Nitrous Oxide Production and Mitigation Through Nitrification Inhibitors in Agricultural Soils: A Mechanistic Understanding and Comprehensive Evaluation of Influencing Factors
Abstract
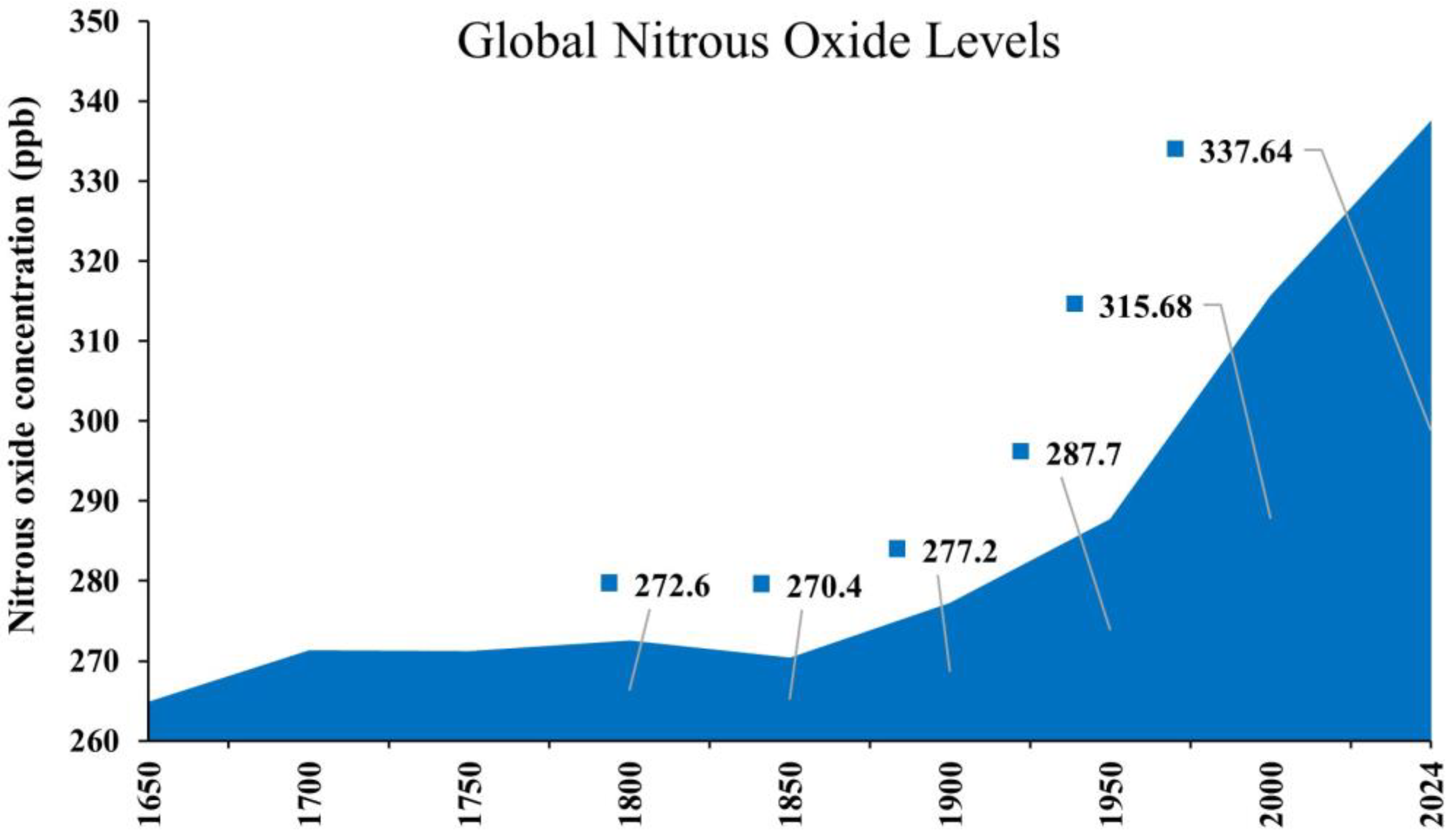
1. Introduction
2. Methodology
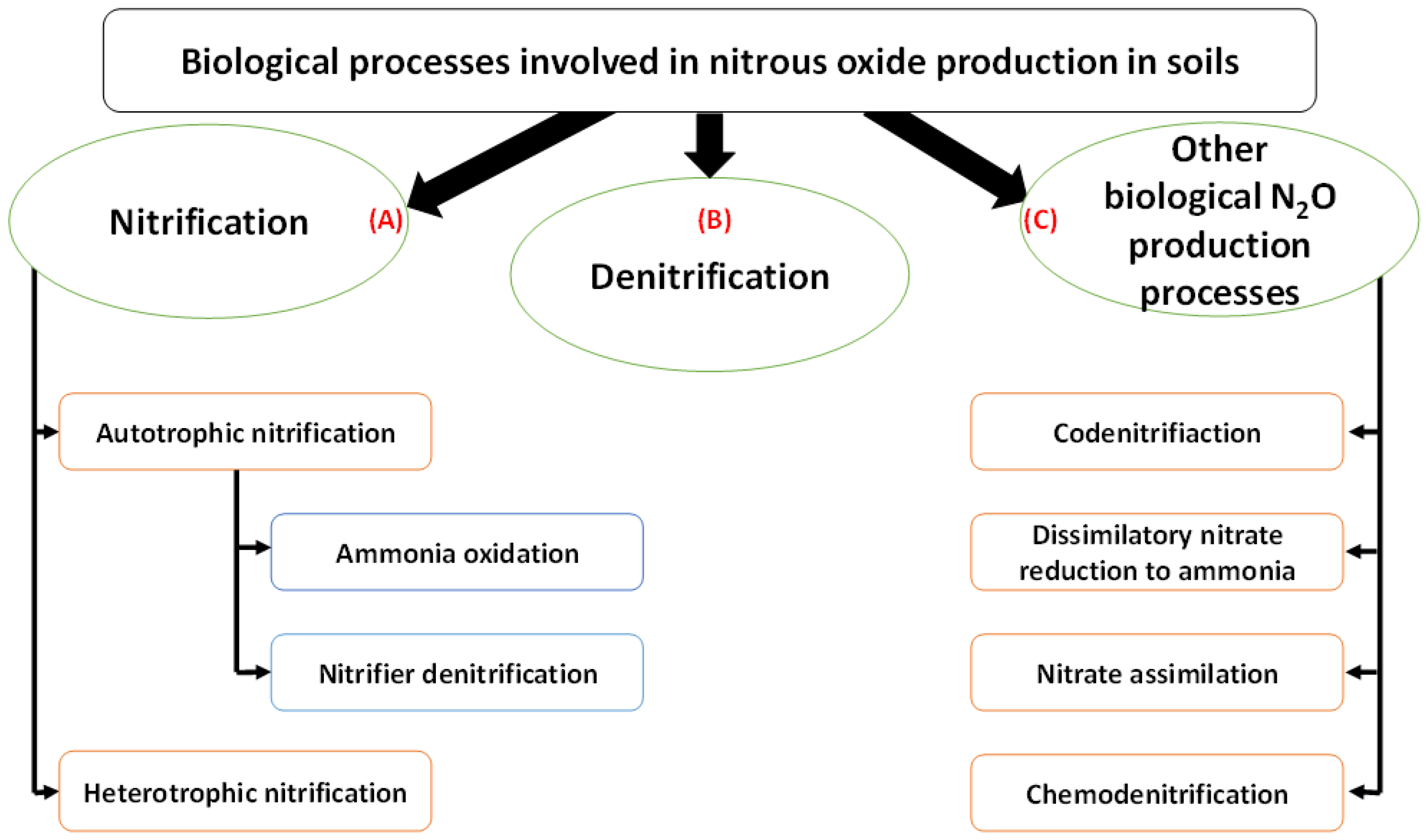
3. Nitrous Oxide Production Processes
3.1. Nitrification
3.1.1. Autotrophic Nitrification
Ammonia Oxidation
Nitrifier Denitrification
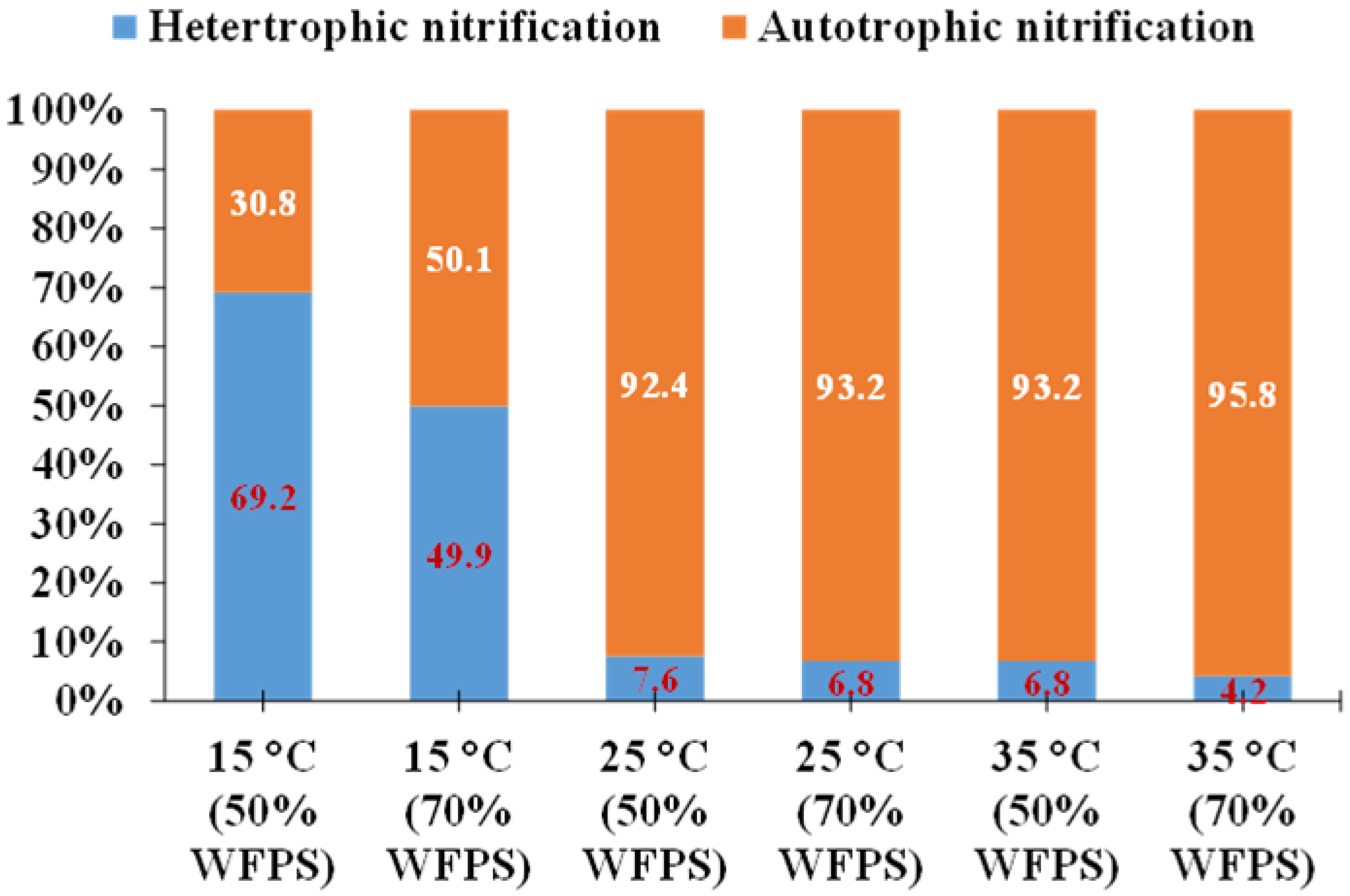
3.1.2. Heterotrophic Nitrification
3.2. Factors Affecting the Nitrification Process
3.2.1. Soil pH
3.2.2. Soil Temperature
3.2.3. Soil Moisture and Oxygen Concentration
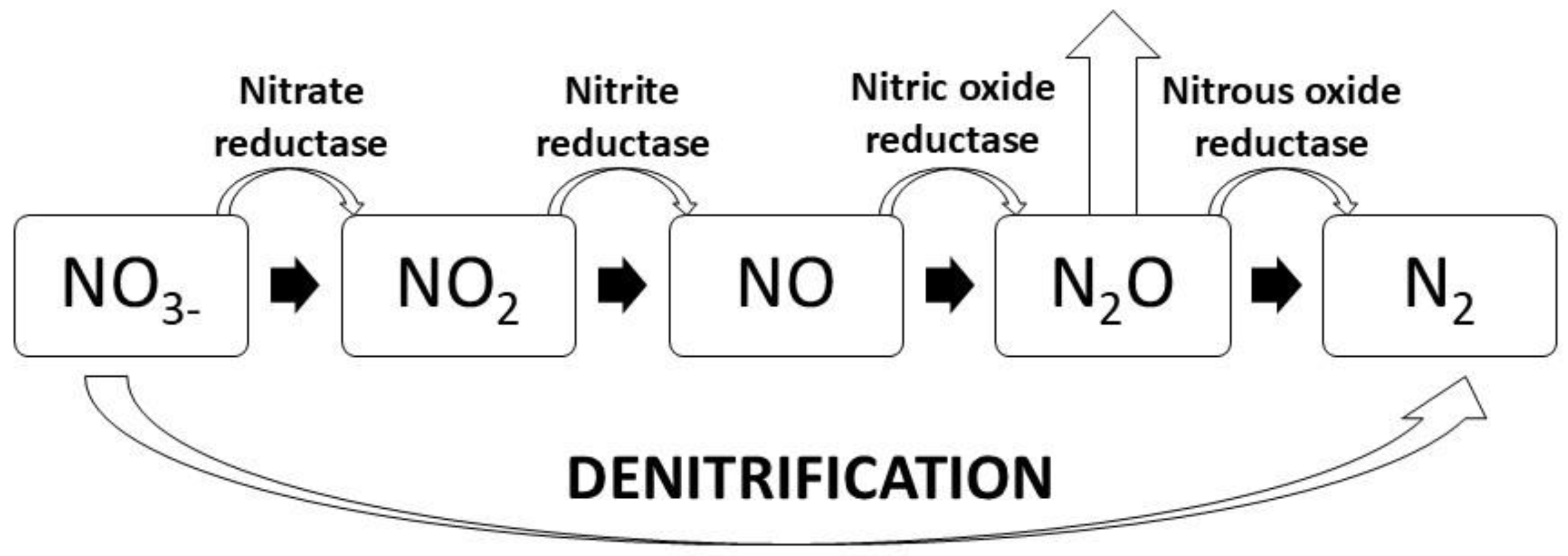
3.3. Denitrification
3.3.1. Oxygen
3.3.2. Soil pH
3.3.3. Carbon
3.3.4. Nitrogen
3.3.5. Temperature
3.4. Other Biological N2O Production Processes in Soil
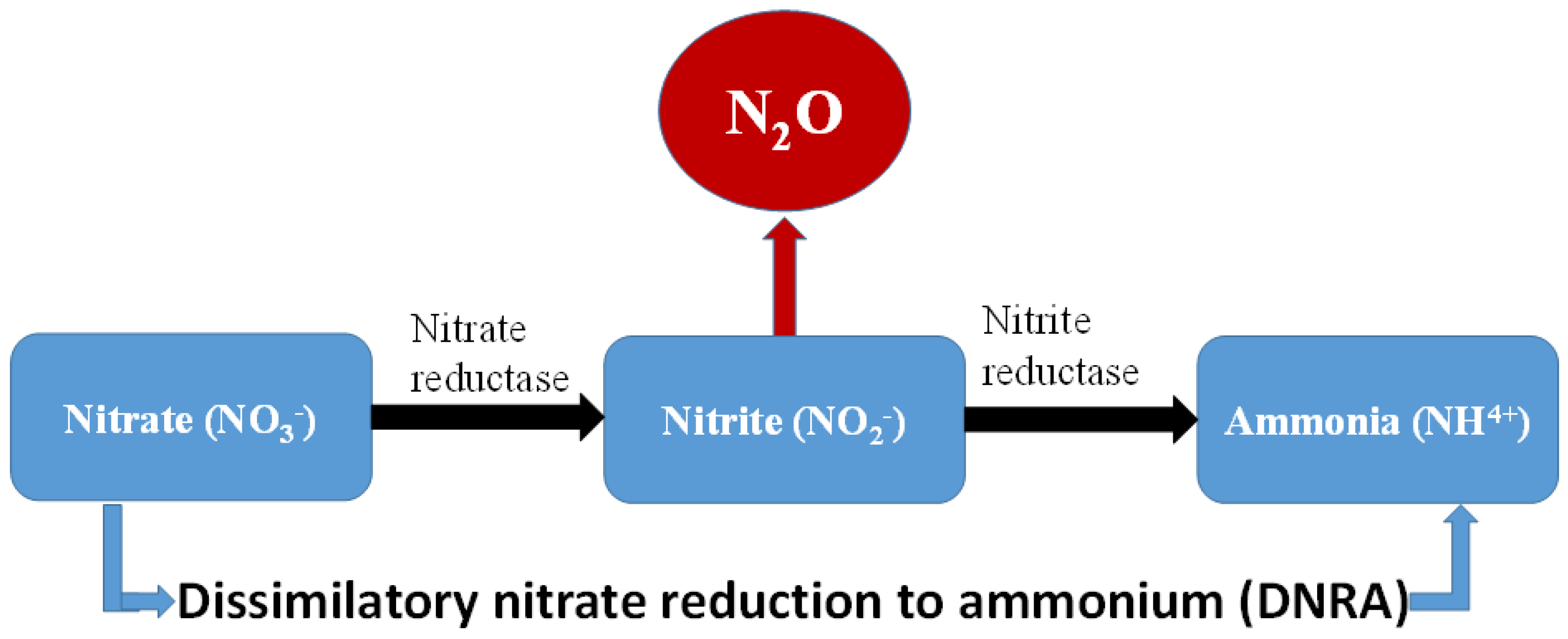
3.4.1. Dissimilatory Nitrate Reduction to Ammonium (DNRA)
3.4.2. Codenitrification
3.4.3. Nitrate Assimilation
3.4.4. Chemodenitrification
4. Role of Nitrification Inhibitors (NIs) in Nitrous Oxide Emissions from Soil
| S. No | Nitrification Inhibitors | Type (Biological or Chemical) | References |
|---|---|---|---|
| 01 | 1,9-decanediol | Biological | Lu et al. [82] |
| 02 | Polyaspartic acid | Polymer material | Yang et al. [94] |
| 03 | 3,4-dimethylprazol phosphate (DMPP) | Chemical | Zhao et al. [95]; Taghizadeh-Toosi et al. [96]; Bozal-Leorri et al. [97]; Muller et al. [98]; Huerfano et al. [99]; Barrena et al. [100] |
| 04 | Dimethylphenylpiperazinium | Chemical | Peixoto and Petersen [101]; Affendi et al. [102] |
| 05 | Pronitridine | Chemical | Nelson, [103] |
| 06 | Nitrapyrin | Chemical | Tariq et al. [104]; Tao et al. [105]; Khodabin et al. [106]; Mirkhani et al. [107]; Peixoto and Petersen [101]; Niu et al. [108] |
| 07 | Dicyandiamide (DCD) | Chemical | Li et al. [109]; Wang et al. [110]; Qu et al. [111]; Yaru et al. [112]; Ren et al. [113] |
| 08 | Neem cake | Biological | Pathak and Bhatia [114] |
| 09 | Neem oil | Biological | Chakraborty et al. [115]; Pathak and Bhatia [114] |
| 10 | Nimin | Biological | Datta and Adhya [85] |
| 11 | Thiosulphate | Chemical | Pathak and Bhatia [114] |
| 12 | 3,4-dimethyl pyrazole succinic (DMPSA) | Chemical | Huérfano et al. [99] |
| 13 | Karanjin | Biological | Paul et al. [84]; Datta and Adhya [85] |
| 14 | Chlorinated pyridine | Chemical | Ma et al. [25] |
| 15 | S-benzylisothiouronium butanoate (SBT-butanoate) | Chemical | Bhatia et al. [116] |
| 16 | S-benzylisothiouronium furoate (SBT-furoate) | Chemical | Bhatia et al. [116] |
| 17 | Mentha spicata oil | Biological | Patra et al. [117] |
| 18 | Calcium carbide | Chemical | Malla et al. [86] |
| 19 | Carbofuran | Chemical | Sahrawat [29] |
| 20 | Aminopurine | Chemical | Bharati et al. [118] |
| 21 | Ammonium thiosulphate | Chemical | Bharati et al. [118] |
| 22 | Pyridine | Chemical | Bharati et al. [118] |
| 23 | Sodium azide | Chemical | Bharati et al. [118] |
| 24 | Pyrazole | Chemical | Peixoto and Petersen [101] |
| 25 | 4-Methyl-1-(prop-2-yn-1-yl)-1H-1,2,3-triazole (MPT) | Chemical | Yildirim et al. [119] |
| 26 | Phosphate rock and epoxy resin-coated urea | Chemical | Ge et al. [112] |
| 27 | Methyl 3-(4-hydroxyphenyl) propionate | Biological | Huang et al. [120]; Lan et al. [121] |
| 28 | 3-methylpyrazole 1,2,4-triazole (Piadin) | Chemical | Muller et al. [98] |
| 29 | eNtrench | Chemical | Wood et al. [122] |
| 30 | 4-amino-l,2,4-triazole | Chemical | Anwar et al. [123] |
| 31 | 2-chloro-6-(trichloromethyl) pyridine | Chemical | Li et al. [124] |
| 32 | Sugarcane root exudate | Biological | Mawan and Kaewpradit [87]; Mawan and Kaewpradit [125] |
| 33 | Oxalic acid | Biological | Wang et al. [126] |
| 34 | Protocatechuic aldehyde | Biological | Wang et al. [126] |
| 34 | 1,9-decanediol | Biological | Dongwei et al. [127] |
| 35 | Leymus chinensis | Biological | Wang et al. [126] |
| 36 | Limus | Chemical | Paul et al. [84] |
| 37 | Moringa (Moringa oleifera Lam) seed extract | Biological | Yang et al. [128] |
| 38 | Sorghum roots release | Biological | Zhang et al. [129] |
| Biological Processes |
|---|
|
| Physical and chemical processes |
|
4.1. Mitigation of Nitrous Oxide Emissions Through Nitrification Inhibitors in Wheat
| Study Location | References | Treatment (Dose and Source) | N2O Emission | Mitigation (%) |
|---|---|---|---|---|
| China | Zong et al. [142] | Urea (U) | 977.6–1806.2 g N2O-N ha−1 | Control (C) |
| Urea (0.5% of urea-N by nitrapyrin) under elevated CO2 | - | 32.2–71.3 | ||
| Urea (0.5% of urea-N by nitrapyrin) under elevated temperature | - | 16.5–30.4 | ||
| Denmark | Peixoto and Petersen [101] | Pig slurry (PS) under ploughing | 0.15 kg N2O-N ha−1 | C |
| PS + DMPP under ploughing | 0.02 kg N2O-N ha−1 | 86.67 | ||
| PS + DMPP under direct seeding | 0.08 kg N2O-N ha−1 | 46.67 | ||
| PS + NP under ploughing | 0.07 kg N2O-N ha−1 | 53.33 | ||
| PS + NP under seeding | 0.03 kg N2O-N ha−1 | 80.00 | ||
| PS + Piadin under ploughing | 0.06 kg N2O-N ha−1 | 60.00 | ||
| PS + Piadin under seeding | 0.05 kg N2O-N ha−1 | 66.67 | ||
| Germany | Ni et al. [143] | Urea (U) | 710 g N ha−1 | C |
| U + DCD + 1H-1,2,4-Triazol (2% of Urea content m w/w) | 295 g N ha−1 | 58.45 | ||
