Effect of Time of Nitrogen Fertilization on Use of Root Reserves in Megathyrsus maximus Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Local and Experimental Design
2.2. Soil and Sowing
2.3. Standardization and Data Collection
2.4. Root Carbohydrate and Nitrogen Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galindo, F.S.; Buzetti, S.; Teixeira Filho, M.C.M.; Dupas, E.; Ludkiewicz, M.G.Z. Dry matter and nutrients accumulation in mombasa guineagrass in function of nitrogen fertilization management. Rev. De Agric. Neotrop. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Seixas, A.A.; Fries, D.D.; Dias, D.L.S.; Santos, I.A.P.S.; Cruz, N.T.; Teixeira, F.A.; Bonomo, P.; Amaral Júnior, F.P. Carbohydrate and protein metabolism of marandu grass affected by nitrogen fertilisation and number of cuts. Agron. Res. 2023, 21, 1581–1596. [Google Scholar] [CrossRef]
- Homem, B.G.C.; Lima, I.B.G.; Spasiani, P.P.; Guimarães, B.C.; Guimarães, G.D.; Bernardes, T.F.; Rezende, C.D.P.; Boddey, R.M.; Casagrande, D.R. N-fertiliser application or legume integration enhances N cycling in tropical pastures. Nutr. Cycl. Agroecosyst. 2021, 121, 167–190. [Google Scholar] [CrossRef]
- Davies, B.; Coulter, J.A.; Pagliari, P.H. Timing and rate of nitrogen fertilization influence maize yield and nitrogen use efficiency. PLoS ONE 2020, 15, e0233674. [Google Scholar] [CrossRef]
- Nilahyane, A.; Islam, M.A.; Mesbah, A.O.; Garcia y Garcia, A. Effect of Irrigation and Nitrogen Fertilization Strategies on Silage Corn Grown in Semi-Arid Conditions. Agronomy 2018, 8, 208. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, H.; You, Y.; Wu, R.; Liu, G.; Sun, Z.; Bademuqiqige; Zhang, Y. Effects of Intercropping, Nitrogen Fertilization and Corn Plant Density on Yield, Crude Protein Accumulation and Ensiling Characteristics of Silage Corn Interseeded into Alfalfa Stand. Agriculture 2022, 12, 357. [Google Scholar] [CrossRef]
- Delevatti, L.M.; Cardoso, A.S.; Barbero, R.P.; Romanzini, E.P.; Ruggieri, A.C.; Reis, R.A. Effect of nitrogen application rate on yield, forage quality, and animal performance in a tropical pasture. Sci. Rep. 2019, 9, 7596. [Google Scholar] [CrossRef]
- Oliveira, J.K.S.; Corrêa, D.C.d.C.; Cunha, A.M.Q.; Rêgo, A.C.d.; Faturi, C.; Silva, W.L.d.; Domingues, F.N. Effect of Nitrogen Fertilization on Production, Chemical Composition and Morphogenesis of Guinea Grass in the Humid Tropics. Agronomy 2020, 10, 1840. [Google Scholar] [CrossRef]
- Zanine, A.; Farias, L.; Ferreira, D.; Farias, L.; Ribeiro, M.; Souza, A.; Rodrigues, R.; Santos, E.; Oliveira, J.; Sousa, L.; et al. Effect of Season and Nitrogen Fertilization on the Agronomic Traits and Efficiency of Piatã Grass in Brazilian Savanna. Agriculture 2020, 10, 337. [Google Scholar] [CrossRef]
- Gomide, C.A.M.; Paciullo, D.S.C.; Morenz, M.J.F.; Costa, I.A.; Lanzoni, C.L. Productive and morphophysiological responses of Panicum maximum Jacq. cv. BRS Zuri to timing and doses of nitrogen application and defoliation intensity. Grassl. Sci. 2019, 65, 93–100. [Google Scholar] [CrossRef]
