1. Introduction
Nitrogen (N) is a key element for the life of all terrestrial organisms, being used by them in the production of complex biological molecules essential for their development, such as amino acids, proteins, enzymes, and nucleic acids [
1].
Although it exists in great abundance in the atmosphere, with about 78% by volume in the N
2 molecular form (non-reactive), N is not assimilated in this form by most living organisms [
2]. For example, plants uptake N in the forms NH
3 (ammonia), NH
4+ (ammonium), NO
3− (nitrate), and (NH
2)
2CO (urea) but assimilate N only in the reactive form of NH
3 (ammonia) into glutamine [
2], which makes N a scarce resource and a limiting factor for plant growth in many ecosystems [
3].
Throughout its biogeochemical cycle, N assumes different reactive molecular forms called Nr (collective of N compounds, except N
2). In a simplified way, the N cycle contemplates the following macroprocesses: (a) fixation of N
2 in the forms HNO
3 (nitric acid) and NO
3− via atmospheric lightning discharge; (b) biological fixation of N
2 in the forms NH
3/NH
4+ as carried out by associative and symbiotic bacteria; (c) industrial fixation of N
2 in the form of synthetic nitrogen fertilizers; (d) nitrification (conversion of ammonium into nitrate) carried out by soil bacteria; (e) assimilation of mineralized N by plants in the reactive forms NH
3, NH
4+, and NO
3−; (f) mineralization of soil organic N to the NH
4+ form carried out by decomposing bacteria; and (g) denitrification or reduction of nitrates and nitrites to N
2O (nitrous oxide) and N
2 forms [
4].
In the pre-industrial historical period, the N cycle remained in a relative state of dynamic equilibrium, with the balancing of N fluxes between terrestrial ecosystems and the atmosphere, that is, with the equivalence of global rates of N
2 fixation with global denitrification rates [
5].
With the advent of the Industrial Revolution, this balance began to be gradually broken. Between 1860 and 2000, the annual rate of anthropogenic Nr emissions increased from 15 Tg [N] (1 Tg = 10
12 g) to 165 Tg [N] [
6]. But it was from the second half of the twentieth century onwards that anthropogenic Nr emissions intensified, producing profound transformations in the N cycle. The determining factor for these transformations was the acceleration of the atmospheric N
2 fixation rate because of three human activities on a global scale: (a) industrial N
2 fixation via the production and use of synthetic nitrogen fertilizers; (b) biological N
2 fixation (BNF) via the large-scale cultivation of plants of the Fabaceae family; and (c) fossil fuel burning [
1].
In 2010, these three activities jointly produced around 210 Tg [N], of which 120 Tg [N] came from synthetic fertilizers, 60 Tg [N] from BNF, and 30 Tg [N] from burning fossil fuels [
7]. The Nr emitted in 2010 by these three anthropogenic sources surpassed the Nr emitted by strictly natural sources in the same year, estimated at 203 Tg [N] [
7].
Global agricultural activity accounted for about 80% of global anthropogenic Nr, and of all N applied in agriculture via synthetic fertilizer, only about 50% on average were incorporated into crop biomass [
8,
9,
10]. The other half (not assimilated by crops) goes into the environment in the form of Nr [
11].
The scarcity of N constitutes a limiting factor for the primary productivity of ecosystems, whereas its excessive presence constitutes a risk factor for the sustainability of ecosystems. For some decades, the global academic literature has been warning about the effects of the growth of anthropogenic Nr emissions: (a) There has been a 20% increase in the atmospheric concentration of N
2O gas in relation to the pre-industrial level [
12], which is currently responsible for about 5% of the atmospheric greenhouse effect [
13]. N
2O is also identified as the main cause of stratospheric ozone depletion [
14], responsible for adverse effects on human health [
12]; (b) There has been an increase in atmospheric emissions of nitrogen oxides N
2O and NO
2, polluting gases responsible for increasing the concentration of ozone in the troposphere and which are harmful to human health [
6,
12,
13]; (c) There has been a reduction in biodiversity in terrestrial ecosystems and an acidification of soils, lakes, and water bodies in different parts of the world [
15]; (d) There is eutrophication of coastal areas (rivers, lakes, and estuaries), caused by excess of nutrients, generally nitrogen and phosphorus, responsible for the proliferation of algae, cyanobacteria, and aquatic plants. Between 1960 and 2000, human activity increased the N flux in the Mississippi River basin 4-fold and in the rivers of the American Northeast 8-fold, with most of these fluxes of nitrates coming from the use of synthetic nitrogenous fertilizers [
16]; and (e) Other studies [
12,
17,
18] warn of the risks of the increasing imbalance in the N biogeochemical cycle, as well as the need for global actions to reduce anthropogenic Nr emissions.