| Germany | Guzman-Bustamante et al. [89] | Calcium ammonium nitrate | 2.9 kg N2O ha−1 | Control |
| Calcium ammonium nitrate + DMPSA | 2.1 kg N2O ha−1 | 27.58 | ||
| Pakistan | Dawar et al. [144] | Urea | 0.71 kg N2O ha−1 | Control |
| Biochar-6tons (B6) | 0.17 kg N2O ha−1 | 76.05 | ||
| Biochar-12tons (B12) | 0.26 kg N2O ha−1 | 63.38 | ||
| Urea + B6 | 0.54 kg N2O ha−1 | 23.94 | ||
| Urea + B12 | 0.57 kg N2O ha−1 | 19.72 | ||
| Urea + B6 + nitrapyrin (700 g ha−1) | 0.35 kg N2O ha−1 | 50.70 | ||
| Urea + B12 + nitrapyrin (700 g ha−1) | 0.46 kg N2O ha−1 | 35.21 | ||
| Canada | An et al. [145] | Urea–ammonium nitrate | 0.734 kg N2O-N ha−1 | Control |
| Urea–ammonium nitrate + DCD | 0.527 kg N2O-N ha−1 | 28.20 | ||
| Spain | Recio et al. [83] | Control (120 kg N ha−1 by urea[U]) | 498.8 g N ha−1 | Control |
| DMPSA (120 kg N ha−1, 99.2% by U, and 0.8% is DMPSA) | 143.2 g N ha−1 | 71.29 | ||
| India | Fagodiya et al. [135] | Control (120 kg N ha−1 by U) | 0.98 kg N2O ha−1 | Control |
| DCD (120 kg N ha−1, 108 and 12 kg N ha−1 by U and DCD, respectively) | 0.74 | 24.49 | ||
| NOCU (120 kg N ha−1 by NOCU) | 0.77 | 21.43 | ||
| Australia | Li et al. [137] | Control (100 kg N ha−1 by Urea) | 80.9 g N2O-N ha−1 | Control |
| NBPT (100 kg N ha−1 U coated by 0.045% w/w NCPT) | 81.5 g N2O-N ha−1 | −0.74 | ||
| DMPP (100 kg N ha−1 U coated by 0.16% w/w NCPT) | 53.9 g N2O-N ha−1 | 33.50 | ||
| China | He et al. [138] | Optimal nitrogen (ON)- [125 kg N ha−1 by U] | 1.59 kg N ha−1 | Control |
| ONB1-ON + Biochar (7.5 t ha−1) | 1.74 kg N ha−1 | −9.43 | ||
| ONB2-ON + biochar (15 t ha−1) | 2.15 kg N ha−1 | −35.22 | ||
| ONI-ON + DCD (0.5% w/w) + HQ (0.3% w/w) | 1.41 kg N ha−1 | 11.31 | ||
| ONIB1-ONB1 + DCD (0.5% w/w) + HQ (0.3% w/w) | 0.80 kg N ha−1 | 49.69 | ||
| ONIB2-DCD (0.5% w/w) + HQ (0.3% w/w) | 0.80 kg N ha−1 | 49.69 | ||
| Spain | Guardia et al. [139] | Control (120 kg N ha−1 by U in one dose) | 421.65 g N ha−1 | Control |
| NBPT (120 kg N ha−1 by coated U in one dose) | 352 g N ha−1 | 17.69 | ||
| DMPSA (120 kg N ha−1, 99.2% by U, and 0.8% is DMPSA) | 263.45 g N ha−1 | 37.52 | ||
| NBPT + DMPSA (120 kg N ha−1, 98.15% by U, 0.8% is DMPSA, and 0.35% is DMPSA) | 280 g N ha−1 | 33.59 | ||
| Nitrapyrin (120 kg N ha−1, 99.65% by U, and 0.35% by DMPSA) | 322.6 g N ha−1 | 23.49 | ||
| Australia | Jamali et al. [146] | Control (172.5 kg N ha−1, 22.5 by DAP, and 150 by U) | 496 g N2O-N ha−1 | Control |
| DMPP (172.5 kg N ha−1, 22.5 by DAP, 150 by U + 17.6% dimethyl pyrazole solution in 1L of water and sprinkled on the soil surface) | 414 g N2O-N ha−1 | 16.53 | ||
| China | Ma et al. [147] | CT-U (200 kg N ha−1) | 1.66 | C |
| CT-U + DCD (200 kg N ha−1, 97% by U, and 3% by DCD) | 1.17 | 29.52 | ||
| CT-U + CP (200 kg N ha−1, 99.76% by U, and 0.24% by CP) | 0.85 | 48.80 | ||
| NT-U (200 kg N ha−1) | 1.98 | C | ||
| NT-U + DCD (200 kg N ha−1, 97% by U, and 3% by DCD) | 1.36 | 31.31 | ||
| NT-U + CP (200 kg N ha−1, 99.76% by U, and 0.24% by CP) | 0.92 | 53.54 | ||
| China | Liu et al. [43] | Urea | 4.49 kg N ha−1 | C |
| DCD | 2.93 kg N ha−1 | 35 | ||
| DMPP | 2.78 kg N ha−1 | 38 | ||
| India | Bhatia et al. [116] | Control (120 kg N ha−1) | 778 g N2O-N ha−1 | Control |
| DCD (120 kg N ha−1, 90% by U and 10% by DCD) | 704 g N2O-N ha−1 | 9.51 | ||
| SBT-butanoate (120 kg N ha−1, 90% by U and 10% by SBT-butanoate) | 708 g N2O-N ha−1 | 9.00 | ||
| SBT-furoate (120 kg N ha−1, 90% by U, and 10% by SBT-furoate) | 673 g N2O-N ha−1 | 13.50 | ||
| India | Malla et al. [86] | U (120 kg N ha−1) | 0.66 kg N2O ha−1 | Control |
| U (120 kg N ha−1) + HQ (120 kg N ha−1) | 0.62 kg N2O ha−1 | 06.06 | ||
| U (108 kg N ha−1) + neem cake (12 kg N ha−1) | 0.52 kg N2O ha−1 | 21.21 | ||
| U (120 kg N ha−1) + thiosulphate (12 kg N ha−1) | 0.48 kg N2O ha−1 | 27.27 | ||
| U coated with calcium carbide (120 kg N ha−1) | 0.58 kg N2O ha−1 | 12.12 | ||
| NOCU (120 kg N ha−1) | 0.56 kg N2O ha−1 | 15.15 | ||
| U (108 kg N ha−1) + DCD (12 kg N ha−1) | 0.47 kg N2O ha−1 | 28.79 | ||
| China (Pot experiment) | Boeckx et al. [148] | U (345 kg N ha−1) | 2.11 mg N2O-N kg−1 soil | C |
| U+ HQ (0.3% of applied U) | 1.87 mg N2O-N kg−1 soil | 11.37 | ||
| U + DCD (0.5% of applied U) | 1.64 mg N2O-N kg−1 soil | 22.27 | ||
| U + HQ (0.3% of applied U) + DCD (0.5% of applied U) | 1.58 mg N2O-N kg−1 soil | 25.12 | ||
| India | Majumdar et al. [140] | U (120 kg N ha−1) | 1.43 kg N2O-N ha−1 | C |
| U + DCD (120 kg N ha−1, 85% by U, and 15% by DCD) | 1.09 kg N2O-N ha−1 | 23.78 | ||
| Nimin-coated U (120 kg N ha−1) | 1.00 kg N2O-N ha−1 | 30.07 | ||
| NOCU (120 kg N ha−1) | 1.36 kg N2O-N ha−1 | 4.90 | ||
| U + thiosulphate (120 kg N ha−1, 90% by U, and 10% by thiosulphate) | 1.19 kg N2O-N ha−1 | 16.78 |
4.2. Role of Nitrification Inhibitors (NIs) in Nitrous Oxide Mitigation in Maize
4.3. Nitrous Oxide Mitigation Through Nitrification Inhibitors in Rice
4.4. Nitrous Oxide Mitigation Through NIs in Orchards, Grasslands, and Others
| References and Location [Crop] | Treatment | N2O Emission | Mitigation (%) | |
|---|---|---|---|---|
| Li et al. [124] | Jiangsu Province (China), Vegetable Soil | Urea (U) | 0.47 mg N2O-N kg−1 soil | C |
| U + BC | 0.21 mg N2O-N kg−1 soil | 55.32 | ||
