Assessment of the Nitrification Inhibitor Nitrapyrin on Nitrogen Losses and Brassica oleracea Growth: A Preliminary Sustainable Research
Abstract
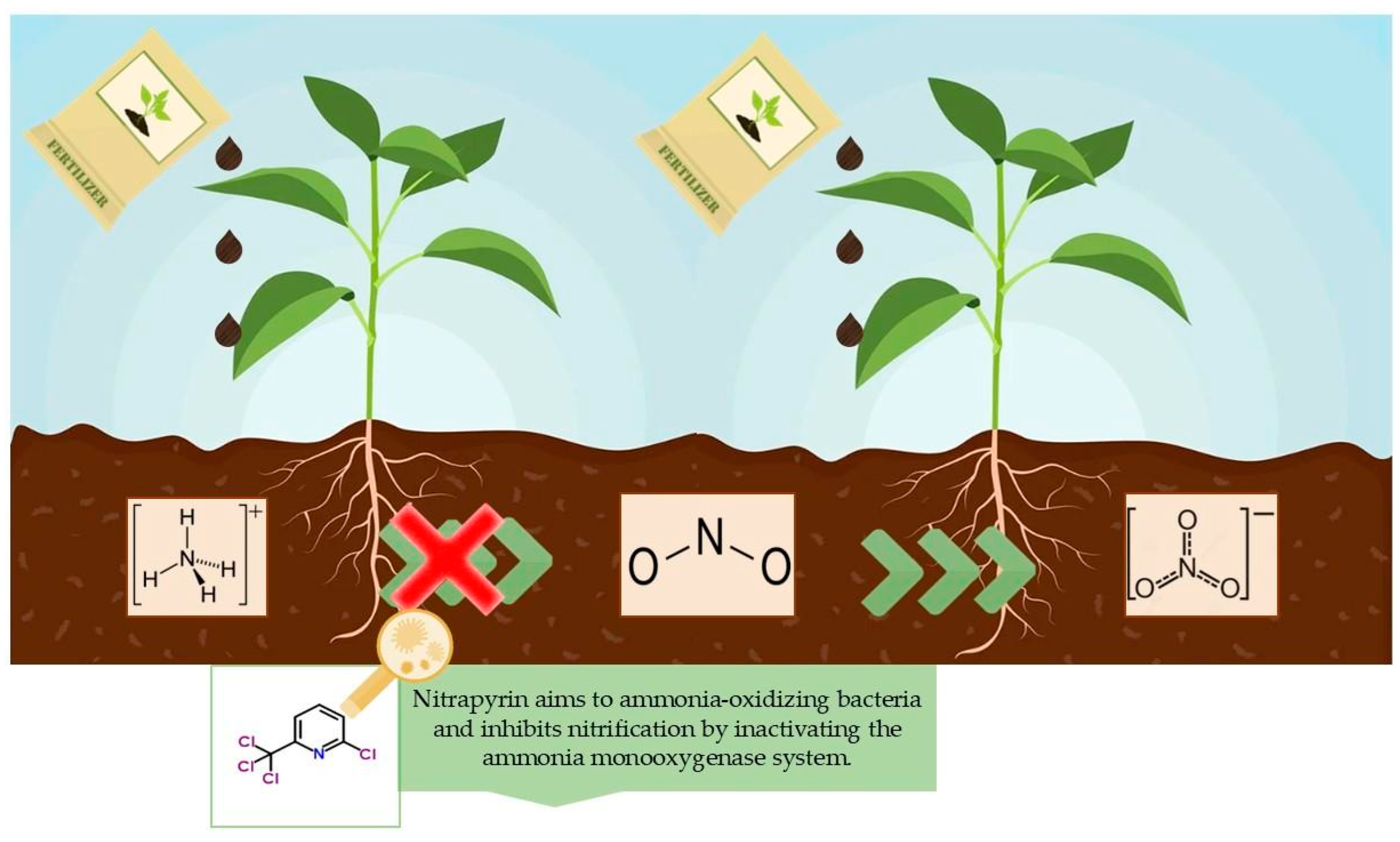
1. Introduction
2. Materials and Methods
2.1. Experimental Setup, Treatments, and Sampling
2.2. Soil Properties
2.3. Ammonium and Nitrate Measurements in Soil and Percolation Water
2.4. Yield Parameters
2.5. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.6. Statistical Analysis
3. Results
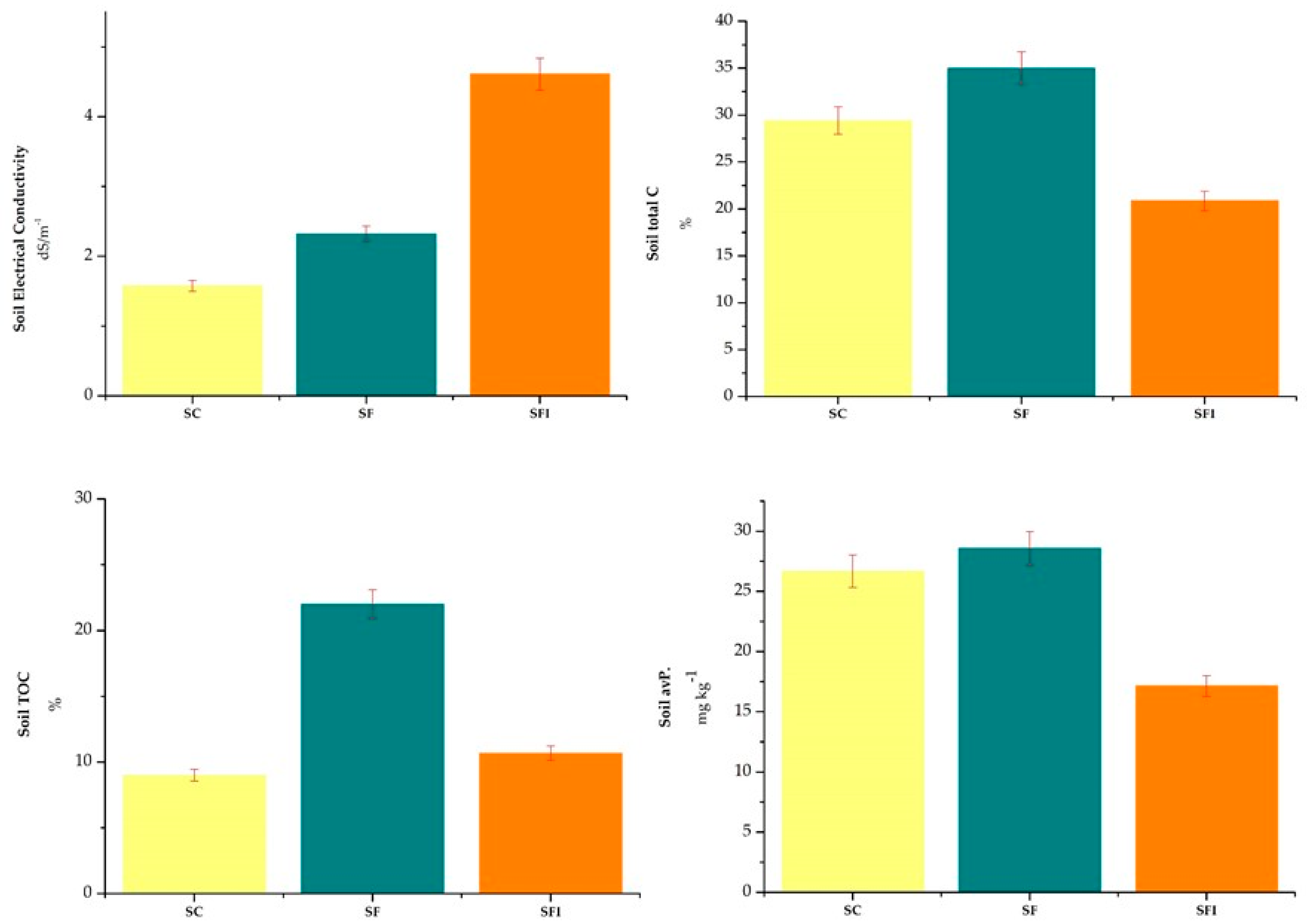
3.1. The Impact of N-Stabilizer on the Soil Properties in the System of Cauliflower Mesocosm
3.2. Nitrogen Dynamics in Environmental Matrices
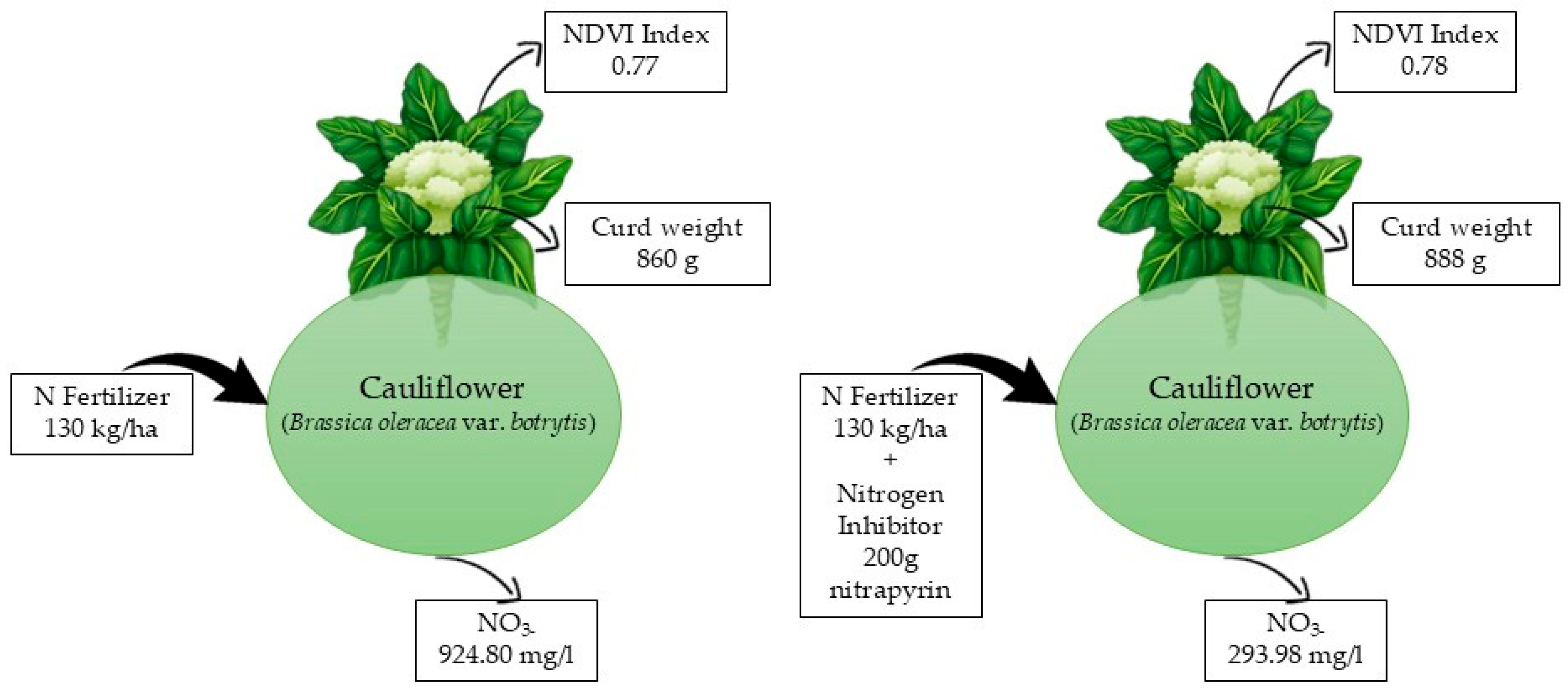
3.3. Effects of N-Stabilizer on Cauliflower Yield
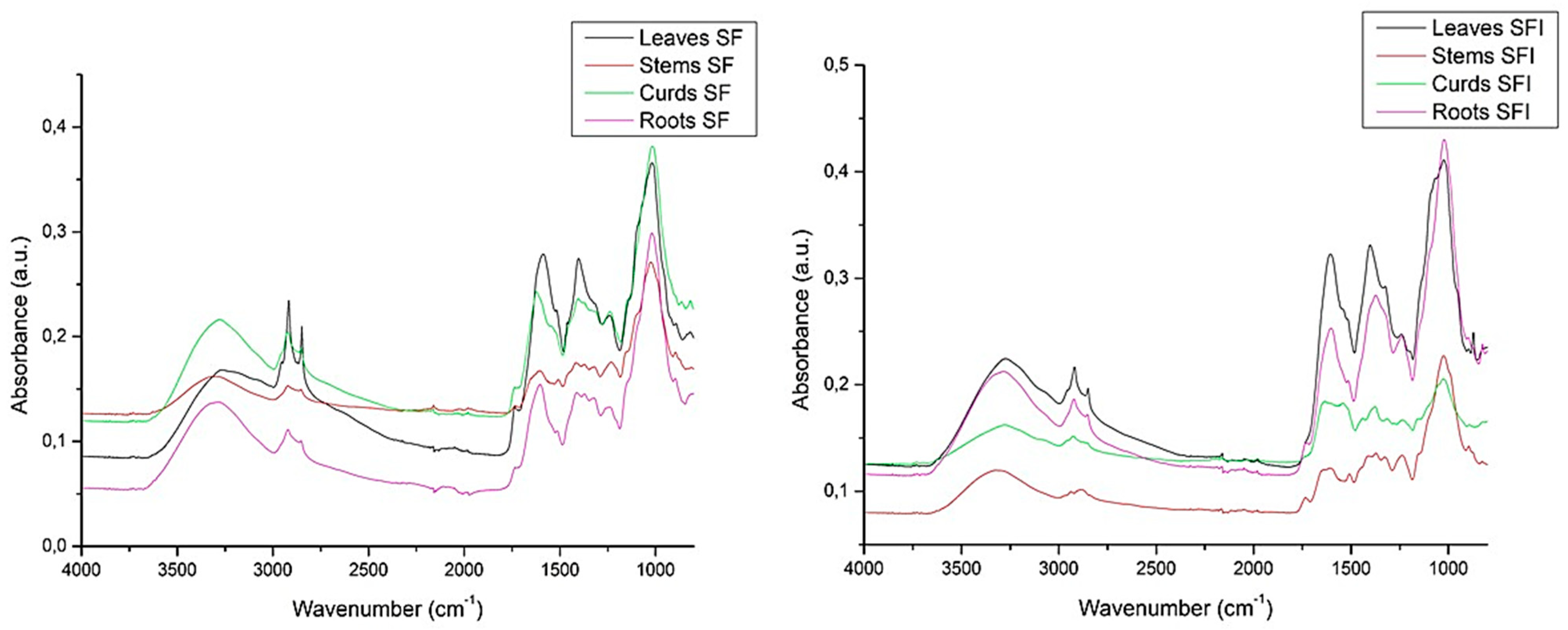
