Removal of Nitrogen, Phosphates, and Chemical Oxygen Demand from Community Wastewater by Using Treatment Wetlands Planted with Ornamental Plants in Different Mineral Filter Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Design and Operation of TWs
2.2. Physical–Chemical Parameters and Plant Growth Measurement
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Characteristics of Water in the Influent and Effluent of the Microcosm Treatment Wetlands
3.2. Pollutant Removal in Treatment Wetlands
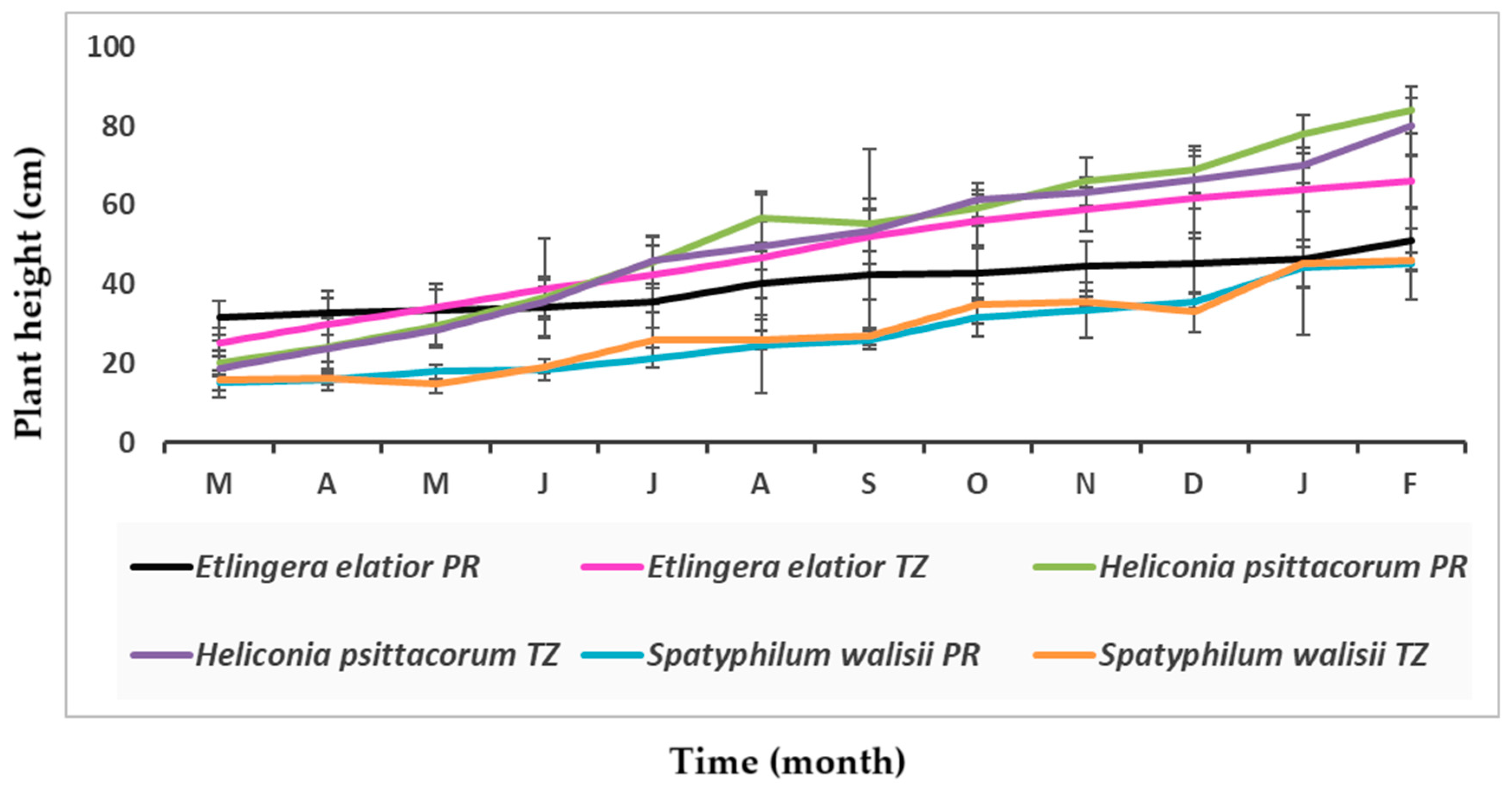
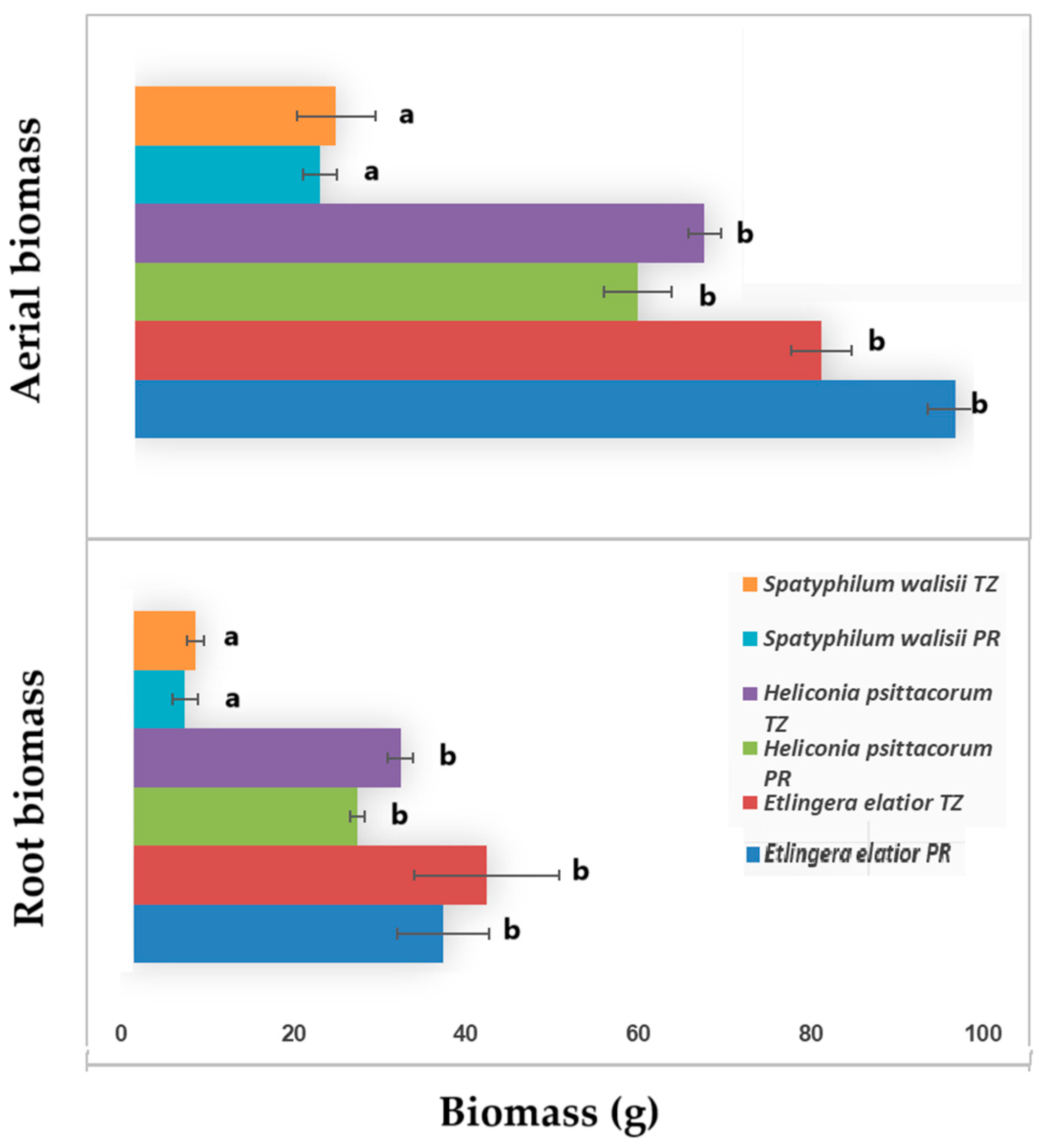
3.3. Plant Height and Biomass Changes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Olguín, E.J.; Sánchez-Galván, G.; González-Portela, R.E.; López-Vela, M. Constructed wetland mesocosms for the treatment of diluted sugarcane molasses stillage from ethanol production using Pontederia sagittata. Water Res. 2008, 42, 3659–3666. [Google Scholar] [CrossRef]
- Khoshnavaz, S.; Nasab, S.; Moazed, H.; Naseri, A.; Izadpanah, Z. Phosphate removal from karun agro-industry INC agricultural wastewater through vetiver planation, and within free water surface constructed wetland. Iran. J. Soil Water Res. 2015, 46, 509–518. [Google Scholar] [CrossRef]
- Montoya, A.; Tejeda, A.; Sulbarán-Rangel, B.; Zurita, F. Treatment of tequila vinasse mixed with domestic wastewater in two types of constructed wetlands. Water Sci. Technol. 2023, 87, 3072. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Hernández, M.E.; Bayona, J.; Morales, L.; Ruiz, P. Removal of selected organic pollutants and coliforms in pilot constructed wetlands in southeastern Mexico. Int. J. Environ. Anal. Chem. 2011, 91, 680–692. [Google Scholar] [CrossRef]
- Sandoval, L.; Marín-Muñiz, J.L.; Adame-García, J.; Fernández-Lambert, G.; Zurita, F. Effect of Spathiphyllum blandum on the removal of ibuprofen and conventional pollutants from polluted river water, in fully saturated constructed wetlands at mesocosm level. J. Water Health 2020, 18, 224–228. [Google Scholar] [CrossRef]
- Tejeda, A.; Torres-Bojorges, Á.; Zurita, F. Carbamazepine removal in three pilot-scale hybrid wetlands planted with ornamental species. Ecol. Eng. 2017, 98, 410–417. [Google Scholar] [CrossRef]
- Nani, G.; Sandoval-Herazo, M.; Martínez-Reséndiz, G.; Marín-Peña, O.; Zurita, F.; Sandoval, L.C. Influence of bed depth on the development of tropical ornamental plants in subsurface flow treatment wetlands for municipal wastewater treatment: A pilot-scale case. Plants 2024, 13, 1958. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Gonzalo, O.G.; Yáñez, S.; Soto, M. Influence of nutrients and pH on the efficiency of vertical flow constructed wetlands treating winery wastewater. J. Water Proc. Eng. 2021, 42, 102103. [Google Scholar] [CrossRef]