| U + DMPP | 0.12 mg N2O-N kg−1 soil | 74.47 | ||
| U + BC + DMPP | 0.09 mg N2O-N kg−1 soil | 80.85 | ||
| Tea Soil | Urea (U) | 0.24 mg N2O-N kg−1 soil | C | |
| U + BC | 0.21 mg N2O-N kg−1 soil | 12.50 | ||
| U + DMPP | 0.10 mg N2O-N kg−1 soil | 58.33 | ||
| U + BC + DMPP | 0.11 mg N2O-N kg−1 soil | 54.17 | ||
| Peach Soil (1 Year) | Urea (U) | 0.13 mg N2O-N kg−1 soil | C | |
| U + BC | 0.15 mg N2O-N kg−1 soil | −15.38 | ||
| U + DMPP | 0.05 mg N2O-N kg−1 soil | 61.54 | ||
| U + BC + DMPP | 0.06 mg N2O-N kg−1 soil | 53.85 | ||
| Peach Soil (7 Years) | Urea (U) | 0.18 mg N2O-N kg−1 soil | C | |
| U + BC | 0.20 mg N2O-N kg−1 soil | −11.11 | ||
| U + DMPP | 0.07 mg N2O-N kg−1 soil | 61.11 | ||
| U + BC + DMPP | 0.08 mg N2O-N kg−1 soil | 55.56 | ||
| Vilarrasa-Nogué, et al. [172] | Aitona (Spain) [Peach] | N25 (25 kg N ha−1) | 0.45 kg N2O-N ha−1 | C |
| N25+ DMPP (1% w/w of N) | −0.05 kg N2O-N ha−1 | 111.2 | ||
| N50 (25 kg N ha−1) | 0.99 kg N2O-N ha−1 | C | ||
| N50 + DMPP (1% w/w of N) | 0.55 kg N2O-N ha−1 | 44.67 | ||
| N100 (25 kg N ha−1) | 4.47 kg N2O-N ha−1 | C | ||
| N100 + DMPP (1% w/w of N) | 3.36 kg N2O-N ha−1 | 24.94 | ||
| Cardenas et al. [173] | Crichton (UK) [Grassland] | Urea (U) (320 kg N ha−1) | 4.24 kg N2O-N ha−1 | C |
| U + DCD (320 kg N ha−1) | 3.26 kg N2O-N ha−1 | 23.11 | ||
| Drayton (UK) [Grassland] | U (320 kg N ha−1) | 1.00 kg N2O-N ha−1 | C | |
| U + DCD (320 kg N ha−1) | 0.49 kg N2O-N ha−1 | 55.35 | ||
| North Wyke (UK) [Grassland] | U (320 kg N ha−1) | 3.07 kg N2O-N ha−1 | C | |
| U + DCD (320 kg N ha−1) | 2.02 kg N2O-N ha−1 | 34.21 | ||
| Hillsborough (UK) [Grassland] | U (320 kg N ha−1) | 1.18 kg N2O-N ha−1 | C | |
| U + DCD (320 kg N ha−1) | 0.31 kg N2O-N ha−1 | 73.78 | ||
| Pwllpeiran (UK) [Grassland] | U (320 kg N ha−1) | 2.06 kg N2O-N ha−1 | C | |
| U + DCD (320 kg N ha−1) | 0.46 kg N2O-N ha−1 | 77.72 | ||
| Zhang et al. [39] | Beijing (China) [Tomato–Cabbage] | U (460 kg N ha−1 yr−1) | 9.58 kg N2O-N ha−1 | C |
| U (95%) + DCD (5%) (460 kg N ha−1 yr−1) | 7.11 kg N2O-N ha−1 | 25.78 | ||
| Vinzeet et al. [178] | Munich (Germany) [Rapeseed] | Ammonium sulphate nitrate (200 kg N ha−1) | 0.43 kg N2O-N ha−1 | C |
| Urea (200 kg N ha−1) | 0.40 kg N2O-N ha−1 | 6.98 | ||
| Urea + NI (200 kg N ha−1) | 0.33 kg N2O-N ha−1 | 23.26 | ||
| Cantú et al. [179] | Federal University of Santa Maria (Brazil) [Lettuce] | Urea (175 kg N ha−1) | 6.71 | C |
| Urea + NBPT (0.003 w/w of urea) + DCD (0.031 w/w of urea) | 2.29 | 65.87 | ||
| PSC (500 kg N ha−1) | 1.46 | 78.24 | ||
| APSC (500 kg N ha−1) | 1.81 | 73.03 | ||
| Riches et al. [175] | Melbourne (Australia) [Lettuce] | Urea (125 kg N ha−1) | 337 g N2O-N ha−1 | C |
| DMPP urea (125 kg N ha−1) | 215 g N2O-N ha−1 | 36.80 | ||
| Melbourne (Australia) [Lettuce] | NP (121 kg N ha−1) | 296 g N2O-N ha−1 | C | |
| DMPP NP (121 kg N ha−1) | 210 g N2O-N ha−1 | 29.05 | ||
| DCD NP (121 kg N ha−1) | 292 g N2O-N ha−1 | 1.35 | ||
| Treweek et al. [177] | Canterbury Region (New Zealand) [Fodder Crop—Brassica] | Urine (700 kg N ha−1) | 14.7 kg N2O-N ha−1 | C |
| UD-[Urine + DCD (20 kg ha−1)] | 5.1 kg N2O-N ha−1 | 65.31 | ||
| UDB-[UD + biochar (5 t ha−1)] | 13.3 kg N2O-N ha−1 | C | ||
| UDB + DCD (20 kg ha−1) | 6.1 kg N2O-N ha−1 | 54.14 | ||
| Zhang et al. [129] | Gaoqiaomen Town, Jiangsu Province (China) [Seven Different Vegetables in Two Years] | Urea (1112 kg N ha−1) | 32.1 kg N ha−1 yr−1 | C |
| DCD (5% of urea) | 30.1 kg N ha−1 yr−1 | 06.23 | ||
| Nitrapyrin (0.24% of Urea) | 26.8 kg N ha−1 yr−1 | 16.51 | ||
| Biological NI | 21.1 kg N ha−1 yr−1 | 34.27 | ||
| Scheer et al. [176] | Queensland (Australia) [Broccoli] | CONV (120 kg N ha−1, 54 by Nitrophoska®, and 66 by U) | 411.2 g N2O-N ha−1 | C |
| DMPP (120 kg N ha−1, 54 by Nitrophoska Entec®, and 66 by U) | 298.1 g N2O-N ha−1 | 27.50 | ||
| DMPP-red (108 kg N ha−1, by 49 by Nitrophoska Entec®, and 59 by U) | 323.9 g N2O-N ha−1 | 21.23 | ||
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dix, B.A.; Hauschild, M.E.; Niether, W.; Wolf, B.; Gattinger, A. Regulating soil microclimate and greenhouse gas emissions with rye mulch in cabbage cultivation. Agric. Ecosyst. Environ. 2024, 367, 108951. [Google Scholar] [CrossRef]
- Portmann, R.W.; Daniel, J.S.; Ravishankara, A.R. Stratospheric ozone depletion due to nitrous oxide: Influences of other gases. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1256–1264. [Google Scholar] [CrossRef]
- Ranjan, R.; Yadav, R. Targeting nitrogen use efficiency for sustained production of cereal crops. J. Plant Nutr. 2019, 42, 1086–1113. [Google Scholar] [CrossRef]
- Fagodiya, R.K.; Pathak, H.; Kumar, A.; Bhatia, A.; Jain, N. Global temperature change potential of nitrogen use in agriculture: A 50-year assessment. Sci. Rep. 2017, 7, 44928. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Bhatia, A.; Kumar, A.; Das, T.K.; Jain, N.; Tomer, R.; Malyan, S.K.; Fagodiya, R.K.; Dubey, R.; Pathak, H. Mitigation of greenhouse gas emission from rice-wheat system of the Indo-Gangetic plains: Through tillage, irrigation and fertilizer management. Agric. Ecosyst. Environ. 2016, 230, 1–9. [Google Scholar] [CrossRef]
- IPCC (Intergorvernmental Panel on Climate Change), Synthesis Report 5; IPPC: Geneva, Switzerland, 2014.