3.4. Molecular Chemistry of Plant Tissues by Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Losacco, D.; Tumolo, M.; Cotugno, P.; Leone, N.; Massarelli, C.; Convertini, S.; Tursi, A.; Uricchio, V.F.; Ancona, V. Use of Biochar to Improve the Sustainable Crop Production of Cauliflower (Brassica oleracea L.). Plants 2022, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, J.; Liu, W.; Li, X.; Zhang, W.; Wang, A. Effects of Alkaline Biochar on Nitrogen Transformation with Fertilizer in Agricultural Soil. Environ. Res. 2023, 233, 116084. [Google Scholar] [CrossRef] [PubMed]
- Losacco, D.; Campanale, C.; Triozzi, M.; Massarelli, C.; Uricchio, V.F. Application of Wood and Vegetable Waste-Based Biochars in Sustainable Agriculture: Evaluation on Nitrate Leaching, Pesticide Fate, Soil Properties, and Brassica oleracea Growth. Environments 2024, 11, 13. [Google Scholar] [CrossRef]
- Losacco, D.; Ancona, V.; De Paola, D.; Tumolo, M.; Massarelli, C.; Gatto, A.; Uricchio, V.F. Development of Ecological Strategies for the Recovery of the Main Nitrogen Agricultural Pollutants: A Review on Environmental Sustainability in Agroecosystems. Sustainability 2021, 13, 7163. [Google Scholar] [CrossRef]
- Losacco, D.; Campanale, C.; Tumolo, M.; Ancona, V.; Massarelli, C.; Uricchio, V.F. Evaluating the Influence of Nitrogen Fertilizers and Biochar on Brassica oleracea L. Var. Botrytis by the Use of Fourier Transform Infrared (FTIR) Spectroscopy. Sustainability 2022, 14, 11985. [Google Scholar] [CrossRef]
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem. Sustainability 2021, 13, 2400. [Google Scholar] [CrossRef]
- Woodward, E.E.; Edwards, T.M.; Givens, C.E.; Kolpin, D.W.; Hladik, M.L. Widespread Use of the Nitrification Inhibitor Nitrapyrin: Assessing Benefits and Costs to Agriculture, Ecosystems, and Environmental Health. Environ. Sci. Technol. 2021, 55, 1345–1353. [Google Scholar] [CrossRef]
- The Italian Institute of Statistics ISTAT. Fertilizzanti Distribuiti in Italia per Stato Liquido o Solido-Reg; The Italian Institute of Statistics ISTAT: Rome, Italy, 2021. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_FERTILIZZANTI (accessed on 3 December 2024).
- Xiong, L.; Sun, W.J.; Cai, H.Y.; Yang, Y.; Zhu, J.; Zhao, B.-W. Correlation of Enhancement Degree on Contrast-Enhanced Ultrasound with Histopathology of Carotid Plaques and Serum High Sensitive C-Reactive Protein Levels in Patients Undergoing Carotid Endarterectomy. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2017, 37, 425–428. [Google Scholar] [CrossRef]
- FAO. Inorganic Fertilizers 1990–2020; FAOSTAT Analytical Brief, 47. 2022. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/4e3d141d-4659-49d9-8e85-6dc36a72c53a/content (accessed on 3 December 2024).