- Sandoval, L.; Alvarado-Lassman, A.; Marín-Muñiz, J.L.; Rodríguez-Miranda, J.P.; Fernández-Lambert, G. A critical review of mineral substrates used as filter media in subsurface constructed wetlands: Costs as a selection criterion. Environ. Technol. Rev. 2023, 12, 251–271. [Google Scholar] [CrossRef]
- Toro-Vélez, A.F.; Madera-Parra, C.A.; Peña-Varón, M.R.; Lee, W.Y.; Bezares, J.C.; Walker, W.S.; Cárdenas-Henao, H.; Quesada-Calderón, S.; García-Hernández, H.; Lens, P.N.L. BPA and NP removal form municipal wastewater by tropical horizontal constructed wetlands. Sci. Total Environ. 2016, 542, 93–101. [Google Scholar] [CrossRef]
- Vymazal, J. The historical development of constructed wetlands for wastewater treatment. Land 2022, 11, 174. [Google Scholar] [CrossRef]
- Sandoval, L.C.; Marín-Muniz, L.C.; Alvarado-Lassman, A.; Zurita, F.; Marín-Pena, O.; Sandoval-Herazo, M. Full-scale constructed wetlands planted with ornamental species and pet as a substitute for filter media for municipal wastewater treatment: An experience in a Mexican community. Water 2023, 15, 2280. [Google Scholar] [CrossRef]
- Zamora-Castro, S.; Marín-Muñiz, J.L.; Sandoval, L.; Vidal-Álvarez, M.; Carrión-Delgado, J. Effect of ornamental plants, seasonality, and filter media material in fill-and-drain constructed wetlands treating rural community wastewater. Sustainability 2019, 11, 2350. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Hernández, M.E.; Gallegos-Pérez, M.P.; Amaya-Tejeda, S.I. Plant growth and pollutant removal from wastewater in domiciliary constructed wetland microcosms with monoculture and polyculture of tropical ornamental plants. Ecol. Eng. 2020, 147, 105658. [Google Scholar] [CrossRef]
- Sánchez-Olivares, E.; Marín-Muñiz, J.L.; Hernández-Alarcón, M.E. Liberación de oxígeno radial por las raíces de las plantas nativas de humedales tropicales costeros de Veracruz en respuesta a diferentes condiciones de inundación. Bot. Sci. 2019, 97, 202–210. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, S.; Wang, P.; Qian, J. Effect of vegetation on the removal of contaminants in aquatic environments: A review. J. Hydrodyn. Ser. B 2014, 26, 497–511. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Sandoval, L.C.; López-Méndez, M.C.; Sandoval-Herazo, M.; Meléndez-Armenta, R.A.; González-Moreno, H.R.; Zamora, S. Treatment wetlands in Mexico for control of wastewater contaminants: A review of experiences during the last twenty-two years. Processes 2023, 11, 359. [Google Scholar] [CrossRef]