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Kumar, A.; Medhi, K.; Fagodiya, R.K.; Subrahmanyam, G.; Mondal, R.; Raja, P.; Malyan, S.K.; Gupta, D.K.; Gupta, C.K.; Pathak, H. Molecular and ecological perspectives of nitrous oxide producing microbial communities in agro-ecosystems. Rev. Environ. Sci. Biotechnol. 2020, 19, 717–750. [Google Scholar] [CrossRef]
- Braker, G.; Conrad, R. Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functioning? Adv. Appl. Microbiol. 2011, 75, 33–70. [Google Scholar]
- Broucek, J. Nitrous oxide production from soil and manure application: A review. Slovak J. Anim. Sci. 2017, 50, 21–32. [Google Scholar]
- Sigurdarson, J.J.; Svane, S.; Karring, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol. 2018, 17, 241–258. [Google Scholar] [CrossRef]
- Richardson, D.; Felgate, H.; Watmough, N.; Thomson, A.; Baggs, E. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle—Could enzymic regulation hold the key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Peng, S.; Fahad, S.; Khaliq, A.; Huang, J.; Cui, K.; Nie, L. Rice management interventions to mitigate greenhouse gas emissions: A review. Environ. Sci. Pollut. Res. 2015, 22, 3342–3360. [Google Scholar] [CrossRef]
- Unique, A. Global N2O levels. Angew. Chem. Int. Ed. 2016, 6, 951–952. [Google Scholar]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Pilegaard, K.; Sutton, M.A.; Ambus, P.; Raivonen, M.; Duyzer, J.; Simpson, D.; Fagerli, H.; Fuzzi, S.; Schjoerring, J.K.; et al. Atmospheric composition change: Ecosystems-Atmosphere interactions. Atmos. Environ. 2009, 43, 5193–5267. [Google Scholar] [CrossRef]
- Rao, D.L.N.; Balachandar, D. Nitrogen Inputs From Biological Nitrogen Fixation in Indian Agriculture. In The Indian Nitrogen Assessment. Sources of Reactive Nitrogen, Environmental and Climate Effects, Management Options, and Policies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 117–132. [Google Scholar] [CrossRef]
- Kumar, S.S.; Malyan, S. Nitrification Inhibitors: A Perspective tool to Mitigate Greenhouse Gas Emission from Rice Soils. Curr. World Environ. 2016, 11, 423–428. [Google Scholar] [CrossRef]
- Baggs, E.M.; Smales, C.L.; Bateman, E.J. Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biol. Fertil. Soils 2010, 46, 793–805. [Google Scholar] [CrossRef]
- Corbet, A.S. The formation of hyponitrous acid as an intermediate compound in the biological or photochemical oxidation of ammonia to nitrous acid. Biochem. J. 1935, 29, 1086–1096. [Google Scholar] [CrossRef]
- Bremner, J.M. Sources of nitrous oxide in soils. Nutr. Cycl. Agroecosystems 1997, 49, 7–16. [Google Scholar] [CrossRef]
- Kalininskaia, T.A. Biological fixation of nitrogen. Vestn. Akad. Nauk SSSR 1962, 32, 44–50. [Google Scholar]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Lawton, T.J.; Bowen, K.E.; Sayavedra-Soto, L.A.; Arp, D.J.; Rosenzweig, A.C. Characterization of a nitrite reductase involved in nitrifier denitrification. J. Biol. Chem. 2013, 288, 25575–25583. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, L.; Zhang, X.; Yang, B.; Wang, J.; Yin, B.; Yan, X.; Xiong, Z. Mitigation of nitrous oxide emissions from paddy soil under conventional and no-till practices using nitrification inhibitors during the winter wheat-growing season. Biol. Fertil. Soils 2013, 49, 627–635. [Google Scholar] [CrossRef]
- Poth, M.; Focht, D.D. 15N kinetic analysis of N2O production by Nitrosomonas europaea: An examination of nitrifier denitrification. Appl. Environ. Microbiol. 1985, 49, 1134–1141. [Google Scholar] [CrossRef]
- Kool, D.M.; Dolfing, J.; Wrage, N.; Van Groenigen, J.W. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 2011, 43, 174–178. [Google Scholar] [CrossRef]
- Wagner, S. Biological Nitrogen Fixation. Nature Education Knowledge. 2011. Available online: https://www.nature.com/scitable/knowledge/library/biological-nitrogen-fixation-23570419/ (accessed on 24 January 2025).
- Sahrawat, K.L. Terminal electron acceptors for controlling methane emissions from submerged rice soils. Commun. Soil Sci. Plant Anal. 2004, 35, 1401–1413. [Google Scholar] [CrossRef]
- Maag, M.; Vinther, F.P. Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Appl. Soil Ecol. 1996, 4, 5–14. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Forge, T.A.; Goyer, C.; Brin, L.D. Effect of soil acidification on nitrification in soil. Can. J. Soil Sci. 2015, 95, 359–363. [Google Scholar] [CrossRef]
- Kyveryga, P.M.; Blackmer, A.M.; Ellsworth, J.W.; Isla, R. Soil pH Effects on Nitrification of Fall-Applied Anhydrous Ammonia. Soil Sci. Soc. Am. J. 2004, 68, 545. [Google Scholar] [CrossRef]
- Jiang, X.; Hou, X.; Zhou, X.; Xin, X.; Wright, A.; Jia, Z. pH regulates key players of nitrification in paddy soils. Soil Biol. Biochem. 2015, 81, 9–16. [Google Scholar] [CrossRef]
- Malyan, S.K. Reducing Methane Emission from Rice Soil Through Microbial Interventions; ICAR-Indian Agricultural Research Institute: New Delhi, India, 2017; Available online: https://krishikosh.egranth.ac.in/handle/1/5810074885 (accessed on 24 January 2025).
- Gubry-Rangin, C.; Novotnik, B.; Mandič-Mulec, I.; Nicol, G.W.; Prosser, J.I. Temperature responses of soil ammonia-oxidising archaea depend on pH. Soil Biol. Biochem. 2017, 106, 61–68. [Google Scholar] [CrossRef]
- Tan, X.; Shao, D.; Gu, W. Effects of temperature and soil moisture on gross nitrification and denitrification rates of a Chinese lowland paddy field soil. Paddy Water Environ. 2018, 16, 687–698. [Google Scholar] [CrossRef]
- Lai, T.V.; Farquharson, R.; Denton, M.D. High soil temperatures alter the rates of nitrification, denitrification and associated N2O emissions. J. Soils Sediments 2019, 19, 2176–2189. [Google Scholar] [CrossRef]
- Osborne, B.B.; Baron, J.S.; Wallenstein, M.D. Moisture and temperature controls on nitrification differ among ammonia oxidizer communities from three alpine soil habitats. Front. Earth Sci. 2016, 10, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Zheng, X.; Cai, Z.; Misselbrook, T.; Carswell, A.; Müller, C.; Zhang, J. Soil N transformation mechanisms can effectively conserve N in soil under saturated conditions compared to unsaturated conditions in subtropical China. Biol. Fertil. Soils 2018, 54, 495–507. [Google Scholar] [CrossRef]
- Szukics, U.; Abell, G.C.J.; Hödl, V.; Mitter, B.; Sessitsch, A.; Hackl, E.; Zechmeister-Boltenstern, S. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Ecol. 2010, 72, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Kou, Y.; Yang, W.; Chen, G.; Xu, H. Effects of urease and nitrification inhibitors on nitrous oxide emissions and nitrifying/denitrifying microbial communities in a rainfed maize soil: A 6-year field observation. Soil Tillage Res. 2018, 180, 82–90. [Google Scholar] [CrossRef]
- Liu, R.; Suter, H.; He, J.; Hayden, H.; Chen, D. Influence of temperature and moisture on the relative contributions of heterotrophic and autotrophic nitrification to gross nitrification in an acid cropping soil. J. Soils Sediments 2015, 15, 2304–2309. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Zheng, X. Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat-maize cropping system. Biogeosciences 2013, 10, 2427–2437. [Google Scholar] [CrossRef]
- Giles, M.; Morley, N.; Baggs, E.M.; Daniell, T.J. Soil nitrate reducing processes—Drivers, mechanisms for spatial variation, and significance for nitrous oxide production. Front. Microbiol. 2012, 3, 30024. [Google Scholar] [CrossRef]
- Mogge, B.; Kaiser, E.A.; Munch, J.C. Nitrous oxide emissions and denitrification N-losses from agricultural soils in the Bornhoved Lake region: Influence of organic fertilizers and land-use. Soil Biol. Biochem. 1999, 31, 1245–1252. [Google Scholar] [CrossRef]
- Smith, K.A. Greenhouse gas fluxes between land surfaces and the atmosphere. Prog. Phys. Geogr. 1990, 14, 349–372. [Google Scholar] [CrossRef]
- Weier, K.L.; Doran, J.W.; Power, J.F.; Walters, D.T. Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sci. Soc. Am. J. 1993, 57, 66–72. [Google Scholar] [CrossRef]
- Thomson, A.J.; Giannopoulos, G.; Pretty, J.; Baggs, E.M.; Richardson, D.J. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Šimek, M.; Cooper, J.E. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Čuhel, J.; Šimek, M.; Laughlin, R.J.; Bru, D.; Chèneby, D.; Watson, C.J.; Philippot, L. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef]
- Knowles, R. Denitrification. Microbiol. Rev. 1982, 46, 43–70. [Google Scholar] [CrossRef]
- Yamulki, S.; Harrison, R.M.; Goulding, K.W.T.; Webster, C.P. N2O, NO and NO2 fluxes from a grassland: Effect of soil pH. Soil Biol. Biochem. 1997, 29, 1199–1208. [Google Scholar] [CrossRef]
- Peterjohn, W.T. Denitrification: Enzyme content and activity in desert soils. Soil Biol. Biochem. 1991, 23, 845–855. [Google Scholar] [CrossRef]
- Martens, D.A. Nitrogen cycling under different soil management systems. Adv. Agron 2001, 70, 143–192. [Google Scholar]
- Xu, Y.; Xu, Z.; Cai, Z.; Reverchon, F. Review of denitrification in tropical and subtropical soils of terrestrial ecosystems. J. Soils Sediments 2013, 13, 699–710. [Google Scholar] [CrossRef]
- Pérez, C.A.; Carmona, M.R.; Fariña, J.M.; Armesto, J.J. Effects of Nitrate and Labile Carbon on Denitrification of Southern Temperate Forest Soils. Chil. J. Agric. Res. 2010, 70, 251–258. [Google Scholar] [CrossRef]
- Chu, C.Y.; Ko, T.H. Evaluation of Acid Leaching on the Removal of Heavy Metals and Soil Fertility in Contaminated Soil. J. Chem. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Henry, S.; Texier, S.; Hallet, S.; Bru, D.; Dambreville, C.; Chèneby, D.; Bizouard, F.; Germon, J.C.; Philippot, L. Disentangling the rhizosphere effect on nitrate reducers and denitrifiers: Insight into the role of root exudates. Environ. Microbiol. 2008, 10, 3082–3092. [Google Scholar] [CrossRef] [PubMed]
- Dodla, S.K.; Wang, J.J.; DeLaune, R.D.; Cook, R.L. Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. Sci. Total Environ. 2008, 407, 471–480. [Google Scholar] [CrossRef]
- Romain, V.; Sylvie, D.; David, B. Water residence time and pesticide removal in pilot-scale wetlands. Ecol. Eng. 2015, 85, 76–84. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Khera, T.S.; Doran, J.W. Mineralization and denitrification in upland, nearly saturated and flooded subtropical soil II. Effect of organic manures varying in N content and C:N ratio. Biol. Fertil. Soils 2000, 31, 168–174. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Mulvaney, C.S. Nitrogen fertilizers promote denitrification. Biol. Fertil. Soils 1997, 24, 211–220. [Google Scholar] [CrossRef]