- Subbarao, G.V.; Rondon, M.; Ito, O.; Ishikawa, T.; Rao, I.M.; Nakahara, K.; Lascano, C.; Berry, W.L. Biological Nitrification Inhibition (BNI)-Is It a Widespread Phenomenon? Plant Soil 2007, 294, 5–18. [Google Scholar] [CrossRef]
- Gilsanz, C.; Báez, D.; Misselbrook, T.H.; Dhanoa, M.S.; Cárdenas, L.M. Development of Emission Factors and Efficiency of Two Nitrification Inhibitors, DCD and DMPP. Agric. Ecosyst. Environ. 2016, 216, 1–8. [Google Scholar] [CrossRef]
- Wolt, J.D. A Meta-Evaluation of Nitrapyrin Agronomic and Environmental Effectiveness with Emphasis on Corn Production in the Midwestern USA. Nutr. Cycl. Agroecosystems 2004, 69, 23–41. [Google Scholar] [CrossRef]
- Subbarao, G.; Ito, O.; Sahrawat, K.; Berry, W.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondon, M.; Rao, I. Scope and Strategies for Regulation of Nitrification in Agricultural Systems-Challenges and Opportunities. CRC. Crit. Rev. Plant Sci. 2006, 25, 303–335. [Google Scholar] [CrossRef]
- Hao, X.; Sun, L.; Zhou, B.; Ma, X.; Wang, S.; Liu, S.; Ji, J.; Kuang, E.; Qiu, S. Change in Maize Yield, N Use Efficiencies and Climatic Warming Potential after Urea Combined with Nitrapyrin and NBPT during the Growing Season in a Black Soil. Soil Tillage Res. 2023, 231, 105721. [Google Scholar] [CrossRef]
- Luo, Y.; Benke, M.B.; Hao, X. Nutrient Uptake and Leaching from Soil Amended with Cattle Manure and Nitrapyrin. Commun. Soil Sci. Plant Anal. 2017, 48, 1438–1454. [Google Scholar] [CrossRef]
- Borzouei, A.; Mander, U.; Teemusk, A.; Sanz-Cobena, A.; Zaman, M.; Kim, D.G.; Muller, C.; Kelestanie, A.A.; Amin, P.S.; Moghiseh, E.; et al. Effects of the Nitrification Inhibitor Nitrapyrin and Tillage Practices on Yield-Scaled Nitrous Oxide Emission from a Maize Field in Iran. Pedosphere 2021, 31, 314–322. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Powlson, D.; Min, J.; Shi, W. Rice Production, Nitrous Oxide Emission and Ammonia Volatilization as Impacted by the Nitrification Inhibitor 2-Chloro-6-(Trichloromethyl)-Pyridine. Field Crop. Res. 2015, 173, 1–7. [Google Scholar] [CrossRef]
- Burzaco, J.P.; Ciampitti, I.A.; Vyn, T.J. Nitrapyrin Impacts on Maize Yield and Nitrogen Use Efficiency with Spring-Applied Nitrogen: Field Studies vs. Meta-Analysis Comparison. Agron. J. 2014, 106, 753–760. [Google Scholar] [CrossRef]
- Borzouei, A.; Karimzadeh, H.; Müller, C.; Sanz-Cobena, A.; Zaman, M.; Kim, D.G.; Ding, W. Relationship between Nitrapyrin and Varying Nitrogen Application Rates with Nitrous Oxide Emissions and Nitrogen Use Efficiency in a Maize Filed. Sci. Rep. 2022, 12, 18424. [Google Scholar] [CrossRef]
- Wang, G.; Gou, Z.; Tian, G.; Sima, W.; Zhou, J.; Bo, Z.; Zhang, Z.; Gao, Q. Study on the Effectiveness and Mechanism of a Sustainable Dual Slow-Release Model to Improve N Utilization Efficiency and Reduce N Pollution in Black Soil. Sci. Total Environ. 2024, 907, 168033. [Google Scholar] [CrossRef]
- Giacometti, C.; Mazzon, M.; Cavani, L.; Ciavatta, C.; Marzadori, C. A Nitrification Inhibitor, Nitrapyrin, Reduces Potential Nitrate Leaching through Soil Columns Treated with Animal Slurries and Anaerobic Digestate. Agronomy 2020, 10, 865. [Google Scholar] [CrossRef]
- Papp, Z. The Role and Impact of N-Lock (N-Stabilizer) to the Utilization of N in the Main Arable Crops. Acta Agrar. Debreceniensis 2014, 62, 51–55. [Google Scholar] [CrossRef]
- Giunta, P. Gazzetta Ufficiale 2009, 2003, 2004–2006. Available online: https://www.gazzettaufficiale.it/eli/gu/2004/03/13/61/so/42/sg/pdf (accessed on 3 December 2024).