- Hernández, M.E.; Lagunes, G. Remoción de contaminantes y crecimiento de plantas ornamentales en humedales a escala piloto con diferente tipo de sustrato. In Book of Abstracts IV Panamerican Conference of Wetland Systems for Treatment and Improvement of Water Quality; Hupanam: Lima, Peru, 2018; Available online: https://hupanam.com/wp-content/uploads/2022/04/Memoria-IV-Conferencia-Peru.pdf (accessed on 4 June 2024). (In Spanish)
- Nakase, C.; Zurita, F.; Nani, G.; Reyes, G.; Fernández-Lambert, G.; Cabrera-Hernández, A.; Sandoval, L. Nitrogen removal from domestic wastewater and the development of tropical ornamental plants in partially saturated mesocosm-scale constructed wetlands. Int. J. Environ. Res. Public Health 2019, 16, 4800. [Google Scholar] [CrossRef]
- Hernández-Castelán, D.; Zurita, F.; Marín-Peña, O.; Betanzo-Torres, E.; Sandoval-Herazo, M.; Castellanos-Rivera, S.; Sandoval, L.C. Effect of monocultures and polycultures of Typha latifolia and Heliconia psittacorum on the treatment of river waters contaminated with landfill leachate/domestic wastewater in partially saturated vertical constructed wetlands. Int. J. Phytoremed. 2024, 1–12. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Zitácuaro-Contreras, I.; Ortega-Pineda, G.; López-Roldán, A.; Vidal-Álvarez, M.; Martínez-Aguilar, K.E.; Álvarez-Hernández, L.M.; Zamora-Castro, S. Phytoremediation performance with ornamental plants in monocultures and polycultures conditions using constructed wetlands technology. Plants 2024, 13, 1051. [Google Scholar] [CrossRef]
- Zurita, F.; Belmont, M.A.; De Anda, J.; Cervantes-Martínez, J. Stress detection by laser-induced fluorescence in Zantedeschia aethiopica planted in subsurface-flow treatment wetlands. Ecol. Eng. 2008, 33, 110–118. [Google Scholar] [CrossRef]
- Stefanatou, A.; Schiza, S.; Petousi, I.; Rizzo, A.; Masi, F.; Stasinakis, A.; Fyllas, N.; Fountoulakis, M. Use of climbing and ornamental plants in vertical flow constructed wetlands treating greywater. J. Water Process Eng. 2023, 53, 103832. [Google Scholar] [CrossRef]
- Gray, S.; Kinross, J.; Read, P.; Marland, A. The nutrient assimilative capacity of maerl as a substrate in constructed wetland systems for waste treatment. Water Res. 2000, 34, 2183–2190. [Google Scholar] [CrossRef]
- Brooks, A.S.; Rozenwald, M.N.; Geohring, L.D.; Lion, L.W.; Steenhuis, T.S. Phosphorus removal by wollastonite: A constructed wetland substrate. Ecol. Eng. 2000, 15, 121–132. [Google Scholar] [CrossRef]
- Akratos, C.; Tsihrintzis, V. Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecol. Eng. 2007, 29, 173–191. [Google Scholar] [CrossRef]