Modern agriculture represents the main factor for transgressing the tolerable limits of human interference in the global N cycle, especially due to the growth in the use of synthetic nitrogen fertilizer [
17]. In this sense, more efficient use of N in agro-systems is a global need of great relevance [
19,
20,
21].
Among the crops that consume the most synthetic nitrogen fertilizers, corn ranks first in the world [
22]. Brazil produced 109 million tons of corn in 2022 (9% of global production) [
23] and is currently the third largest corn producer in the world [
24]. Around 27% of all synthetic N fertilizer consumed in 2018 in Brazil was utilized for corn crops [
25]. However, studies on the agri-environmental efficiency of N use in this crop at a regional level are scarce. Brazil produced 121 million tons of soybeans in 2022 (35% of world production) and is currently the largest soybean producer in the world [
23]. Unlike corn, the soybean production system relies only on biological N fixation as a N source. One study on macronutrients applied to sixteen main Brazilian crops [
24] estimated negative results in the N balance in agricultural soils in all five regions of the country, including the midwest region, the largest producer states of soybeans and corn in Brazil.
The objective of this study was to carry out an exploratory analysis on the efficiency of N use in the soil–plant system of the soybean-corn succession (here designated by ) in the Brazilian states of Mato Grosso (MT) and Mato Grosso do Sul (MS). For the most part, these crops are large scale, with soybeans and corn being cultivated under direct seeding (no tillage). In recent decades, there has been a significant expansion of this agricultural modality in these states, where soybean represents the main summer crop and corn the secondary crop in succession (locally called “second season corn” or “off season corn”). The efficiency in the use of N was analyzed based on the results of N fluxes in the as well as the results of the agro-environmental indicators.
2. Materials and Methods
The choice of the soybean-corn succession in MT and MS as the object of interest for the present study was based on the common attributes of these crops in both states: (a) The regional amplitude of the total sown area, with more than seven million hectares in 2020 [
26]. Regional analyses of large agricultural areas make it possible to more clearly identify the existence of surplus and deficit of nutrients in the soil–plant system than analyses at the local level; (b) Taking into account that all corn harvested in MT and MS is grown as second crop corn in succession to the soybean crop grown in the summer, we can assume that the cultivated area of the soybean-corn succession is equivalent to the total area of second corn crop cultivated in MT and MS; (c) The similarity between environmental characteristics (“Cerrado” biome) and agricultural management of soybean-corn succession crops in MT and MS [
27]; (d) The rainy tropical climate between October and May with a defined dry season, annual rainfall ranging between 1500 and 1900 mm, average annual temperature between 22 °C and 26 °C, and latitudes between 10° S and 23° S) [
28]; (e) Soils with a predominance of red Oxisols with medium and clayey texture [
29]; (f) Soybean sowing typically performed between mid-October and mid-December, and corn between January and mid-March, right after the soybean harvest [
27]; and (g) Soybean harvest between January and March and corn harvest between June and August.
In the soybean-corn succession analyzed here, cover crops such as
Brachiaria spp.,
Crotalaria spp., and others eventually cultivated in intercropping with corn (second harvest) were not considered due to the unavailability of quantitative data on their cultivation.