- Lang, F.; Krüger, J.; Amelung, W.; Willbold, S.; Frossard, E.; Bünemann, E.K.; Bauhus, J.; Nitschke, R.; Kandeler, E.; Marhan, S.; et al. Soil Phosphorus Supply Controls P Nutrition Strategies of Beech Forest Ecosystems in Central Europe. Biogeochemistry 2017, 136, 5–29. [Google Scholar] [CrossRef]
- Hautman, D.P.; Munch, D.J. EPA Method 300.1, Revision 1.0: Determination of Inorganic Anions in Drinking Water by Ion Chromatography; Environmental Monitoring Systems Laboratory, Office of Research and Development, US Environmental Protection Agency: Cincinnati, OH, USA, 1997.
- O’Dell, J.W. EPA Method 350.1, Revision 2.0: Determination of Ammonia Nitrogen by Semi-Automated Colorimetry; Environmental Monitoring Systems Laboratory, Office of Research and Development, US Environmental Protection Agency: Cincinnati, OH, USA, 1993.
- Shahar, B.; Dolma, N.; Chongtham, N. Phytochemical Analysis, Antioxidant Activity and Identification of Bioactive Constituents from Three Wild Medicinally Important Underutilized Plants of Ladakh, India Using GCMS and FTIR Based Metabolomics Approach. Food Humanit. 2023, 1, 430–439. [Google Scholar] [CrossRef]
- Berthomieu, C.; Hienerwadel, R. Fourier Transform Infrared (FTIR) Spectroscopy. Photosynth. Res. 2009, 101, 157–170. [Google Scholar] [CrossRef]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Xiao, F.; Zhang, L.; Song, Y.; Li, Y.; Zheng, Y.; Zhang, J.; et al. Effects of Nitrification Inhibitors on Soil Nitrification and Ammonia Volatilization in Three Soils with Different Ph. Agronomy 2021, 11, 1674. [Google Scholar] [CrossRef]
- Wang, S.; Shan, J.; Xia, Y.; Tang, Q.; Xia, L.; Lin, J.; Yan, X. Different Effects of Biochar and a Nitrification Inhibitor Application on Paddy Soil Denitrification: A Field Experiment over Two Consecutive Rice-Growing Seasons. Sci. Total Environ. 2017, 593–594, 347–356. [Google Scholar] [CrossRef]
- Hayden, H.L.; Phillips, L.A.; Marshall, A.J.; Condon, J.R.; Doran, G.S.; Wells, G.S.; Mele, P.M. Nitrapyrin Reduced Ammonia Oxidation with Different Impacts on the Abundance of Bacterial and Archaeal Ammonia Oxidisers in Four Agricultural Soils. Appl. Soil Ecol. 2021, 157, 103759. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, L.; Hu, S.; Compton, J.E.; Greaver, T.L.; Li, Q. How Inhibiting Nitrification Affects Nitrogen Cycle and Reduces Environmental Impacts of Anthropogenic Nitrogen Input. Glob. Change Biol. 2015, 21, 1249–1257. [Google Scholar] [CrossRef]
- Noor Affendi, N.M.; Yusop, M.K.; Othman, R. Efficiency of Coated Urea on Nutrient Uptake and Maize Production. Commun. Soil Sci. Plant Anal. 2018, 49, 1394–1400. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Inhibition of Nitrification to Mitigate Nitrate Leaching and Nitrous Oxide Emissions in Grazed Grassland: A Review. J. Soils Sediments 2016, 16, 1401–1420. [Google Scholar] [CrossRef]
- Yang, R.; Yang, Z.; Yang, S.; Chen, L.l.; Xin, J.; Xu, L.; Zhang, X.; Zhai, B.; Wang, Z.; Zheng, W.; et al. Nitrogen Inhibitors Improve Soil Ecosystem Multifunctionality by Enhancing Soil Quality and Alleviating Microbial Nitrogen Limitation. Sci. Total Environ. 2023, 880, 163238. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, J.J.; Wei, Z.; Dodla, S.K.; Fultz, L.M.; Gaston, L.A.; Xiao, R.; Park, J.-h.; Scaglia, G. Nitrification Inhibitors Reduce Nitrogen Losses and Improve Soil Health in a Subtropical Pastureland. Geoderma 2021, 388, 114947. [Google Scholar] [CrossRef]