- INEGI. Instituto Nacional de Estadística y Geografía. Censo de Población y Vivienda. México. 2020. Available online: http://www.inegi.org.mx/ (accessed on 10 July 2024). (In Spanish).
- Zhang, H.H.; Tian, J.S.; Zhang, Y.M.; Wu, Z.L.; Hu, Y.; Li, D.L. Removal of phosphorus and nitrogen from domestic wastewater using a mineralized refuse-based bioreactor. Environ. Technol. 2012, 33, 173–181. [Google Scholar] [CrossRef]
- Abhiram, G.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C.; McCurdy, M. Iron-rich sand promoted nitrate reduction in a study for testing og lignite based new slow-release fertilisers. Sci. Total Environ. 2023, 864, 160949. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.E.; Marín-Muñiz, J.L.; Olguín, E.J. Effect of flood frequency and nutrient addition on plant growth and total petroleum hydrocarbons removal in mangrove microcosm. J. Water Res, Prot. 2014, 6, 1716–1730. [Google Scholar] [CrossRef][Green Version]
- Wieczorek, D.; Zyska-Haberecht, B.; Kafka, A.; Lipok, J. Determination of phosphorous compounds in plant tissues: From colourimetry to advanced instrumental analytical chemistry. Plant Methods 2022, 18, 22. [Google Scholar] [CrossRef]
- Msimbira, L.; Smith, D. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Cases, I.; de Lorenzo, V. Genetically modified organisms for the environment: Stories of success and failure and what we have learned from them. Int. Microbiol. 2005, 8, 213–222. Available online: https://goo.gl/3oaxJT (accessed on 10 June 2024). [PubMed]
- Liu, H.; Hu, Z.; Zhang, J.; Ngo, H.; Guo, W.; Liang, S.; Fan, J.; Lu, S.; Wu, H. Optimizations on supply and distribution of dissolved oxygen in constructed wetlands: A review. Bioresour. Technol. 2016, 214, 797–805. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Shukla, R.; Gupta, D.; Singh, G.; Mishra, V. Performance of horizontal flow constructed wetland for secondary treatment of domestic wastewater in a remote tribal area of Central India. Sustain. Environ. Res. 2021, 31, 13. [Google Scholar] [CrossRef]
- Alburquerque, A.; Oliveira, J.; Semitela, S.; Amaral, L. Evaluation of the effectiveness of horizontal subsurface flow constructed wetlands for different media. J. Environ. Sci. 2010, 22, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Rocha, D.; Yoshie, L.; Moraes, D.; Valquíria, M.; Pedrosa, M. Phytoremediation by ornamental plants: A beautiful and ecological alternative. Environ. Sci. Pollut. Res. 2022, 29, 3336–3354. [Google Scholar] [CrossRef]