- Wang, H.; Gilbert, J.A.; Zhu, Y.; Yang, X. Salinity is a key factor driving the nitrogen cycling in the mangrove sediment. Sci. Total Environ. 2018, 631–632, 1342–1349. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Bodenbender, J.; Wassmann, R.; Rennenberg, H. Methane transport capacity of rice plants. II. Variations among different rice cultivars and relationship with morphological characteristics. Nutr. Cycl. Agroecosystems 2000, 58, 367–375. [Google Scholar] [CrossRef]
- Philippot, L. Tracking nitrate reducers and denitrifiers in the environment. Biochem. Soc. Trans. 2005, 33, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Bu, C.; Wang, Y.; Ge, C.; Ahmad, H.A.; Gao, B.; Ni, S.Q. Dissimilatory Nitrate Reduction to Ammonium in the Yellow River Estuary: Rates, Abundance, and Community Diversity. Sci. Rep. 2017, 7, 6830. [Google Scholar] [CrossRef]
- Lam, P.; Kuypers, M.M.M. Microbial Nitrogen Cycling Processes in Oxygen Minimum Zones. Ann. Rev. Mar. Sci. 2011, 3, 317–345. [Google Scholar] [CrossRef]
- Rütting, T.; Boeckx, P.; Müller, C.; Klemedtsson, L. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences 2011, 8, 1779–1791. [Google Scholar] [CrossRef]
- Tiedje, J.M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Environ. Microbiol. Anaerobes 1988, 717, 179–244. [Google Scholar]
- Silver, W.L.; Herman, D.J.; Firestone, M.K. Dissimilatory Nitrate Reduction to Ammonium in Upland Tropical Forest Soils. Ecology 2015, 82, 2410–2416. [Google Scholar] [CrossRef]
- Yin, S.X.; Chen, D.; Chen, L.M.; Edis, R. Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils. Soil Biol. Biochem. 2002, 34, 1131–1137. [Google Scholar] [CrossRef]
- Yin, S.; Shen, Q.; Tang, Y.; Cheng, L. Reduction of nitrate to ammonium in selected paddy soils of China. Chemosphere 1998, 8, 221–228. [Google Scholar]
- Baggs, E.M. Soil microbial sources of nitrous oxide: Recent advances in knowledge, emerging challenges and future direction. Curr. Opin. Environ. Sustain. 2011, 3, 321–327. [Google Scholar] [CrossRef]
- Spott, O.; Russow, R.; Stange, C.F. Formation of hybrid N2O and hybrid N2 due to codenitrification: First review of a barely considered process of microbially mediated N-nitrosation. Soil Biol. Biochem. 2011, 43, 1995–2011. [Google Scholar] [CrossRef]
- Tanimoto, T.; Hatano, K.-I.; Kim, D.-H.; Uchiyama, H.; Shoun, H. Co-denitrification by the denitrifying system of the fungus Fusarium oxysporum. FEMS Microbiol. Lett. 1992, 93, 177–180. [Google Scholar] [CrossRef][Green Version]
- Kumon, Y.; Sasaki, Y.; Kato, I.; Takaya, N.; Shoun, H.; Beppu, T. Codenitrification and denitrification are dual metabolic pathways through which dinitrogen evolves from nitrate in Streptomyces antibioticus. J. Bacteriol. 2002, 184, 2963–2968. [Google Scholar] [CrossRef]
- Lin, J.T.; Stewart, V. Nitrate assimilation by bacteria. Adv. Microb. Physiol. 1997, 39, 1–30. [Google Scholar]
- Wankel, S.D.; Ziebis, W.; Buchwald, C.; Charoenpong, C.; de Beer, D.; Dentinger, J.; Xu, Z.; Zengler, K. Evidence for fungal and chemodenitrification based N2O flux from nitrogen impacted coastal sediments. Nat. Commun. 2017, 8, 15595. [Google Scholar] [CrossRef]
- Chalk, P.M.; Smith, C.J. Chemodenitrification. In Gaseous Loss of Nitrogen from Plant-Soil Systems; Springer: Dordrecht, The Netherlands, 1983; pp. 65–89. [Google Scholar] [CrossRef]
- Kesik, M.; Blagodatsky, S.; Papen, H.; Butterbach-Bahl, K. Effect of pH, temperature and substrate on N2O, NO and CO2 production by Alcaligenes faecalis p. J. Appl. Microbiol. 2006, 101, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, X.; Jiang, J.; Kronzucker, H.J.; Shen, W.; Shi, W. Effects of the biological nitrification inhibitor 1,9-decanediol on nitrification and ammonia oxidizers in three agricultural soils. Soil Biol. Biochem. 2019, 129, 48–59. [Google Scholar] [CrossRef]
- Recio, J.; Vallejo, A.; Le-Noë, J.; Garnier, J.; García-Marco, S.; Álvarez, J.M.; Sanz-Cobena, A. The effect of nitrification inhibitors on NH3 and N2O emissions in highly N fertilized irrigated Mediterranean cropping systems. Sci. Total Environ. 2018, 636, 427–436. [Google Scholar] [CrossRef]
- Paul, A.; Bhatia, A.; Tomer, R.; Kumar, V.; Sharma, S.; Pal, R.; Mina, U.; Kumar, R.; Manjaiah, K.M.; Chakrabarti, B.; et al. Dual inhibitors for mitigating greenhouse gas emissions and ammonia volatilization in rice for enhancing environmental sustainability. Clean. Environ. Syst. 2024, 13, 100199. [Google Scholar] [CrossRef]
- Datta, A.; Adhya, T.K. Effects of organic nitrification inhibitors on methane and nitrous oxide emission from tropical rice paddy. Atmos. Environ. 2014, 92, 533–545. [Google Scholar] [CrossRef]
- Malla, G.; Bhatia, A.; Pathak, H.; Prasad, S.; Jain, N.; Singh, J. Mitigating nitrous oxide and methane emissions from soil in rice-wheat system of the Indo-Gangetic plain with nitrification and urease inhibitors. Chemosphere 2005, 58, 141–147. [Google Scholar] [CrossRef]
- Mawan, N.; Kaewpradit, W. Sugarcane exudates as nitrification inhibitors; improvement of soybean nitrogen recovery and yield by reducing soil nitrification and N2O emission using 15N tracing techniques. Rhizosphere 2024, 29, 100871. [Google Scholar] [CrossRef]
- Fagodiya, R.K.; Singh, A.; Prajapat, K.; Chandra, P.; Malyan, S.K.; Verma, K.; Verma, V.K.; Rai, A.K.; Yadav, R.K.; Biswas, A.K. Conservation agriculture practices for carbon sequestration and greenhouse gas mitigation. In Waste Management for Sustainable and Restored Agricultural Soil; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2000, pp. 323–343. ISBN 9780443184864. [Google Scholar]
- Guzman-Bustamante, I.; Schulz, R.; Müller, T.; Ruser, R. Split N application and DMP based nitrification inhibitors mitigate N2O losses in a soil cropped with winter wheat. Nutr. Cycl. Agroecosystems 2022, 123, 119–135. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Cassman, N.A.; Pijl, A.S.; van Veen, J.A.; Cantarella, H.; Kuramae, E.E. Nitrosospira sp. govern nitrous oxide emissions in a tropical soil amended with residues of bioenergy crop. Front. Microbiol. 2018, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Barth, K.R.; Isabella, V.M.; Clark, V.L. Biochemical and genomic analysis of the denitrification pathway within the genus Neisseria. Microbiology 2009, 155, 4093–4103. [Google Scholar] [CrossRef]
- Rodgers, G.A. Nitrification inhibitors in agriculture. J. Environ. Sci. Heal. Part A Environ. Sci. Eng. 1986, 21, 701–722. [Google Scholar] [CrossRef]
- Irigoyen, I.; Muro, J.; Azpilikueta, M.; Aparicio-Tejo, P.; Lamsfus, C. Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures. Soil Res. 2003, 41, 1177. [Google Scholar] [CrossRef]
- Yang, J.; Liu, T.; Liu, H.; Zhang, D.; Zhai, L.; Liu, J.; Wang, M.; Chen, Y.; Chen, B.; Wang, H. Biodegradable PASP can effectively inhibit nitrification, moderate NH3 emission, and promote crop yield. Arch. Agron. Soil Sci. 2019, 65, 1273–1286. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, G.; Zhang, X.; Tan, Y.; Meng, F.; Bol, R. Nitrification inhibitor 3,4-dimethylpyrazole phosphate alleviates the dissolution of soil inorganic carbon caused by nitrogen fertilization. Geoderma 2024, 441, 116742. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Baral, K.R.; Sørensen, P.; Petersen, S.O. Short-Term Nitrous Oxide Emissions from Cattle Slurry for Silage Maize: Effects of Placement and the Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP). Sustainability 2023, 15, 15810. [Google Scholar] [CrossRef]
- Bozal-Leorri, A.; Corrochano-Monsalve, M.; Arregui, L.M.; Aparicio-Tejo, P.M.; González-Murua, C. Evaluation of a crop rotation with biological inhibition potential to avoid N2O emissions in comparison with synthetic nitrification inhibition. J. Environ. Sci. 2023, 127, 222–233. [Google Scholar] [CrossRef]
- Muller, J.; De Rosa, D.; Friedl, J.; De Antoni Migliorati, M.; Rowlings, D.; Grace, P.; Scheer, C. Combining nitrification inhibitors with a reduced N rate maintains yield and reduces N2O emissions in sweet corn. Nutr. Cycl. Agroecosystems 2023, 125, 107–121. [Google Scholar] [CrossRef]
- Huérfano, X.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Torralbo, F.; González-Murua, C.; Menéndez, S. DMPSA and DMPP equally reduce N2O emissions from a maize-ryegrass forage rotation under Atlantic climate conditions. Atmos. Environ. 2018, 187, 255–265. [Google Scholar] [CrossRef]
- Barrena, I.; Menéndez, S.; Correa-Galeote, D.; Vega-Mas, I.; Bedmar, E.J.; González-Murua, C.; Estavillo, J.M. Soil water content modulates the effect of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on nitrifying and denitrifying bacteria. Geoderma 2017, 303, 1–8. [Google Scholar] [CrossRef]
- Peixoto, L.; Petersen, S.O. Efficacy of three nitrification inhibitors to reduce nitrous oxide emissions from pig slurry and mineral fertilizers applied to spring barley and winter wheat in Denmark. Geoderma Reg. 2023, 32, e00597. [Google Scholar] [CrossRef]
- Noor Affendi, N.M.; Yusop, M.K.; Othman, R. Efficiency of Coated Urea on Nutrient Uptake and Maize Production. Commun. Soil Sci. Plant Anal. 2018, 49, 1394–1400. [Google Scholar] [CrossRef]
- Habibullah, H.; Nelson, K.A.; Motavalli, P.P. Management of nitrapyrin and pronitridine nitrification inhibitors with urea ammonium nitrate for winter wheat production. Agronomy 2018, 8, 204. [Google Scholar] [CrossRef]
- Tariq, A.; Hansen, L.V.; Braendholt, A.; Stoumann Jensen, L.; Bruun, S. Assessing nitrous oxide mitigation efficiency of three nitrification inhibitors with 2 synthetic and organic fertilisers in Eastern Denmark 3. Environ. Technol. Innov. 2025, 37, 103952. [Google Scholar] [CrossRef]
- Tao, Z.; Liu, Y.; Li, S.; Li, B.; Fan, X.; Liu, C.; Hu, C.; Liu, H.; Li, Z. Global warming potential assessment under reclaimed water and livestock wastewater irrigation coupled with co-application of inhibitors and biochar. J. Environ. Manag. 2024, 353, 120143. [Google Scholar] [CrossRef]
- Khodabin, G.; Jalilian, A.; Zandi Esfahan, E.; Shahbazi, N.; Amini, F.; Ghaznavi, S.; Heidarzadeh, A. The Effect of Nitrification Inhibitor on Grain Yield of Wheat Cultivars and Some Soil Properties under Conventional and No-Tillage Systems. Iran. J. Field Crop Sci. 2023, 54, 31–46. [Google Scholar] [CrossRef]
- Mirkhani, R.; Shorafa, M.; Roozitalab, M.H.; Heng, L.K.; Dercon, G. Ammonia emission and nitrogen use efficiency with application of nitrification inhibitor and plant growth regulator in a calcareous soil (Karaj, Iran). Geoderma Reg. 2023, 35, e00718. [Google Scholar] [CrossRef]