- Yang, M.; Hou, Z.; Guo, N.; Yang, E.; Sun, D.; Fang, Y. Effects of Enhanced-Efficiency Nitrogen Fertilizers on CH4 and CO2 Emissions in a Global Perspective. Field Crop. Res. 2022, 288, 108694. [Google Scholar] [CrossRef]
- Hou, E.; Chen, C.; Wen, D.; Liu, X. Phosphatase Activity in relation to Key Litter and Soil Properties in Mature Subtropical Forests in China. Sci. Total Environ. 2015, 515–516, 83–91. [Google Scholar] [CrossRef]
- Poblete-Grant, P.; Suazo-Hernández, J.; Condron, L.; Rumpel, C.; Demanet, R.; Malone, S.L.; Mora, M.d.L.L. Soil Available P, Soil Organic Carbon and Aggregation as Affected by Long-Term Poultry Manure Application to Andisols under Pastures in Southern Chile. Geoderma Reg. 2020, 21, e00271. [Google Scholar] [CrossRef]
- Ramotowski, D.; Shi, W. Nitrapyrin-Based Nitrification Inhibitors Shaped the Soil Microbial Community via Controls on Soil PH and Inorganic N Composition. Appl. Soil Ecol. 2022, 170, 104295. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, C.H.; Li, Q.L.; Li, B.; Zhu, Y.Y.; Xiong, Z.Q. A 2-Yr Field Assessment of the Effects of Chemical and Biological Nitrification Inhibitors on Nitrous Oxide Emissions and Nitrogen Use Efficiency in an Intensively Managed Vegetable Cropping System. Agric. Ecosyst. Environ. 2015, 201, 43–50. [Google Scholar] [CrossRef]
- Dawar, K.; Sardar, K.; Zaman, M.; Müller, C.; Sanz-Cobena, A.; Khan, A.; Borzouei, A.; Pérez-Castillo, A.G. Effects of the Nitrification Inhibitor Nitrapyrin and the Plant Growth Regulator Gibberellic Acid on Yield-Scale Nitrous Oxide Emission in Maize Fields under Hot Climatic Conditions. Pedosphere 2021, 31, 323–331. [Google Scholar] [CrossRef]
- Jones, F. Infrared Investigation of Barite and Gypsum Crystallization: Evidence for an Amorphous to Crystalline Transition. CrystEngComm 2012, 14, 8374–8381. [Google Scholar] [CrossRef]
- Da Silva Leite, R.; Hernandéz-Navarro, S.; do Nascimento, M.N.; Potosme, N.M.R.; Carrión-Prieto, P.; dos Santos Souza, E. Nitrogen Fertilization Affects Fourier Transform Infrared Spectra (FTIR) in Physalis L. Species. Comput. Electron. Agric. 2018, 150, 411–417. [Google Scholar] [CrossRef]
- Carrión-Prieto, P.; Hernández-Navarro, S.; Martín-Ramos, P.; Sánchez-Sastre, L.F.; Garrido-Laurnaga, F.; Marcos-Robles, J.L.; Martín-Gil, J. Mediterranean Shrublands as Carbon Sinks for Climate Change Mitigation: New Root-to-Shoot Ratios. Carbon Manag. 2017, 8, 67–77. [Google Scholar] [CrossRef]
- Anil Kumar, V.S.; Dinesh Babu, K.V.; Salini, R.; Eapen, J.; Deepa, M.S. FTIR Spectroscopy data as a Fingerprint of Withania somnifera root tissues: A case study with accessions of the species from Kerala, south India. Indo Am. J. Pharm. Res. 2021, 6, 5748–5756. [Google Scholar]


| Soil Properties | Treatments | ||
|---|---|---|---|
| Control Soil | Soil Fertilized | Soil Fertilized Inhibitor-Based | |
| SC | SF | SFI | |
| pH (H2O) | 5.14 ± 0.12 | 5.23 ± 0.02 | 5.38 ± 0.12 |
| Electrical conductivity (dS/m) | 1.58 ± 0.01 | 2.32 ± 0.03 | 4.61 ± 0.03 |
| Water content (%) | 19.04 ± 2.18 | 14.03 ± 1.10 | 15 ± 0.68 |