- Sandoval, L.; Zamora-Castro, S.A.; Vidal-Álvarez, M.; Marín-Muñiz, J.L. Role of wetland plants and use of ornamental flowering plants in constructed wetlands for wastewater treatment: A review. Appl. Sci. 2019, 9, 685. [Google Scholar] [CrossRef]
- Khatiwada, N.R.; Polprasert, C. Assessment of effective specific surface area for free water surface constructed wetlands. Water Sci. Technol. 1999, 40, 83–89. [Google Scholar] [CrossRef]
- Lee, C.; Fletcher, T.; Sun, G. Nitrogen removal in constructed wetland systems. Eng. Life Sci. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Matolisi, E.; Damiri, N.; Sodik, M.; Hasyim, H. Performance of horizontal subsurface flow constructed wetland in domestic wastewater treatment using different media. J. Ecol. Eng. 2024, 25, 107–119. [Google Scholar] [CrossRef]
- Shved, O.; Petrina, R.; Chervetsova, V.; Novikov, V. Enhancing efficiency of nitrogen removal from wastewater in constructed wetlands. East-Eur. J. Enterp. Technol. 2015, 3, 63–68. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.; Anderson, C.J.; Fennessy, M.S. Wetlands, 6th ed.; John Wiley and Sons Inc.: New York, NY, USA, 2023. [Google Scholar]
- Dell´Osbel, N.; Stolzenberg, G.; Alves, G.; Souza, M.; Vieira, C.; Machado, Ê. Bibliometric analysis of phosphorous removal through constructed wetlands. Water Air Soil Pollut. 2020, 231, 117. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L. Removal of wastewater pollutant in artificial wetlands implemented in Actopan, Veracruz, Mexico. Rev. Mex. Ing. Quím. 2016, 15, 553–563. Available online: http://www.redalyc.org/articulo.oa?id=62046829021 (accessed on 20 July 2024). (In Spanish). [CrossRef]
- Zitácuaro-Contreras, I.; Vidal-ÁLvarez, M.; Hernández y Orduña, M.; Zamora-Castro, S.; Betanzo-Torres, E.; Marín-Muñiz, J.; Sandoval-Herazo, L. Environmental, economic, and social potentialities of ornamental vegetation cultivated in constructed wetlands of Mexico. Sustainability 2021, 13, 6267. [Google Scholar] [CrossRef]
- Maucieri, C.; Salvato, M.; Borin, M. Vegetation contribution on phosphorous removal in constructed wetlands. Ecol. Eng. 2020, 152, 105853. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ahmad, Z.; Asgher, M.; Bhat, J.A.; Sharma, M.; Kumar, A.; Sharma, V.; Kumar, A.; Pant, S.; Lukatkin, A.S.; et al. Phytoremediation of Potentially Toxic Elements: Role, Status and Concerns. Plants 2023, 12, 429. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Cerri, M.; Forni, C. Phytoremediation of Pollutants: Applicability and Future Perspective. Plants 2023, 12, 2462. [Google Scholar] [CrossRef]
- Jethwa, K.; Bajpai, S. Role of plants in constructed wetlands (CWS): A review. J. Chem. Pharm. Sci. 2016, 2, 4–10. [Google Scholar]