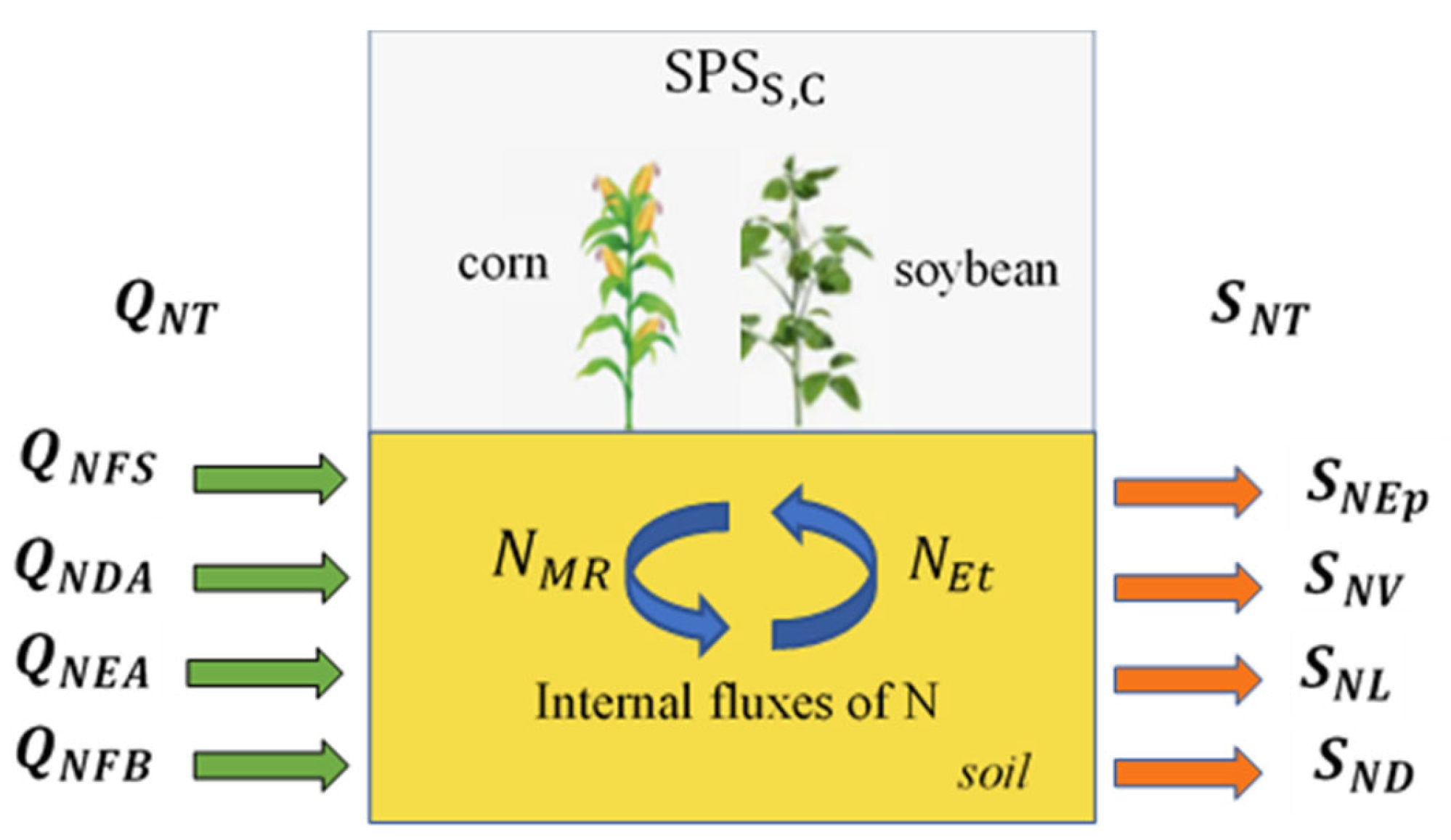
Figure 1 illustrates the
of the MT and MS states. The green and orange arrows indicate, respectively, the inflow and outflow of N. The blue arrows indicate the internal flows.