- Marques, M.F.; Romualdo, L.M.; Martinez, J.F.; Lima, C.G.; Lunardi, L.J.; Luz, P.H.C.; Herling, V.R. Time of nitrogen application and some structural and bromatologic variables of massaigrass. Braz. J. Vet. Anim. Sci. 2016, 68, 776–784. [Google Scholar] [CrossRef][Green Version]
- Faria, D.A.; Avelino, A.C.D.; Cabral, C.E.A.; Abreu, J.G.; Barros, L.V.; Cabral, C.H.A.; Assis, L.M.B. Investigating the Optimal Day for Nitrogen Fertilization on Piatã palisadegrass and Quênia guineagrass after Defoliation. J. Exp. Agric. Int. 2019, 34, 1–11. [Google Scholar] [CrossRef]
- Cabral, C.E.A.; Motta, A.M.; Santos, A.R.M.; Gomes, F.J.; Pedreira, B.C.; Cabral, C.H.A. Effects of timing of nitrogen fertilizer application on responses by tropical grasses. Trop. Grassl. 2021, 9, 182–191. [Google Scholar] [CrossRef]
- Motta, A.M.; Mota, L.G.; Melo, K.K.; Silva, P.R.; Santos, A.R.M.; Motta, L.J.M.; Cabral, C.H.A.; Cabral, C.E.A. Interval between defoliation and nitrogen fertilization of Panicum maximum cultivars. Bull. Anim. Husb. 2021, 78, e1500. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Campos, D.V.B.; Saldanha, M.F.C. Soil pH. In Manual of Soil Analysis Methods, 3rd ed.; Teixeira, P.C., Donagemma, G.K., Fontana, A., Teixeira, W.G., Eds.; Embrapa Solos (INFOTECA-E): Brasilia, Brazil, 2017; Volume 1, pp. 199–202. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/1085209 (accessed on 21 July 2024).
- Teixeira, P.C.; Campos, D.V.B.; Saldanha, M.F.C. Available phosphorus. In Manual of Soil Analysis Methods, 3rd ed.; Teixeira, P.C., Donagemma, G.K., Fontana, A., Teixeira, W.G., Eds.; Embrapa Solos (INFOTECA-E): Brasilia, Brazil, 2017; Volume 1, pp. 203–208. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/1085209 (accessed on 21 July 2024).
- Teixeira, P.C.; Campos, D.V.B.; Bianchi, S.R.; Perez, D.V.; Saldanha, M.F.C. Exchangeable cations. In Manual of Soil Analysis Methods, 3rd ed.; Teixeira, P.C., Donagemma, G.K., Fontana, A., Teixeira, W.G., Eds.; Embrapa Solos (INFOTECA-E): Brasilia, Brazil, 2017; Volume 1, pp. 209–232. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/1085209 (accessed on 21 July 2024).
- Campos, D.V.B.; Teixeira, P.C.; Perez, D.V.; Saldanha, M.F.C. Extractable hydrogen. In Manual of Soil Analysis Methods, 3rd ed.; Teixeira, P.C., Donagemma, G.K., Fontana, A., Teixeira, W.G., Eds.; Embrapa Solos (INFOTECA-E): Brasilia, Brazil, 2017; Volume 1, pp. 238–239. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/1085209 (accessed on 21 July 2024).
- Donagemma, G.K.; Calderano, S.B.; Viana, J.H.M. Separation of soil granulometric fractions for mineralogical analysis. In Manual of Soil Analysis Methods, 3rd ed.; Teixeira, P.C., Donagemma, G.K., Fontana, A., Teixeira, W.G., Eds.; Embrapa Solos (INFOTECA-E): Brasilia, Brazil, 2017; Volume 1, pp. 439–442. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/1085209 (accessed on 21 July 2024).