- Yousaf, A.; Khalid, N.; Aqeel, M.; Noman, A.; Naeem, N.; Sarfraz, W.; Ejaz, U.; Qaiser, Z.; Khalid, A. Nitrogen Dynamics in Wetland Systems and Its Impact on Biodiversity. Nitrogen 2021, 2, 196–217. [Google Scholar] [CrossRef]
- Dong, J.; Kuang, S. Bibliometric analysis of nitrogen removal in constructed wetlands: Current trends and future research directions. Water 2024, 16, 1453. [Google Scholar] [CrossRef]
- Mesquita, C.; Albulquerque, A.; Amaral, L.; Nogueira, R. Effectiveness and temporal variation of a full-scale horizontal constructed wetland in reducing nitrogen and phosphorus from domestic wastewater. ChemEngineering 2018, 2, 3. [Google Scholar] [CrossRef]
- Vera-Puerto, I.; Marca, N.; Contreras, C.; Zuñiga, F.; López, J.; Sanguesa, C.; Correo, C.; Arias, C.; Valenzuela, M. Performance of vertical and horizontal treatment wetlands planted with ornamental plants in Central Chile: Comparative analysis of initial operations stage for effluent reuse in agriculture. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, J. Variation in photosynthetic characteristics and leaf area contributes to Spathiphylumm cultivar differences in biomass production. Photosynthetica 2003, 41, 443–447. [Google Scholar] [CrossRef]
- Conover, C.A. Foliage plants. In Introduction to Floriculture; Larson, R.A., Ed.; Academic Press: New York, NY, USA, 1992; pp. 569–601. [Google Scholar]
- Yunus, M.; Ismail, N.; Sundram, T.; Zainuddin, Z.; Rosli, N. Commercial potential and agronomi status of Etlingera elatior, a promising horticulture plant from Zingiberaceae family. AGRIVITA J. Agric. Sci. 2021, 43, 665–678. [Google Scholar] [CrossRef]
- Jácome-Chacón, M.A.; Trejo-Télez, L.I.; García-Albarado, J.C.; Cuacua-Temiz, C.; Gómez-Merino, F.C. Consideraciones sobre manejo fitosanitario, nutrimental y postcosecha de heliconias para su comercialización. AGROProductividad 2018, 11, 41–48. (In Spanish) [Google Scholar]
- Carrera-Alvarado, G.; Arévalo-Galarza, M.L.; Velasco-Velasco, J.; Ruiz-Posadas, L.; Salinas-Ruiz, J.; Baltazar-Bernal, O. Postharvest management of Heliconia psittacorum x H. spathocircinata cv. Tropics. AGROProductividad 2020, 13, 99–106. [Google Scholar] [CrossRef]
- Hernández, M.E.; Galindo-Zetina, M.; Hernández-Hernández, J.C. Greenhouse gas emissions and pollutant removal in treatment wetlands with ornamental plants under subtropical conditions. Ecol. Eng. 2018, 114, 88–95. [Google Scholar] [CrossRef]
- Tejeda, A.; Valencia-Botín, A.; Zurita, F. Resistance of Canna indica, Cyperus papyrus, Iris sibirica, and Typha latifolia to phototoxic characteristics of diluted tequila vinasse in wetland microcosms. Int. J. Phytoremediation 2023, 25, 1259–1268. [Google Scholar] [CrossRef]