- Niu, Y.; Luo, J.; Liu, D.; Müller, C.; Zaman, M.; Lindsey, S.; Ding, W. Effect of biochar and nitrapyrin on nitrous oxide and nitric oxide emissions from a sandy loam soil cropped to maize. Biol. Fertil. Soils 2018, 54, 645–658. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Liu, J.; Shen, J.; Kuang, W.; Chen, J.; Zeng, F. Effects of nitrification and urease inhibitors on nitrous oxide emissions and concentrations driven by soil moisture in sandy soils. J. Environ. Manag. 2025, 373, 123066. [Google Scholar] [CrossRef]
- Wang, X.; Cao, B.; Zhou, Y.; Zhao, M.; Chen, Y.; Zhang, J.; Wang, J.; Liang, L. Effects of Long-Term Controlled-Release Urea on Soil Greenhouse Gas Emissions in an Open-Field Lettuce System. Plants 2024, 13, 1071. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Xia, X.; Liu, D.; Dong, H.; Pan, T.; Feng, H.; Lou, Y.; Wang, H.; Yang, Q.; Yang, Z.; et al. Response of Nitrification and Crop Yield to the Presence of NBPT and DCD in a Wheat-Corn Double Cropping System. Agronomy 2024, 14, 285. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, M.; Dong, Y.; Dai, X.; He, M. Enhanced-Efficiency Urea Coated with Ground Phosphate Rock Powder, Inhibitor and Epoxy Resin: Preparation and Effects on Soil Nitrogen Supply Capacity, Wheat Yield and Nitrogen Use Efficiency. Eurasian Soil Sci. 2024, 57, 1033–1047. [Google Scholar] [CrossRef]
- Ren, B.; Huang, Z.; Liu, P.; Zhao, B.; Zhang, J. Urea ammonium nitrate solution combined with urease and nitrification inhibitors jointly mitigate NH3 and N2O emissions and improves nitrogen efficiency of summer maize under fertigation. Field Crop. Res. 2023, 296, 108909. [Google Scholar] [CrossRef]
- Pathak, H.; Bhatia, A. Reactive Nitrogen and Its Impacts on Climate Change. In The Indian Nitrogen Assessment; Elsevier: Amsterdam, The Netherlands, 2017; pp. 383–401. ISBN 9780128119044. [Google Scholar]
- Chakraborty, R.; Purakayastha, T.J.; Pendall, E.; Dey, S.; Jain, N.; Kumar, S. Nitrification and urease inhibitors mitigate global warming potential and ammonia volatilization from urea in rice-wheat system in India: A field to lab experiment. Sci. Total Environ. 2023, 898, 165479. [Google Scholar] [CrossRef]
- Bhatia, A.; Sasmal, S.; Jain, N.; Pathak, H.; Kumar, R.; Singh, A. Mitigating nitrous oxide emission from soil under conventional and no-tillage in wheat using nitrification inhibitors. Agric. Ecosyst. Environ. 2010, 136, 247–253. [Google Scholar] [CrossRef]
- Patra, A.K.; Le Roux, X.; Abbadie, L.; Clays-Josserand, A.; Poly, F.; Loiseau, P.; Louault, F. Effect of microbial activity and nitrogen mineralization on free-living nitrogen fixation in permanent grassland soils. J. Agron. Crop Sci. 2007, 193, 153–156. [Google Scholar] [CrossRef]
- Bharati, K.; Mohanty, S.R.; Singh, D.P.; Rao, V.R.; Adhya, T.K. Influence of incorporation or dual cropping of Azolla on methane emission from a flooded alluvial soil planted to rice in eastern India. Agric. Ecosyst. Environ. 2000, 79, 73–83. [Google Scholar] [CrossRef]
- Yildirim, S.C.; Nathanael, J.G.; Frindte, K.; Leal, O.d.A.; Walker, R.M.; Roessner, U.; Knief, C.; Nicolas, B.; Wille, U. 4-Methyl-1-(prop-2-yn-1-yl)-1H-1,2,3-triazole (MPT): A Novel, Highly Efficient Nitrification Inhibitor for Agricultural Applications. ACS Agric. Sci. Technol. 2024, 4, 255–265. [Google Scholar] [CrossRef]
- Huang, X.; Zou, Y.; Qiao, C.; Liu, Q.; Liu, J.; Kang, R.; Ren, L.; Wu, W. Effects of Biological Nitrification Inhibitor on Nitrous Oxide and nosZ, nirK, nirS Denitrifying Bacteria in Paddy Soils. Sustainability 2023, 15, 5348. [Google Scholar] [CrossRef]
- Lan, T.; Chen, X.; Liu, S.; Zhou, M.; Gao, X. Biological and chemical nitrification inhibitors exhibited different effects on soil gross N nitrification rate and N2O production: A 15N microcosm study. Environ. Sci. Pollut. Res. Int. 2023, 30, 116162–116174. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Gao, X.; Tiessen, K.H.D.; Tenuta, M.; Flaten, D.N. Enhanced efficiency urea fertilizers and timing effects on N2O emissions from spring wheat production in Manitoba. Agron. J. 2024, 116, 51–72. [Google Scholar] [CrossRef]
- Anwar, A.M. Efficiency of Nitrogen Fertilizer in Wheat As Influenced by Different Nitrification Inhibitors. Agrobiol. Rec. 2023, 11, 67–77. [Google Scholar] [CrossRef]
- Li, Z.; Xu, P.; Han, Z.; Wu, J.; Bo, X.; Wang, J.; Zou, J. Effect of biochar and DMPP application alone or in combination on nitrous oxide emissions differed by soil types. Biol. Fertil. Soils 2023, 59, 123–138. [Google Scholar] [CrossRef]
- Mawan, N.; Kaewpradit, W. Sugarcane root exudate impact on the potential nitrification rate and N dynamics in the rhizosphere. Rhizosphere 2022, 23, 100551. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Wang, C.; Xie, T.; Wang, W.; Wang, D.; Zhang, G. Two newly-identified biological nitrification inhibitors in Suaeda salsa: Synthetic pathways and influencing mechanisms. Chem. Eng. J. 2023, 454, 140172. [Google Scholar] [CrossRef]
- Dongwei, D.; Mingkun, M.; Xiaoyang, Z.; Yufang, L.; Kronzucker, H.J.; Weiming, S. Potential Secretory Transporters and Biosynthetic Precursors of Biological Nitrification Inhibitor 1,9-Decanediol in Rice as Revealed by Transcriptome and Metabolome Analyses. Rice Sci. 2024, 31, 87–102. [Google Scholar] [CrossRef]
- Yang, M.; Ban, C.; Zhao, T.; Zhao, J.; Zhou, N.; Ma, L.; Zhou, J.; Deng, X. Harnessing moringa seed extract for control of soil nitrate accumulation and nitrous oxide emissions on the Loess Plateau. Appl. Soil Ecol. 2025, 206, 105862. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, C.H.; Li, Q.L.; Li, B.; Zhu, Y.Y.; Xiong, Z.Q. A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system. Agric. Ecosyst. Environ. 2015, 201, 43–50. [Google Scholar] [CrossRef]
- FAOSTAT; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022.
- Cooper, R. Re-discovering ancient wheat varieties as functional foods. J. Tradit. Complement. Med. 2015, 5, 138–143. [Google Scholar] [CrossRef]
- Recio, J.; Alvarez, J.M.; Rodriguez-Quijano, M.; Vallejo, A. Nitrification inhibitor DMPSA mitigated N2O emission and promoted NO sink in rainfed wheat. Environ. Pollut. 2019, 245, 199–207. [Google Scholar] [CrossRef]
- Opoku, A.; Chaves, B.; De Neve, S. Neem seed oil: A potent nitrification inhibitor to control nitrate leaching after incorporation of crop residues. Biol. Agric. Hortic. 2014, 30, 145–152. [Google Scholar] [CrossRef]
- Kiran, U.; Patra, D. Influence of natural essential oils and their by-products as nitrification retarders in regulating nitrogen utilization for Japanese mint in sandy loam soils of subtropical central India. Agric. Ecosyst. Environ. 2003, 94, 237–245. [Google Scholar] [CrossRef]
- Fagodiya, R.K.; Pathak, H.; Bhatia, A.; Jain, N.; Gupta, D.K.; Kumar, A.; Malyan, S.K.; Dubey, R.; Radhakrishanan, S.; Tomer, R. Nitrous oxide emission and mitigation from maize–wheat rotation in the upper Indo-Gangetic Plains. Carbon Manag. 2019, 10, 489–499. [Google Scholar] [CrossRef]
- Majumdar, D. Methane and nitrous oxide emission from irrigated rice fields: Proposed mitigation strategies. Curr. Sci. 2003, 84, 1317–1326. [Google Scholar]
- Li, J.; Li, Y.; Wan, Y.; Wang, B.; Waqas, M.A.; Cai, W.; Guo, C.; Zhou, S.; Su, R.; Qin, X.; et al. Combination of modified nitrogen fertilizers and water saving irrigation can reduce greenhouse gas emissions and increase rice yield. Geoderma 2018, 315, 1–10. [Google Scholar] [CrossRef]
- He, T.; Liu, D.; Yuan, J.; Luo, J.; Lindsey, S.; Bolan, N.; Ding, W. Effects of application of inhibitors and biochar to fertilizer on gaseous nitrogen emissions from an intensively managed wheat field. Sci. Total Environ. 2018, 628–629, 121–130. [Google Scholar] [CrossRef]
- Guardia, G.; Vallejo, A.; Cardenas, L.M.; Dixon, E.R.; García-Marco, S. Fate of 15N-labelled ammonium nitrate with or without the new nitrification inhibitor DMPSA in an irrigated maize crop. Soil Biol. Biochem. 2018, 116, 193–202. [Google Scholar] [CrossRef]
- Majumdar, D.; Kumar, S.; Pathak, H.; Jain, M.C.; Kumar, U. Reducing nitrous oxide emission from an irrigated rice field of North India with nitrification inhibitors. Agric. Ecosyst. Environ. 2000, 81, 163–169. [Google Scholar] [CrossRef]
- Ribeiro, P.L.; Pitann, B.; Banedjschafie, S.; Mühling, K.H. Effectiveness of three nitrification inhibitors on mitigating trace gas emissions from different soil textures under surface and subsurface drip irrigation. J. Environ. Manag. 2024, 359, 120969. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Qiu, N.; Li, L.; Zhang, Y.; Shi, X.; Zhang, D.; Hao, X.; Li, P.; Lam, S.K. Effects of the nitrification inhibitor nitrapyrin on N2O emissions under elevated CO2 and rising temperature in a wheat cropping system. Appl. Soil Ecol. 2024, 201, 105501. [Google Scholar] [CrossRef]
- Ni, K.; Vietinghoff, M.; Pacholski, A. Targeting yield and reducing nitrous oxide emission by use of single and double inhibitor treated urea during winter wheat season in Northern Germany. Agric. Ecosyst. Environ. 2023, 347, 108391. [Google Scholar] [CrossRef]
- Dawar, K.; Khan, H.; Zaman, M.; Muller, C.; Alam, S.S.; Fahad, S.; Alwahibi, M.S.; Alkahtani, J.; Saeed, B.; Saud, S.; et al. The Effect of Biochar and Nitrogen Inhibitor on Ammonia and Nitrous Oxide Emissions and Wheat Productivity. J. Plant Growth Regul. 2021, 40, 2465–2475. [Google Scholar] [CrossRef]
- An, H.; Owens, J.; Stoeckli, J.; Hao, X.; Beres, B.; Li, Y. Nitrous oxide emissions following split fertilizer application on winter wheat grown on Mollisols of Southern Alberta, Canada. Geoderma Reg. 2020, 21, e00272. [Google Scholar] [CrossRef]
- Jamali, H.; Quayle, W.; Scheer, C.; Baldock, J. Mitigation of N2O emissions from surface-irrigated cropping systems using water management and the nitrification inhibitor DMPP. Soil Res. 2016, 54, 481–493. [Google Scholar] [CrossRef]
- Ma, J.; Ji, Y.; Zhang, G.; Xu, H.; Yagi, K. Timing of midseason aeration to reduce CH4 and N2O emissions from double rice cultivation in China. Soil Sci. Plant Nutr. 2013, 59, 35–45. [Google Scholar] [CrossRef]
- Boeckx, P.; Xu, X.; Van Cleemput, O. Mitigation of N2O and CH4 emission from rice and wheat cropping systems using dicyandiamide and hydroquinone. Nutr. Cycl. Agroecosystems 2005, 72, 41–49. [Google Scholar] [CrossRef]