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences: Vienna, Austria, 2022. [Google Scholar]
- Euclides, V.P.B.; Carpejani, G.C.; Montagner, D.B.; Nascimento Junior, D.; Barbosa, R.A.; Difante, G.S. Maintaining post-grazing sward height of Panicum maximum (cv. Mombaça) at 50 cm led to higher animal performance compared with post-grazing height of 30 cm. Grass Forage Sci. 2017, 73, 174–182. [Google Scholar] [CrossRef]
- Tesk, C.R.M.; Cavalli, J.; Pina, D.S.; Pereira, D.H.; Pedreira, C.G.S.; Jank, L.; Sollenberger, L.E.; Pedreira, B.C. Herbage responses of Tamani and Quenia guineagrasses to grazing intensity. Agron. J. 2020, 112, 2081–2091 . [Google Scholar] [CrossRef]
- Passos, L.P. Analytical and Laboratory Methods in Plant Physiology; Embrapa-CNPGL: Coronel Pacheco, Brazil, 1996. [Google Scholar]
- Association of Official Analytical Chemists International (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists International (AOAC): Washington, DC, USA, 2000.
- Yang, F.; Schäufele, R.; Liu, H.T.; Ostler, U.; Schnyder, H.; Gong, X.Y. Gross and net nitrogen export from leaves of a vegetative C4 grass. J. Plant Physiol. 2020, 244, 153093. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.E.A.; Mota, L.G.; Gomes, L.D.; Silva, P.H.G.; Duarte, C.F.D.; Cabral, C.H.A. How many days after harvesting must there be nitrogen fertilization of BRS Ipyporã grass? Nativa 2023, 11, 448–453. [Google Scholar] [CrossRef]