- Sandoval-Herazo, M.; Martínez-Reséndiz, G.; Fernández, E.; Fernández-Lambert, G.; Sandoval, L.C. Plant biomass production in constructed wetlands treating swine wastewater in tropical climates. Fermentation 2021, 7, 296. [Google Scholar] [CrossRef]
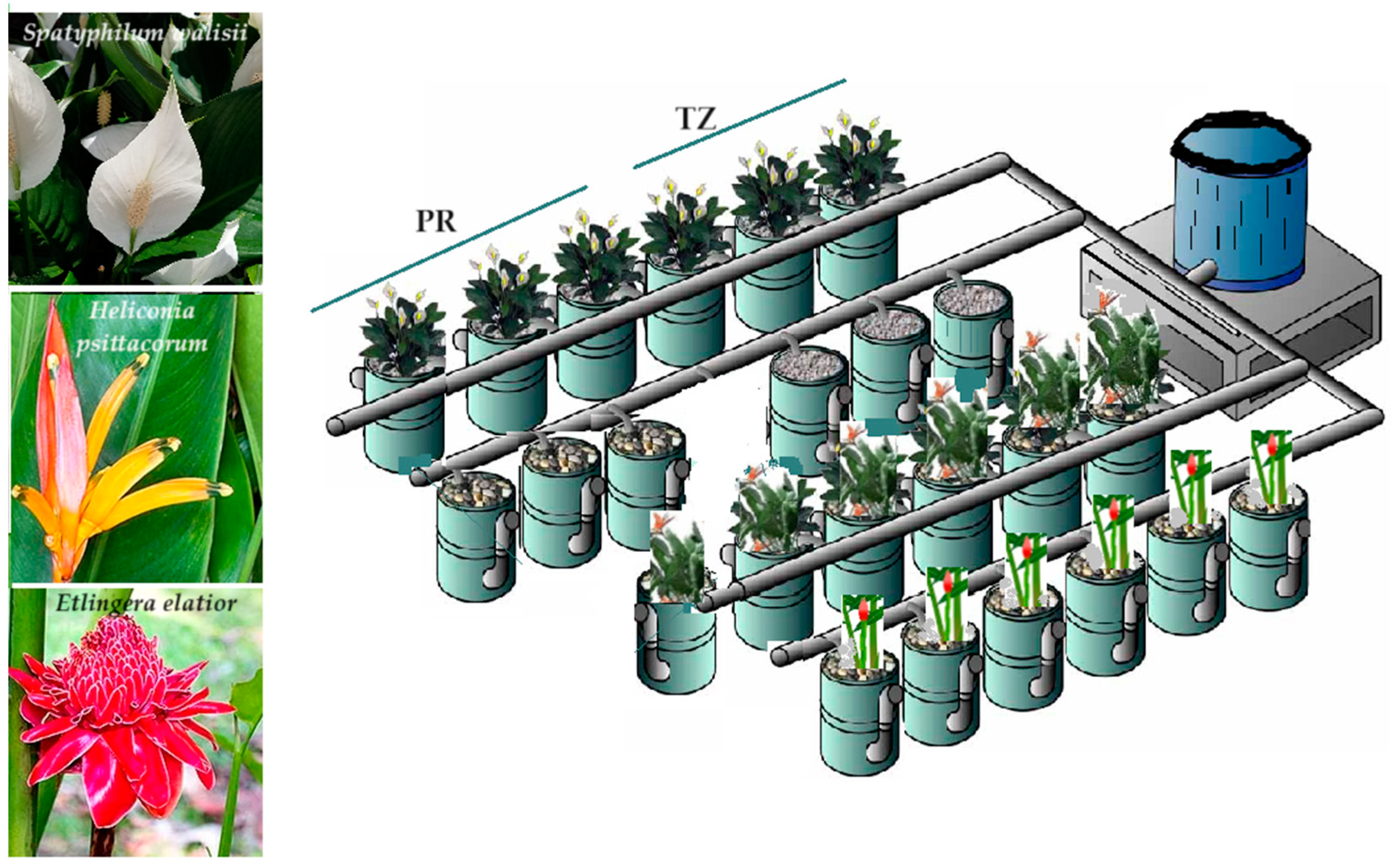
| Parameter | Wetland Plants in Different Substrates | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Influent | Heliconia psittacorum | Etlingera elatior | Spatyphilum wallisii | Control | |||||
| PR | TZ | PR | TS | PR | TZ | PR | TZ | ||
| pH (pH units) | 7.5 ± 0.3 | 7.2 ± 0.4 | 7.1 ± 0.2 | 7.2 ± 0.1 | 7.2 ± 0.3 | 7.1 ± 0.6 | 6.5 ± 0.1 | 7.5 ± 0.3 | 7.4 ± 0.2 |
| DO (mg L−1) | 1.3 ± 0.4 | 4.1 ± 0.6 | 4.2 ± 0.9 | 3.9 ± 0.5 | 4.6 ± 0.3 | 4.2 ± 0.8 | 3.9 ± 0.7 | 1.7 ± 0.6 | 1.5 ± 0.4 |
| Temperature (°C) | 19.0 ± 0.6 | 19.2 ± 0.9 | 19.4 ± 1.2 | 18.9 ± 1.3 | 17.6 ± 0.9 | 16.9 ± 1.2 | 18.4 ± 0.3 | 19.1 ± 0.4 | 19.8 ± 0.7 |
| EC (µS/cm) | 1151 ± 143 | 1150 ± 099 | 1209 ± 102 | 1124 ± 161 | 1233 ± 52 | 1124.2 ± 98 | 1130.8 ± 110 | 1126 ± 130 | 1012.9 ± 64 |
| Parameter | Wetland Plants in Different Substrates | |||||||
|---|---|---|---|---|---|---|---|---|
| Heliconia psittacorum | Etlingera elatior | Spatyphilum wallisii | Control | |||||
| PR | TZ | PR | TZ | PR | TZ | PR | TZ | |
| COD | ||||||||
| Influent concentration (mg L−1) | 299.2 ± 35.6 | |||||||
| Effluent concentration (mg L−1) | 83.6 ± 19.2 | 82.1 ± 18.1 | 83.1 ± 16.6 | 81.9 ± 11.4 | 96.7 ± 15.4 | 106.2 ± 24.6 | 218.3 ± 21.3 | 216.3 ± 21.1 |
| Removal (%) | 72.1 ± 21.1 a | 72.3 ± 12.8 a | 72.6 ± 14.3 a | 72.6 ± 14.3 a | 67.7 ± 11.9 b | 64.5 ± 10.9 b | 27.0 ± 11.1 c | 27.7 ± 19.4 c |
| NT | ||||||||
| Influent concentration (mg L−1) | 65.4 ± 14.3 | |||||||
| Effluent concentration (mg L−1) | 30.9 ± 05.1 | 22.6 ± 14.3 | 23.6 ± 11.6 | 23.9 ± 11.4 | 29.5 ± 09.1 | 28.6 ± 11.7 | 44.6 ± 11.4 | 45.8 ± 13.2 |
| Removal (%) | 52.8 ± 16.3 a | 65.4 ± 13.1 a | 63.9 ± 16.3 a | 63.5 ± 11.3 a | 54.9 ± 17.3 b | 56.3 ± 16.1 b | 31.8 ± 11.7 c | 30.0 ± 11.8 c |
| NO3-N | ||||||||
| Influent concentration (mg L−1) | 6.48 ± 1.36 | |||||||
| Effluent concentration (mg L−1) | 2.96 ± 0.92 | 2.76 ± 0.14 | 2.26 ± 0.16 | 2.62 ± 0.99 | 2.36 ± 0.82 | 2.84 ± 0.99 | 5.65 ± 0.65 | 5.32 ± 0.19 |
| Removal (%) | 54.3 ± 13.2 a | 57.4 ± 3.6 a | 59.6 ± 13.1 a | 59.6 ± 10.8 a | 63.6 ± 11.2 b | 56.2 ± 11.3 b | 12.8 ± 3.9 c | 17.9 ± 6.3 c |
| NH4-N | ||||||||
| Influent concentration (mg L−1) | 4.01 ± 0.82 | |||||||
| Effluent concentration (mg L−1) | 1.14 ± 0.22 | 1.32 ± 0.62 | 1.3 ± 0.32 | 1.13 ± 0.42 | 1.32 ± 0.11 | 1.21 ± 0.16 | 2.86 ± 0.71 | 2.69 ± 0.45 |
