Sudhir Murthy, and Domenico Santoro. 2025. "Wastewater Denitrification with Solid-Phase Carbon: A Sustainable Alternative to Conventional Electron Donors" Nitrogen 6, no. 2: 22. https://doi.org/10.3390/nitrogen6020022
APA StyleBarkhordari, D., Mathew, J., Haroun, B., Rehmann, L., Murthy, S., & Santoro, D. (2025). Wastewater Denitrification with Solid-Phase Carbon: A Sustainable Alternative to Conventional Electron Donors. Nitrogen, 6(2), 22. https://doi.org/10.3390/nitrogen6020022