- Paiva, H.S.; Sousa, S.V.; Conceição, E.C.A.; Santos, A.C.; Okumura, R.S.; Mezzomo, R.; Santos, P.M.; Maciel, R.P. Recommended fertilization and timing of nitrogen fertilization influences the morphogenesis, structural characteristics, and production efficiency of Mombaça grass. Acta Sci. Anim. Sci. 2023, 45, e60704. [Google Scholar] [CrossRef]
- Silva, L.S.; Silva, V.J.; Yasuoka, J.I.; Sollenberger, L.E.; Pedreira, C.G.S. Tillering dynamics of ‘Mulato II’ brachiariagrass under continuous stocking. Crop Sci. 2019, 60, 1105–1112. [Google Scholar] [CrossRef]
- Sousa, B.M.L.; Rizato, C.A.; Fagundes, J.L.; Fontes, P.T.N.; Backes, A.A.; Oliveira Junior, L.F.G.; Nascimento, C.S. Tillering dynamics of digit grass subject to different defoliation frequencies. Pesqui. Agropecuária Bras. 2019, 54, e00668. [Google Scholar] [CrossRef]
- Tshewang, S.; Rengel, Z.; Siddique, K.H.M.; Solaiman, Z.M. Growth, Rhizosphere Carboxylate Exudation, and Arbuscular Mycorrhizal Colonisation in Temperate Perennial Pasture Grasses Varied with Phosphorus Application. Agronomy 2020, 10, 2017. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B. Physiological and Morphometric Response of Forage Grass Species and Their Biomass Distribution Depending on the Term and Frequency of Water Deficiency. Agronomy 2021, 11, 2471. [Google Scholar] [CrossRef]
- Dar, B.A.; Assaeed, A.M.; Al-Rowaily, S.L.; Al-Doss, A.A.; Habib, M.M.; Malik, J.A.; Abd-ElGawad, A.M. Effect of Simulated Grazing on Morphological Plasticity and Resource Allocation of Aeluropus lagopoides. Agronomy 2024, 14, 144. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G. Defoliation, Shoot Plasticity, Sward Structure and Herbage Utilization in Pasture: Review of the Underlying Ecophysiological Processes. Agriculture 2015, 5, 1146–1171. [Google Scholar] [CrossRef]