| Removal (%) | 71.6 ± 13.6 a | 67.1 ± 09.2 a | 67.6 ± 14.2 a | 71.8 ± 11.3 a | 67.1 ± 16.6 b | 69.8 ± 13.1 b | 28.7 ± 2.9 c | 32.9 ± 02.1 c |
| PO4-P | ||||||||
| Influent concentration (mg L−1) | 10.6 ± 1.3 | |||||||
| Effluent concentration (mg L−1) | 4.20 ± 0.36 | 4.11 ± 0.14 | 3.01 ± 0.33 | 3.15 ± 0.72 | 4.01 ± 0.97 | 4.11 ± 0.32 | 8.26 ± 0.32 | 7.92 ± 0.36 |
| Removal (%) | 60.4 ± 3.9 a | 61.2 ± 3.6 a | 71.6 ± 3.2 a | 70.3 ± 3.4 a | 62.2 ± 3.6 b | 61.2 ± 6.9 b | 22.1 ± 3.8 c | 25.3 ± 3.6 c |
| Plant | Filter Media | Wilting Degree (Number of Plants) | Growth Characteristics Stems–Leaves–Flowers a | Diseases and Pests b | Number of Flowers during the Study |
|---|---|---|---|---|---|
| Heliconia psittacorum | PR | 0 | - | - | 20 |
| TZ | 0 | - | - | 22 | |
| Etlingera elatior | PR | 2 | XX | X | 0 |
| TZ | 2 | XX | X | 0 | |
| Spatiphylum wallisii | PR | 0 | - | - | 8 |
| TZ | 0 | - | - | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Muñiz, J.L.; Ortega-Pineda, G.; Zitácuaro-Contreras, I.; Vidal-Álvarez, M.; Martínez-Aguilar, K.E.; Álvarez-Hernández, L.M.; Zamora-Castro, S. Removal of Nitrogen, Phosphates, and Chemical Oxygen Demand from Community Wastewater by Using Treatment Wetlands Planted with Ornamental Plants in Different Mineral Filter Media. Nitrogen 2024, 5, 903-914. https://doi.org/10.3390/nitrogen5040058
Marín-Muñiz JL, Ortega-Pineda G, Zitácuaro-Contreras I, Vidal-Álvarez M, Martínez-Aguilar KE, Álvarez-Hernández LM, Zamora-Castro S. Removal of Nitrogen, Phosphates, and Chemical Oxygen Demand from Community Wastewater by Using Treatment Wetlands Planted with Ornamental Plants in Different Mineral Filter Media. Nitrogen. 2024; 5(4):903-914. https://doi.org/10.3390/nitrogen5040058
Chicago/Turabian StyleMarín-Muñiz, José Luis, Gonzalo Ortega-Pineda, Irma Zitácuaro-Contreras, Monserrat Vidal-Álvarez, Karina E. Martínez-Aguilar, Luis M. Álvarez-Hernández, and Sergio Zamora-Castro. 2024. "Removal of Nitrogen, Phosphates, and Chemical Oxygen Demand from Community Wastewater by Using Treatment Wetlands Planted with Ornamental Plants in Different Mineral Filter Media" Nitrogen 5, no. 4: 903-914. https://doi.org/10.3390/nitrogen5040058
APA StyleMarín-Muñiz, J. L., Ortega-Pineda, G., Zitácuaro-Contreras, I., Vidal-Álvarez, M., Martínez-Aguilar, K. E., Álvarez-Hernández, L. M., & Zamora-Castro, S. (2024). Removal of Nitrogen, Phosphates, and Chemical Oxygen Demand from Community Wastewater by Using Treatment Wetlands Planted with Ornamental Plants in Different Mineral Filter Media. Nitrogen, 5(4), 903-914. https://doi.org/10.3390/nitrogen5040058