- Pengthamkeerati, P.; Modtad, A. Nitrification Inhibitor Effects on Nitrous Oxide Emission, Nitrogen Transformation, and Maize (Zea mays L.) Yield in Loamy Sand Soil in Thailand. Commun. Soil Sci. Plant Anal. 2016, 47, 875–887. [Google Scholar] [CrossRef]
- Hadi, A.; Jumadi, O.; Inubushi, K.; Yagi, K. Mitigation options for N2O emission from a corn field in Kalimantan, Indonesia. Soil Sci. Plant Nutr. 2008, 54, 644–649. [Google Scholar] [CrossRef]
- Skowrońska, M.; Kuśmierz, S.; Walczak, J. Selected Carbon and Nitrogen Compounds in a Maize Agroecosystem under the Use of Nitrogen Mineral Fertilizer, Farmyard Manure, Urease, and Nitrification Inhibitors. Agriculture 2024, 14, 274. [Google Scholar] [CrossRef]
- Guardia, G.; García-Gutiérrez, S.; Vallejo, A.; Ibáñez, M.A.; Sanchez-Martin, L.; Montoya, M. Nitrous oxide emissions and N-cycling gene abundances in a drip-fertigated (surface versus subsurface) maize crop with different N sources. Biol. Fertil. Soils 2024, 60, 375–391. [Google Scholar] [CrossRef]
- Dong, D.; Yang, W.; Sun, H.; Kong, S.; Xu, H. Effects of animal manure and nitrification inhibitor on N2O emissions and soil carbon stocks of a maize cropping system in Northeast China. Sci. Rep. 2022, 12, 15202. [Google Scholar] [CrossRef]
- Borzouei, A.; Mander, U.; Teemusk, A.; Sanz-Cobena, A.; Zaman, M.; Kim, D.G.; Muller, C.; Kelestanie, A.A.; Amin, P.S.; Moghiseh, E.; et al. Effects of the nitrification inhibitor nitrapyrin and tillage practices on yield-scaled nitrous oxide emission from a maize field in Iran. Pedosphere 2021, 31, 314–322. [Google Scholar] [CrossRef]
- Jumadi, O.; Hala, Y.; Iriany, R.N.; Makkulawu, A.T.; Baba, J.; Hartono; Hiola, S.F.; Inubushi, K. Combined effects of nitrification inhibitor and zeolite on greenhouse gas fluxes and corn growth. Environ. Sci. Pollut. Res. 2020, 27, 2087–2095. [Google Scholar] [CrossRef]
- Omonode, R.A.; Vyn, T.J. Tillage and Nitrogen Source Impacts on Relationships between Nitrous Oxide Emission and Nitrogen Recovery Efficiency in Corn. J. Environ. Qual. 2019, 48, 421–429. [Google Scholar] [CrossRef]
- Song, Y.; Tan, M.; Zhang, Y.; Li, X.; Liu, P.; Mu, Y. Effect of fertilizer deep placement and nitrification inhibitor on N2O, NO, HONO, and NH3 emissions from a maize field in the North China Plain. Atmos. Environ. 2024, 334, 120684. [Google Scholar] [CrossRef]
- Du, Y.; Lu, Y.; Guo, S.; Wang, R.; Song, X.; Ju, X. Enhanced efficiency nitrogen fertilizers (EENFs) can reduce nitrous oxide emissions and maintain high grain yields in a rain-fed spring maize cropping system. Field Crop. Res. 2024, 312, 109408. [Google Scholar] [CrossRef]
- Drury, C.F.; Yang, X.; Reynolds, W.D.; Calder, W.; Oloya, T.O.; Woodley, A.L. Combining Urease and Nitrification Inhibitors with Incorporation Reduces Ammonia and Nitrous Oxide Emissions and Increases Corn Yields. J. Environ. Qual. 2017, 46, 939–949. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Sánchez-Martín, L.; García-Torres, L.; Vallejo, A. Gaseous emissions of N2O and NO and NO3− leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop. Agric. Ecosyst. Environ. 2012, 149, 64–73. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Fagodiya, R.K.; Kumar, S.S.; Kumar, A.; Gupta, D.K.; Tomer, R.; Harit, R.C.; Kumar, V.; Jain, N.; et al. Plummeting global warming potential by chemicals interventions in irrigated rice: A lab to field assessment. Agric. Ecosyst. Environ. 2021, 319, 107545. [Google Scholar] [CrossRef]
- Guo, C.; Ren, T.; Li, P.; Wang, B.; Zou, J.; Hussain, S.; Cong, R.; Wu, L.; Lu, J.; Li, X. Producing more grain yield of rice with less ammonia volatilization and greenhouse gases emission using slow/controlled-release urea. Environ. Sci. Pollut. Res. 2019, 26, 2569–2579. [Google Scholar] [CrossRef]
- Ghosh, S.; Majumdar, D.; Jain, M.C. Methane and nitrous oxide emissions from an irrigated rice of North India. Chemosphere 2003, 51, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Xu, H.; Cai, Z.; Yagi, K. Methane and nitrous oxide emissions from rice paddy soil as influenced by timing of application of hydroquinone and dicyandiamide. Nutr. Cycl. Agroecosystems 2009, 85, 31–40. [Google Scholar] [CrossRef]
- Surekha, K.; Pragnya, M.; Manasa, V.; Gobinath, R.; Brajendra, P. Potential agronomic and environmental benefits of neem coated urea (NCU) application in rice (Oryza sativa L.)—A systematic review. Oryza-An Int. J. Rice 2024, 61, 408–419. [Google Scholar] [CrossRef]
- PATTANAIK, I. Relative performance of neem coated urea on the basis of need based nitrogen management using customized leaf colour chart in low land rice (Oryza sativa) of eastern India. Ann. Plant Soil Res. 2022, 24, 536–542. [Google Scholar] [CrossRef]
- He, T.; Yuan, J.; Xiang, J.; Lin, Y.; Luo, J.; Lindsey, S.; Liao, X.; Liu, D.; Ding, W. Combined biochar and double inhibitor application offsets NH3 and N2O emissions and mitigates N leaching in paddy fields. Environ. Pollut. 2022, 292, 118344. [Google Scholar] [CrossRef]
- Cowan, N.; Bhatia, A.; Drewer, J.; Jain, N.; Singh, R.; Tomer, R.; Kumar, V.; Kumar, O.; Prasanna, R.; Ramakrishnan, B.; et al. Experimental comparison of continuous and intermittent flooding of rice in relation to methane, nitrous oxide and ammonia emissions and the implications for nitrogen use efficiency and yield. Agric. Ecosyst. Environ. 2021, 319, 107571. [Google Scholar] [CrossRef]
- Gaihre, Y.K.; Singh, U.; Bible, W.D.; Fugice, J.; Sanabria, J. Mitigating N2O and NO Emissions from Direct-Seeded Rice with Nitrification Inhibitor and Urea Deep Placement. Rice Sci. 2020, 27, 434–444. [Google Scholar] [CrossRef]
- Ali, M.A.; Hoque, M.A.; Kim, P.J. Mitigating global warming potentials of methane and nitrous oxide gases from rice paddies under different irrigation regimes. Ambio 2013, 42, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Rath, A.K.; Ramakrishnan, B.; Sethunathan, N. Effect of application of ammonium thiosulphate on production and emission of methane in a tropical rice soil. Agric. Ecosyst. Environ. 2002, 90, 319–325. [Google Scholar] [CrossRef]
- Vilarrasa-Nogué, M.; Teira-Esmatges, M.R.; Pascual, M.; Villar, J.M.; Rufat, J. Effect of N dose, fertilisation duration and application of a nitrification inhibitor on GHG emissions from a peach orchard. Sci. Total Environ. 2020, 699, 134042. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, L.M.; Bhogal, A.; Chadwick, D.R.; McGeough, K.; Misselbrook, T.; Rees, R.M.; Thorman, R.E.; Watson, C.J.; Williams, J.R.; Smith, K.A.; et al. Nitrogen use efficiency and nitrous oxide emissions from five UK fertilised grasslands. Sci. Total Environ. 2019, 661, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Almaguer-Cantú, V.; Morales-Ramos, L.H.; Balderas-Rentería, I. Biosorption of lead (II) and cadmium (II) using Escherichia coli genetically engineered with mice metallothionein I. Water Sci. Technol. 2011, 63, 1607–1613. [Google Scholar] [CrossRef]
- Riches, D.A.; Mattner, S.W.; Davies, R.; Porter, I.J. Mitigation of nitrous oxide emissions with nitrification inhibitors in temperate vegetable cropping in southern Australia. Soil Res. 2016, 54, 533. [Google Scholar] [CrossRef]
- Scheer, C.; Deuter, P.L.; Rowlings, D.W.; Grace, P.R. Effect of the nitrification inhibitor (DMPP) on soil nitrous oxide emissions and yield in a lettuce crop in Queensland, Australia. Acta Hortic. 2016, 1123, 101–107. [Google Scholar] [CrossRef]
- Treweek, G.; Di, H.J.; Cameron, K.C.; Podolyan, A. Effectiveness of the nitrification inhibitor dicyandiamide and biochar to reduce nitrous oxide emissions. N. Z. J. Agric. Res. 2016, 59, 165–173. [Google Scholar] [CrossRef]
- Vinzent, B.; Fuß, R.; Maidl, F.X.; Hülsbergen, K.J. N2O emissions and nitrogen dynamics of winter rapeseed fertilized with different N forms and a nitrification inhibitor. Agric. Ecosyst. Environ. 2018, 259, 86–97. [Google Scholar] [CrossRef]
- Cantú, R.R.; Aita, C.; Doneda, A.; Giacomini, D.A.; Dessbesell, A.; Arenhardt, M.; De Bastiani, G.G.; Pujol, S.B.; Rochette, P.; Chantigny, M.H.; et al. Alternatives to regular urea for abating N losses in lettuce production under sub-tropical climate. Biol. Fertil. Soils 2017, 53, 589–599. [Google Scholar] [CrossRef]

| Location, Reference | Treatment | N2O (Unit) | Mitigation (%) | Remarks |
|---|---|---|---|---|
| Spain, Guardia et al. [152] | Ammonium sulphate (AS) | 415.15 g N2O-N ha−1 | Control (C) | In this study, the effect of different nitrogen on N2O emissions was investigated. |
| AS + DMPSA | 228.5 g N2O-N ha−1 | 44.96 | ||
| Calcium nitrate | 243.75 g N2O-N ha−1 | 41.29 | ||
| Australia, Muller et al. [98] | Recommended dose of NPK | 312.3 g N2O-N ha−1 | Control | The effect of DMPP and Piadin on N2O was studied. |
| 80% of NPK + DMPP | 152.2 g N2O-N ha−1 | 51.27 | ||
| 80% of NPK + Piadin | 154.6 g N2O-N ha−1 | 50.50 | ||
| China, Dong et al. [153] | Inorganic fertilizer + Manure | 1.06 kg N2O ha−1 | Control | The impacts of manure and nitrification inhibitors were investigated. |
| Inorganic fertilizer + Manure + DMPP | 0.71 kg N2O ha−1 | 33.02 | ||
| Iran, Borzouei et al. [154] | CT | 1.62 kg N2O-N ha−1 | Control | In this study, the effect of NIs on tillage practice was investigated. |
| CT + NI (0.35% of applied N) | 0.95 kg N2O-N ha−1 | 41.36 | ||
| MT | 1.37 kg N2O-N ha−1 | 15.43 | ||
| MT + NI (0.35% of applied N) | 0.93 kg N2O-N ha−1 | 42.59 | ||
| Indonesia, Jumadi et al. [155] | Urea | 4.67 kg N2O-N ha−1 | Control | In this study, the impact of DCD and zeolite on N2O emission from maize soil was studied. |
| Urea + Neem | 3.96 kg N2O-N ha−1 | 15.20 | ||
| Urea + zeolite | 2.07 kg N2O-N ha−1 | 55.68 | ||
| Urea + zeolite + neem | 1.75 kg N2O-N ha−1 | 62.53 | ||
| Urea + zeolite + DCD | 1.10 kg N2O-N ha−1 | 76.45 | ||
| Urea + DCD | 0.89 kg N2O-N ha−1 | 80.94 | ||
| USA, Omonode & Vyn [156] | UAN (220 kg N ha−1 by urea ammonium nitrate) | 37.96 g N ha−1 d−1 | Control | The study was conducted at Purdue University, West Lafayette, USA. |
| UAN + nitrapyrin (2.6 kg ha−1) | 36.01 g N ha−1 d−1 | 5.14 | ||
| India, Fagodiya et al. [135] | Control (120 kg N ha−1 by Urea) | 0.89 kg N2O ha−1 | Control | Two years of studies were conducted in New Delhi, India. |
| DCD (120 kg N ha−1, 108 and 12 kg N ha−1 by U and DCD, respectively) | 0.70 | 21.35 | ||
| NOCU (120 kg N ha−1 by NOCU) | 0.775 | 12.92 | ||
| China, Song et al. [157] | Deep fertilizer (242 kg N ha−1) | 113 ng N m−2 s−1 | Control | In this study, deep fertilizer along with DCD reduces N2O emission significantly. |
| Deep fertilizer + DCD (7% of N) | 11.7 ng N m−2 s−1 | 89.65 | ||