- Yang, D.; Luo, Y.; Kong, X.; Huang, C.; Wang, Z. Interactions between exogenous cytokinin and nitrogen application regulate tiller bud growth via sucrose and nitrogen allocation in winter wheat. J. Plant Growth Regul. 2021, 40, 329–341. [Google Scholar] [CrossRef]
- Mota, L.G.; Righi, R.S.M.; Duarte, C.F.D.; Cabral, C.H.A.; Cabral, C.E.A. Nitrogen fertilization time affects the root reserves of tropical grasses. Pesqui. Agropecuária Trop. 2023, 53, e75444. [Google Scholar] [CrossRef]
- Ferro, M.M.; Zanine, A.M.; Ferreira, D.J.; Souza, A.L.; Geron, L.J.V. Organic Reserves in tropical Grasses under Grazing. Am. J. Plant Sci. 2015, 6, 2329–2338. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Müller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, RS, Brazil, 2017. [Google Scholar]
- Aranjuelo, I.; Molero, G.; Erice, G.; Aldasoro, J.; Arrese-Igor, C.; Nogués, S. Effect of shoot removal on remobilization of carbon and nitrogen during regrowth of nitrogen-fixing alfalfa. Physiol. Plant. 2014, 153, 91–104. [Google Scholar] [CrossRef]
- Lu, X.; Ji, S.; Hou, C.; Qu, H.; Li, P.; Shen, Y. Impact of root C and N reserves on shoot regrowth of defoliated alfalfa cultivars differing in fall dormancy. Grassl. Sci. 2018, 64, 83–90. [Google Scholar] [CrossRef]
- Cruz, N.T.; Jardim, R.R.; Sousa, B.M.L.; Seixas, A.A.; Fries, D.D.; Pires, A.J.V.; Dias, D.L.S.; Bonomo, P.; Ramos, B.L.P.; Alcantara, W.Q.; et al. Energy flows and organic reserves of forage plants. Res. Soc. Dev. 2022, 11, e549111234782. [Google Scholar] [CrossRef]