| Total N (%) | 0.29 ± 0.09 | 0.40 ± 0.01 | 0.15 ± 0.05 |
| Total C (%) | 29.4 ± 2.37 | 35 ± 1.55 | 20.9 ± 2.53 |
| Total organic carbon (%) | 9 ± 0.6 | 22 ± 4.04 | 10.7 ± 0.88 |
| Available P (mg/kg) | 26.7 ± 1.9 | 28.6 ± 0.01 | 17. ± 0.01 |
| C/N | 116 ± 10.44 | 125 ± 4.02 | 77. ± 26.2 |
| Experimental Lines | Soil Ammonium | Soil Nitrate | Water Ammonium | Water Nitrate |
|---|---|---|---|---|
| mg/L | mg/L | |||
| Soil fertilized | 7.04 ± 2.7 | 22 ± 6.42 | 3.15 ± 1.29 | 924.80 ± 149.68 |
| Soil fertilized + nitrification inhibitor | 6.35 ± 2.16 | 7.55 ± 5.15 | 1.71 ± 0.72 | 293.98 ± 52.82 |
| Tissue | Functional Group | Peak Value (cm−1) | ||
|---|---|---|---|---|
| SC | SF | SFI | ||
| Root | O-H | 3284 (0.03) | 3283 (0.13) | 3282.43 (0.21) |
| C-H | 2918 (0.03) | 2920 (0.11) | 2922.18 (0.19) | |
| C=N | 1604 (0.03) | 1603 (0.15) | 1601.78 (0.25) | |
| C=O-N | 1396 (0.03) | 1410 (0.14) | 1374.57 (0.28) | |
| C-O | 1234 (0.03) | 1243 (0.13) | 1238.27 (0.24) | |
| S=N | 1019 (0.08) | 1019 (0.29) | 1022.7 (0.43) | |
| Stem | O-H | n/d | n/d | 3323.67 (0.12) |
| C-H | 2924.97 (0.14) | 2920.79 (0.15) | 2882.11 (0.1) | |
| C=N | 1603.96 (0.15) | 1604.45 (0.17) | 1605.72 (0.12) | |
| N-O | n/d | n/d | 1508.68 (0.12) | |
| C=O-N | 1370.83 (0.15) | 1413.79 (0.18) | 1371.04 (0.14) | |
| C-O | 1238.28 (0.16) | 1231.67 (0.18) | 1236.99 (0.13) | |
| S=N | 1018.87 (0.23) | 1024.12 (0.27) | 1023.48 (0.23) | |
| Leaves | O-H | 3300.77 (0.23) | 3264.93 (0.17) | 3276.12 (0.22) |
| C-H | 2919.43 (0.19) | 2917.44 (0.23) | 2919.43 (0.22) | |
| C=N | 1606.53 (0.22) | 1586.13 (0.28) | 1605.34 (0.32) | |
| C=O-N | n/d | 1401.94 (0.27) | 1401.62 (0.33) | |
| C-O | 1242.25 (0.2) | n/d | n/d | |
| S=N | 1012.33 (0.45) | 1016.76 (0.37) | 1024.1 (0.41) | |
| Inflorescence | O-H | 3278.6 (0.19) | 3277.69 (0.22) | 3277.49 (0.16) |
| C-H | 2919.75 (0.18) | 2922.04 (0.2) | 2925.83 (0.15) | |
| C=N | 1601.9 (0.21) | 1621.92 (0.24) | 1632.45 (0.18) | |
| C=O-N | 1400.94 (0.21) | 1402.19 (0.24) | 1376.21 (0.18) | |
| C-O | 1238.62 (0.2) | 1238.03 (0.22) | n/d | |
| S=N | 1023.52 (0.34) | 1016.11 (0.38) | 1026.99 (0.21) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triozzi, M.; Ilacqua, A.; Tumolo, M.; Ancona, V.; Losacco, D. Assessment of the Nitrification Inhibitor Nitrapyrin on Nitrogen Losses and Brassica oleracea Growth: A Preliminary Sustainable Research. Nitrogen 2025, 6, 15. https://doi.org/10.3390/nitrogen6010015
Triozzi M, Ilacqua A, Tumolo M, Ancona V, Losacco D. Assessment of the Nitrification Inhibitor Nitrapyrin on Nitrogen Losses and Brassica oleracea Growth: A Preliminary Sustainable Research. Nitrogen. 2025; 6(1):15. https://doi.org/10.3390/nitrogen6010015
Chicago/Turabian StyleTriozzi, Mariangela, Annamaria Ilacqua, Marina Tumolo, Valeria Ancona, and Daniela Losacco. 2025. "Assessment of the Nitrification Inhibitor Nitrapyrin on Nitrogen Losses and Brassica oleracea Growth: A Preliminary Sustainable Research" Nitrogen 6, no. 1: 15. https://doi.org/10.3390/nitrogen6010015
APA StyleTriozzi, M., Ilacqua, A., Tumolo, M., Ancona, V., & Losacco, D. (2025). Assessment of the Nitrification Inhibitor Nitrapyrin on Nitrogen Losses and Brassica oleracea Growth: A Preliminary Sustainable Research. Nitrogen, 6(1), 15. https://doi.org/10.3390/nitrogen6010015