| China, Du et al. [158] | Optimized N (160 kg N ha−1) | 1.41 kg N ha−1 | Control | Three years of studies were carried out to enhance the efficiency of nitrogen-based fertilizers. |
| Optimized N + DCD (10% of N) | 1.01 kg N ha−1 | 28.37 | ||
| China, Niu et al. [108] | N (120 kg N ha−1) | 1.00 kg N ha−1 | Control | In this study, the impact of biochar and a nitrification inhibiter (nitrapyrin) was studied in combination and alone also. |
| NB3 (120 kg N ha−1 + BC 3 t ha−1) | 0.84 kg N ha−1 | 16 | ||
| NB6 (120 kg N ha−1 + BC 6 t ha−1) | 0.85 kg N ha−1 | 15 | ||
| NB12 (120 kg N ha−1 + BC 12 t ha−1) | 0.81 kg N ha−1 | 19 | ||
| NI (120 kg N ha−1, 99.74% by urea + 0.26% by nitrapyrin) | 0.87 kg N ha−1 | 13 | ||
| NIB3 (NI + BC 3 t ha−1) | 0.80 kg N ha−1 | 20 | ||
| NIB6 (NI + BC 6 t ha−1) | 0.84 kg N ha−1 | 16 | ||
| NIB12 (NI + BC 12 t ha−1) | 0.75 kg N ha−1 | 25 | ||
| China, Dong et al. [41] | Urea (U) (180 kg N ha−1) | 0.639 (kg N ha−1) | Control | Mean data of six years of maize growing seasons. |
| U + DCD (5.4 kg ha−1) + HQ (1.8 kg ha−1) | 0.528 (kg N ha−1) | 17.37 | ||
| Spain, Guardia et al. [139] | U-S (180 kg N ha−1) | 1.665 | Control | The average data of two years of study conducted in Spain. |
| CAN-S (180 kg N ha−1) | 1.30 | 21.92 | ||
| U + NI-S (180 kg N ha−1) | 0.83 | 50.15 | ||
| CAN + NI-S (180 kg N ha−1) | 0.55 | 66.97 | ||
| Canada, Drury et al. [159] | U (130 kg N ha−1) | 1.69 kg N ha−1 | Control | Average data of two years of study. |
| U + NBPT + DCD (130 kg N ha−1) | 1.49 kg N ha−1 | 11.83 | ||
| Thailand, Pengthamkeerati & Modtad [149] | Chemical fertilizers (CF) 28.125 kg N ha−1 | 48.6 mg N2O-N m−2 | Control | An experiment was conducted in the loamy sandy soil of Thailand. |
| CF + neem oil (5%) | 43.7 mg N2O-N m−2 | 10.48 | ||
| CF + neem oil (10%) | 43.8 mg N2O-N m−2 | 9.88 | ||
| CF + DCD (5%) | 40.5 mg N2O-N m−2 | 16.67 | ||
| CF + DCD (10%) | 36.2 mg N2O-N m−2 | 25.51 | ||
| Spain, Sanz-Cobena et al. [160] | U (250 kg N ha−1) | 0.94 (kg N2O-N ha−1) | Control | The average data are taken from the study. |
| U + NBPT (0.4%) + DCD (3%) | 0.65 (kg N2O-N ha−1) | 30.85 | ||
| U + NBPT (0.4%) | 0.59 (kg N2O-N ha−1) | 37.23 | ||
| Indonesia, Hadi et al. [150] | U (200 kg ha−1) | 691.7 mg N m−2 | Control | The dose of each fertilizer is 90 kg N ha−1 in this experiment. |
| U (170 kg ha−1) + DCD (20 kg ha−1) | 21.1 mg N m−2 | 96.95 | ||
| LP-30 (214 kg ha−1) | 57.3 mg N m−2 | 91.72 | ||
| Indonesia, Jumadi et al. [150] | U (90 kg N ha−1) | 1.87 kg N2O-N ha−1 | Control | In this study, fertilizers are applied in two spilt (45 + 45 kg N ha−1). |
| CRF (90 kg N ha−1) | 1.70 kg N2O-N ha−1 | 9.09 | ||
| DCD (90 kg N ha−1, 90% by U + 10% by DCD) | 1.06 kg N2O-N ha−1 | 43.32 |
| References (Study Location) | Treatment | N2O Emission | Mitigation (%) | |
|---|---|---|---|---|
| Paul et al. [84] (New Delhi, India) | Prilled urea | 0.80 kg N2O-N ha−1 | Control (C) | |
| Neem oil-coated urea (NCU) | 0.68 kg N2O-N ha−1 | 15 | ||
| Karanj oil-coated urea | 0.67 kg N2O-N ha−1 | 16.25 | ||
| Limus + NCU | 0.61 kg N2O-N ha−1 | 23.75 | ||
| Huang et al. [120] (Anhui Province, China) | Conventional fertilizer | 0.1028 mg N2O kg−1 | C | |
| Methyl 3-(4-hydroxyphenyl) propionate | 0.0598 mg N2O kg−1 | 41.83 | ||
| Ren et al. [113] (Shandong Province, China) | Urea | 23.5 kg N2O ha−1 | Control | |
| Urea + DCD | 16.29 kg N2O ha−1 | 30.68 | ||
| Urea + DCD + NBPT | 18.0 kg N2O ha−1 | 23.40 | ||
| He et al. [167] (China) | Conventional urea | 1.49 kg N2O ha−1 | C | |
| Reduced N fertilizer (RN) | 1.14 kg N2O ha−1 | 23.49 | ||
| RN + 7.5 t ha−1 biochar (B1) | 1.37 kg N2O ha−1 | 8.05 | ||
| RN + 15 t ha−1 biochar (B2) | 1.49 kg N2O ha−1 | 0 | ||
| RN + DCD + HQ (RNI) | 0.90 kg N2O ha−1 | 39.60 | ||
| RNI + B1 | 0.97 kg N2O ha−1 | 34.90 | ||
| RNI + B2 | 1.14 kg N2O ha−1 | 23.49 | ||
| Cowan et al. [168] (India) | Prilled urea and continuous flooding | 1.17 kg N2O-N ha−1 | C | |
| Neem oil-coated urea and continuous flooding | 1.07 | 8.55 | ||
| Prilled urea and intermitted irrigation | 1.45 | −23.93 | ||
| Neem oil-coated urea and intermitted irrigation | 1.38 | −17.95 | ||
| Gaihre et al. [169] (USA) | Urea broadcast | 3010 g N2O-N/h m2 | C | |
| Potassium nitrate | 1359 g N2O-N/h m2 | 54.85 | ||
| Urea deep placement | 205 g N2O-N/h m2 | 93.19 | ||
| Urea + DCD | 815 g N2O-N/h m2 | 72.93 | ||
| Guo et al. [162] (Hubei Province, China) | Farmer fertilizer practice (FFP) (195 kg N ha−1) | 3.33 kg N2O ha−1 | C | |
| Polymer-coated controlled urea (195 kg N ha−1) | 2.10 kg N2O ha−1 | 36.99 | ||
| Nitrapyrin-coated urea (nitrapyrin coating 5%) (195 kg N ha−1) | 2.00 kg N2O ha−1 | 39.99 | ||
| Jumadi et al. [155] (Maros District, Indonesia) | Urea granule (UG) (150 kg N ha−1) | 7.2 kg-N ha−1 | C | |
| UGZ-UG + zeolite (10% (w/w) of U) (150 kg N ha−1) | 3.4 kg-N ha−1 | 52.78 | ||
| UGZN-UGZ + neem cake (5% (w/w) of U) (150 kg N ha−1) | 7.0 kg-N ha−1 | 2.78 | ||
| UGZD-UGZ + DCD (5% (w/w) of U) (150 kg N ha−1) | 4.7 kg-N ha−1 | 34.72 | ||
| Li et al. [137] (Jingzhou City, China) | Early rice | U (165 kg N ha−1) + CI * | 1.3 kg N2O ha−1 | C |
| U (165 kg N ha−1) + SWD ** | 1.9 kg N2O ha−1 | −46.15 ! | ||
| CRU *** (165 kg N ha−1) + SWD | 1.4 kg N2O ha−1 | −7.69 | ||
| NU +HQ (165 kg N ha−1) + CI | 1.1 kg N2O ha−1 | 7.69 | ||
| Late rice | U (165 kg N ha−1) + CI * | 1.7 kg N2O ha−1 | C | |
| U (165 kg N ha−1) + SWD ** | 2.5 kg N2O ha−1 | −47.06 | ||
| CRU *** (165 kg N ha−1) + SWD | 2.2 kg N2O ha−1 | −29.41 | ||
| NU + HQ (165 kg N ha−1) + CI | 1.8 kg N2O ha−1 | −5.88 | ||
| Datta & Adhya [85] (Cuttack, India) | Urea (90 kg N ha−1) | 2.05 kg N2O ha−1 | C | |
| U (90 kg N ha−1) + DCD (5 kg ha−1) | 1.62 kg N2O ha−1 | 20.98 | ||
| U (90 kg N ha−1) + nimin (0.9 kg N ha−1) | 0.30 kg N2O ha−1 | 85.37 | ||
| U (90 kg N ha−1) + karanjin (0.9 kg N ha−1) | 1.05 kg N2O ha−1 | 48.78 | ||
| Ali et al. [170] (Mymensingh, Bangladesh) | Continues flooding (CF) + urea (200 kg ha−1) | 0.55 kg N2O ha−1 | Control | |
| CF + Urea (200 kg ha−1) + calcium carbide (30 ppm) | 0.29 kg N2O ha−1 | 47.27 | ||
| Intermittent irrigation (IR) + urea (200 kg ha−1) | 0.98 kg N2O ha−1 | Control | ||
| IR + urea (200 kg ha−1) + calcium carbide (30 ppm) | 0.69 kg N2O ha−1 | 29.59 | ||
| Li et al. [164] (Jurong City, China) | U (150 kg N ha−1) | 3.90 kg N2O-N ha−1 | C | |
| U (150 kg N ha−1) + HQ (0.45 kg ha−1) + DCD (7.5 kg ha−1) HQ and DCD basal | 2.98 kg N2O-N ha−1 | 23.59 | ||
| U (150 kg N ha−1) + HQ (0.45 kg ha−1) + DCD (7.5 kg ha−1) HQ and DCD at tillering | 1.73 kg N2O-N ha−1 | 55.64 | ||
| U (150 kg N ha−1) + HQ (0.45 kg ha−1) + DCD (7.5 kg ha−1) HQ and DCD at panicle initiation | 3.23 kg N2O-N ha−1 | 17.18 | ||
| Malla et al. [86] (New Delhi, India) | U (120 kg N ha−1) | 0.76 kg N2O-N ha−1 | C | |
| U (120 kg N ha−1) + hydroquinone (12 kg ha−1) | 0.73 kg N2O-N ha−1 | 3.95 | ||
| U (108 kg N ha−1) + neem cake (12 kg N ha−1) | 0.68 kg N2O-N ha−1 | 10.53 | ||
| Calcium carbide-coated urea (120 kg N ha−1) | 0.54 kg NO-N ha−1 | 28.95 | ||
| Neem oil-coated urea (120 kg N ha−1) | 0.60 kg N22O-N ha−1 | 21.05 | ||
| U (108 kg N ha−1) + DCD (12 kg N ha−1) | 0.63 kg N2O-N ha−1 | 17.11 | ||
| U (120 kg N ha−1) + thiosulphate (12 kg ha−1) | 0.50 kg N2O-N ha−1 | 34.21 | ||
| Boeckx et al. [148] (Pot experiment, China) | U (345 kg N ha−1) | 8.62 mg N2O-N kg−1 soil | C | |
| U + HQ (0.3% of applied U) | 6.60 mg N2O-N kg−1 soil | 23.43 | ||
| U + DCD (0.5% of applied U) | 4.57 mg N2O-N kg−1 soil | 46.98 | ||
| U + HQ (0.3% of applied U) + DCD (0.5% of applied U) | 3.25 mg N2O-N kg−1 soil | 62.30 | ||
| Ghosh et al. [163] (New Delhi, India) | U (120 kg N ha−1) | 167.9 g N2O ha−1 | C | |
| U (108 kg N ha−1) + DCD (12 kg N ha−1) | 79.5 g N2O ha−1 | 52.65 | ||
| Ammonium sulphate (120 kg N ha−1) | 151.4 g N2O ha−1 | C | ||
| Ammonium sulphate (108 kg N ha−1) + DCD (12 kg N ha−1) | 81.9 g N2O ha−1 | 45.90 | ||
| Potassium nitrate (120 kg N ha−1) | 186.7 g N2O ha−1 | C | ||
| Potassium nitrate (108 kg N ha−1) + DCD (12 kg N ha−1) | 167.5 g N2O ha−1 | 10.28 | ||
| Kumar et al. [171] (New Delhi, India) | U (140 kg N ha−1) | 0.16 kg N2O-N ha−1 | C | |
| U (126 kg N ha−1) + DCD (14 kg N ha−1) | 0.142 kg N2O-N ha−1 | 11.25 | ||
| U (126 kg N ha−1) + thiosulphate (14 kg N ha−1) | 0.147 kg N2O-N ha−1 | 8.13 | ||
| (NH4)2SO4 (126 kg N ha−1) + thiosulphate (14 kg N ha−1) | 0.235 kg N2O-N ha−1 | −46.88 | ||
| (NH4)2SO4 (126 kg N ha−1) + DCD (14 kg N ha−1) | 0.174 kg N2O-N ha−1 | −8.75 | ||
| Majumdar et al. [140] (New Delhi, India) | U (140 kg N ha−1) | 0.060 kg N2O-N ha−1 | C | |
| U (119 kg N ha−1) + DCD (21 kg N ha−1) | 0.049 kg N2O-N ha−1 | 18.33 | ||
| NOCU (140 kg N ha−1) | 0.053 kg N2O-N ha−1 | 11.67 | ||
| Nimin-coated U (140 kg N ha−1) | 0.057 kg N2O-N ha−1 | 5.00 | ||
| Pattanaik et al. [166] (Cuttack, India) | Control (recommended dose through NCU) | 0.58 kg N2O ha−1 | Control | |
| 75% of the recommended dose through NCU | 0.55 kg N2O ha−1 | 5.17 | ||
| 50% of the recommended dose through NCU | 0.51 kg N2O ha−1 | 12.07 | ||
| 100% of the recommended dose through CLCC | 0.53 kg N2O ha−1 | 8.62 | ||
| 75% of the recommended dose through CLCC | 0.47 kg N2O ha−1 | 18.97 | ||
| 50% of the recommended dose through CLCC | 0.43 kg N2O ha−1 | 25.86 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyan, S.K.; Maithani, D.; Kumar, V. Nitrous Oxide Production and Mitigation Through Nitrification Inhibitors in Agricultural Soils: A Mechanistic Understanding and Comprehensive Evaluation of Influencing Factors. Nitrogen 2025, 6, 14. https://doi.org/10.3390/nitrogen6010014
Malyan SK, Maithani D, Kumar V. Nitrous Oxide Production and Mitigation Through Nitrification Inhibitors in Agricultural Soils: A Mechanistic Understanding and Comprehensive Evaluation of Influencing Factors. Nitrogen. 2025; 6(1):14. https://doi.org/10.3390/nitrogen6010014
Chicago/Turabian StyleMalyan, Sandeep Kumar, Damini Maithani, and Vineet Kumar. 2025. "Nitrous Oxide Production and Mitigation Through Nitrification Inhibitors in Agricultural Soils: A Mechanistic Understanding and Comprehensive Evaluation of Influencing Factors" Nitrogen 6, no. 1: 14. https://doi.org/10.3390/nitrogen6010014
APA StyleMalyan, S. K., Maithani, D., & Kumar, V. (2025). Nitrous Oxide Production and Mitigation Through Nitrification Inhibitors in Agricultural Soils: A Mechanistic Understanding and Comprehensive Evaluation of Influencing Factors. Nitrogen, 6(1), 14. https://doi.org/10.3390/nitrogen6010014