| Soil | pH | P | K | Ca | Mg | Al | H | CEC 1 | V 2 | M 3 | Sand | Silt | Clay |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | mg dm−3 | cmolc dm−3 | % | g kg−1 | |||||||||
| Site 1 | 4.7 | 8.5 | 43 | 0.95 | 0.39 | 0.2 | 2.3 | 3.95 | 36.7 | 12.1 | 823 | 43 | 134 |
| Site 2 | 6.0 | 3.4 | 119 | 2.3 | 2.0 | 0.0 | 1.7 | 6.3 | 73.0 | 0.0 | 425 | 150 | 425 |
| Variable | Cultivars | Interval | Cultivars × Interval | SEM |
|---|---|---|---|---|
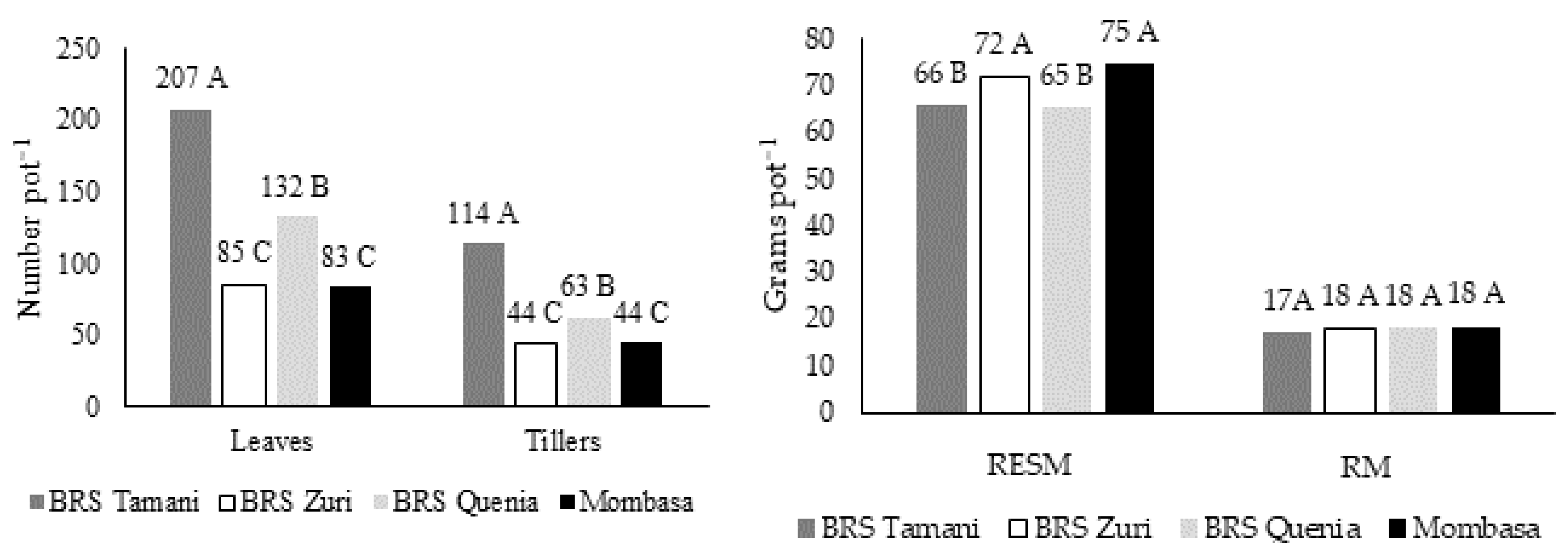
| Number of leaves (leaves pot−1) | <0.001 | 0.064 | 0.891 | 5.678 |
| TPD (tillers pot−1) | <0.001 | 0.204 | 0.754 | 2.662 |
| Forage mass (g pot−1) | 0.002 | 0.015 | 0.043 | 0.536 |
| Residual mass (g pot−1) | 0.006 | 0.648 | 0.688 | 4.394 |
| Root mass (g vaso−1) | 0.845 | 0.723 | 0.876 | 1.868 |
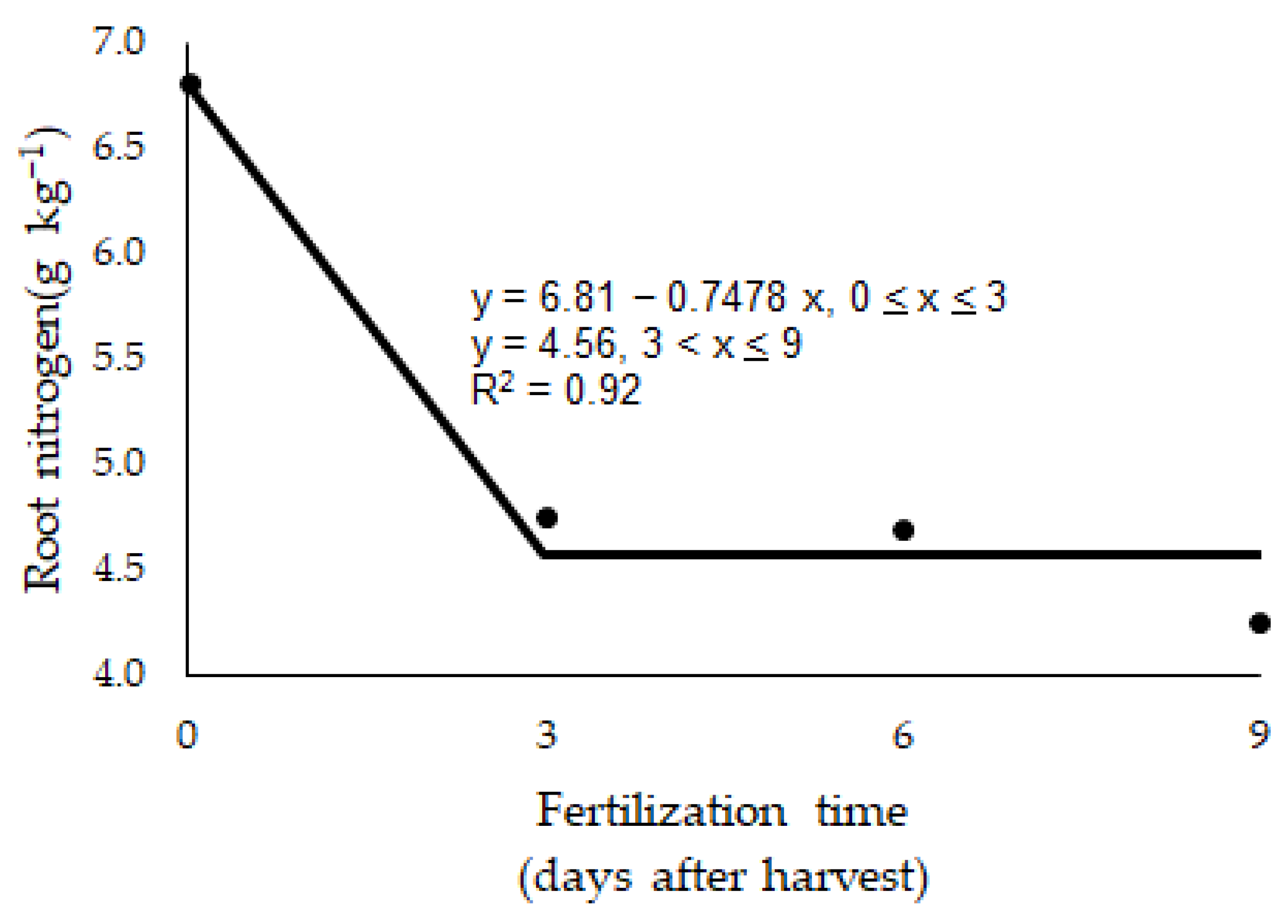
| Root nitrogen (g kg−1) | 0.008 | 0.410 | 0.027 | 0.459 |
| Root WSC (mg g−1) | <0.001 | 0.010 | <0.001 | 1.136 |
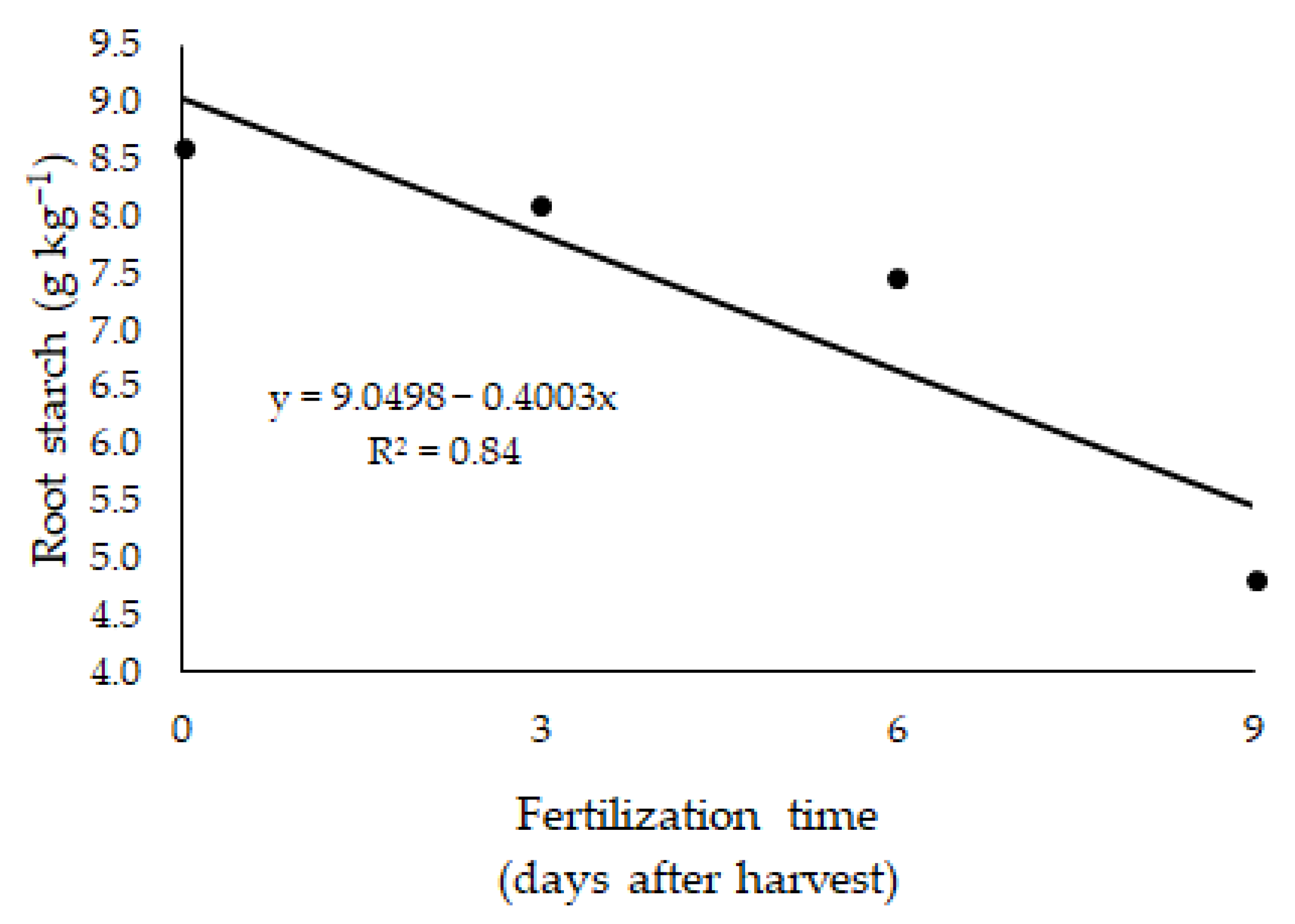
| Root starch (mg g−1) | 0.095 | 0.136 | <0.001 | 0.654 |
| Root TNSC (mg g−1) | <0.001 | 0.002 | <0.001 | 1.303 |
| Chlorophyll index | <0.001 | 0.002 | 0.041 | 0.578 |
| Days after Harvesting | p-Value 1 | |||||
|---|---|---|---|---|---|---|
| Cultivars | 0 | 3 | 6 | 9 | L | Q |
| Forage Mass (g pot−¹) | ||||||
| Mombasa | 14.57 A | 14.77 A | 14.27 A | 14.03 A | 0.353 | 0.662 |
| BRS Quenia | 13.33 A | 13.59 A | 13.97 A | 12.99 AB | 0.756 | 0.185 |
| BRS Tamani | 14.17 A | 13.54 A | 13.42 A | 12.15 B | 0.003 | 0.449 |
| BRS Zuri | 14.00 A | 14.88 A | 14.20 A | 13.59 AB | 0.376 | 0.120 |
| Chlorophyll index | ||||||
| Mombasa | 34.2 B | 30.3 B | 31.8 B | 31.1 BC | 0.136 | 0.165 |
| BRS Quenia | 39.5 A | 36.2 A | 37.7 A | 36.5 A | 0.147 | 0.364 |
| BRS Tamani | 33.4 B | 33.0 AB | 33.7 AB | 33.3 AB | 0.908 | 0.992 |
| BRS Zuri | 35.2 AB | 35.1 A | 32.3 B | 28.8 C | <0.001 | 0.143 |
| Grass | Days after Harvesting | p-Value 1 | ||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | L | Q | |
| Nitrogen (g kg−1) | ||||||
| Mombasa | 4.24 B | 4.35 A | 4.19 A | 4.34 A | 0.936 | 0.958 |
| BRS Quenia | 4.17 B | 4.42 A | 4.27 A | 4.40 A | 0.693 | 0.842 |
| BRS Tamani | 3.97 B | 3.85 A | 4.16 A | 4.24 A | 0.505 | 0.801 |
| BRS Zuri | 6.81 A | 4.75 A | 4.70 A | 4.25 A | <0.001 | 0.034 |
| Water-soluble carbohydrates (mg g−1) | ||||||
| Mombasa | 11.26 B | 8.96 AB | 7.70 B | 14.94 A | 0.057 | <0.001 |
| BRS Quenia | 8.57 B | 6.28 B | 9.23 B | 10.32 AB | 0.108 | 0.139 |
| BRS Tamani | 7.98 B | 8.45 AB | 10.79 B | 5.91 C | 0.447 | 0.020 |
| BRS Zuri | 16.80 A | 12.08 A | 17.62 A | 12.81 B | 0.209 | 0.967 |
| Starch (mg g−1) | ||||||
| Mombasa | 5.64 B | 5.61 B | 6.29 A | 7.95 A | 0.011 | 0.200 |
| BRS Quenia | 8.30 A | 5.51 B | 7.75 A | 7.22 A | 0.737 | 0.086 |
| BRS Tamani | 6.88 AB | 6.32 AB | 6.44 A | 5.99 AB | 0.387 | 0.936 |
| BRS Zuri | 8.60 A | 8.11 A | 7.47 A | 4.81 B | <0.001 | 0.100 |
| Total non-structural carbohydrates (mg g−1) | ||||||
| Mombasa | 16.91 B | 14.58 B | 13.96 B | 22.89 A | 0.003 | <0.001 |
| BRS Quenia | 18.87 B | 11.80 B | 16.98 B | 17.55 B | 0.217 | 0.032 |
| BRS Tamani | 14.87 B | 14.77 B | 17.24 B | 11.91 C | 0.273 | 0.046 |
| BRS Zuri | 25.41 A | 20.20 A | 25.10 A | 17.63 B | 0.002 | 0.386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, A.M.; Motta, L.J.M.; Mota, L.G.; Assis, L.M.B.; Moura, A.B.O.; Borges, L.C.O.; Silva, G.B.A.; Duarte, C.F.D.; Cabral, C.H.A.; Cabral, C.E.A. Effect of Time of Nitrogen Fertilization on Use of Root Reserves in Megathyrsus maximus Cultivars. Nitrogen 2024, 5, 702-711. https://doi.org/10.3390/nitrogen5030046
Motta AM, Motta LJM, Mota LG, Assis LMB, Moura ABO, Borges LCO, Silva GBA, Duarte CFD, Cabral CHA, Cabral CEA. Effect of Time of Nitrogen Fertilization on Use of Root Reserves in Megathyrsus maximus Cultivars. Nitrogen. 2024; 5(3):702-711. https://doi.org/10.3390/nitrogen5030046
Chicago/Turabian StyleMotta, Aline M., Luiz J. M. Motta, Lucas G. Mota, Lucas M. B. Assis, Anna B. O. Moura, Luis C. O. Borges, Gustavo B. A. Silva, Camila F. D. Duarte, Carla H. A. Cabral, and Carlos E. A. Cabral. 2024. "Effect of Time of Nitrogen Fertilization on Use of Root Reserves in Megathyrsus maximus Cultivars" Nitrogen 5, no. 3: 702-711. https://doi.org/10.3390/nitrogen5030046
APA StyleMotta, A. M., Motta, L. J. M., Mota, L. G., Assis, L. M. B., Moura, A. B. O., Borges, L. C. O., Silva, G. B. A., Duarte, C. F. D., Cabral, C. H. A., & Cabral, C. E. A. (2024). Effect of Time of Nitrogen Fertilization on Use of Root Reserves in Megathyrsus maximus Cultivars. Nitrogen, 5(3), 702-711. https://doi.org/10.3390/nitrogen5030046