2.1. Definition of N Entries and Outcomes in the
The amount of N entries (
, kg [
] ha
−1) can be calculated as:
where
refers to N (kg [
] ha
−1) entries as synthetic fertilizers,
to N (kg [
] ha
−1) entries via atmospheric deposition in the molecular forms
(
O,
, and
) and
(
and
),
to N (kg [
] ha
−1) entries via animal excreta, and
(kg [
] ha
−1) entries via biologic fixation of atmospheric N
2 by the soybean crop.
The total N (
, kg [
] ha
−1) outcome can be calculated as:
where
refers to N (kg [
] ha
−1) outcomes via volatilization in the forms
, NO, and
,
to N (kg [
] ha
−1) outcomes via lixiviation/hydrologic runoff in the form
,
to
(kg [
] ha
−1) outcomes via grain export of soybeans and corn, and
to N (kg [
] ha
−1) outcomes via denitrification by eliminating N
2 and
.
The
that leaves
via the export of soybeans and corn
(kg [
] ha
−1) is calculated as:
where
and
refer to the
(kg [
] ha
−1) outcome via the grain export of soybeans and corn, respectively.
2.2. Definition of Internal Fluxes of the
The average annual amount of gross mineralized N from the organic waste of soybean and corn crops (
NMR, kg [N] ha
−1) left in the soil after grain harvest is:
where
refers to the organic N mineralization of the residues of previous crops (soybean and corn) after grain harvest.
represents the annual average mineralized N from the organic root, stem, and leaf residues of the previous crops.
Although considered as an internal flux of the soil–plant system,
represents an extra
supply to the
via mineralization of the organic residues of the previous crops in addition to the entry fluxes
,
,
, and
. Therefore, the total quantity of N supplied to the
, here designated as
, turns out to be:
The average annual amount of organic N (
, kg [N] ha
−1) accumulated in the roots, stems, and leaves of the previous crops of soybean and corn crops until the grain harvest is expressed by the following equation:
where
refers to the organic N accumulated in the roots, stems, and leaves of the predecessor crop of soybeans and
refers to the predecessor crop of corn cultivation.
Considering that
represents the total N extracted from soil by the soybean and corn crops, we have:
Considering that
corresponds to the fraction of the total N extracted by soybean (
) relative to the N accumulated in soybean roots, stems, and leaves, and that
corresponds to the complementary fraction of
NEt,S relative to the N accumulated in soybean grain (
), we have that:
In the same way for corn, we have:
The total accumulated N in grains of soybean and corn (
) is:
and equivalent to the total N that leaves
via grains export is:
2.3. Criteria Assumed in Estimating the Amounts of N in the Soybean-Corn Succession
All N fluxes were estimated on an annual basis within the period from 2008 to 2020 in kg [N] ha
−1 using data extracted from the following primary sources: IBGE (Brazilian Institute of Geography and Statistics—statistical yearbooks) [
26], CONAB (National Supply Company—historical series of soybean and corn harvests) [
30], FAOSTAT (Food and Agriculture Organization Statistical Databases) [
23], IPCC (Intergovernmental Panel on Climate Change) [
31], EMBRAPA (Brazilian Agricultural Research Corporation—third Brazilian inventory of anthropogenic emissions and removals of GHG) [
32], and IFA (International Fertilizer Association) [
22].
Due to the gaps and uncertainties observed in regional agricultural data relating to soybean-corn succession crops in the states of MT and MS, some simplifying assumptions were admitted, as described in the following sub-items. Whenever applicable, N fluxes in
were estimated using the equations and parameters recommended in the “Guidelines” of the Intergovernmental Panel on Climate Change [
31].
Section 2.3.1,
Section 2.3.2,
Section 2.3.3,
Section 2.3.4,
Section 2.3.5,
Section 2.3.6,
Section 2.3.7,
Section 2.3.8 and
Section 2.3.9 detail the assumed calculation criteria.
2.3.1. Amount of N Added via Synthetic Fertilizer
represents the amount of applied synthetic N fertilizers. In Brazil, consumption data on synthetic N fertilizer on a regional scale, by type of crop, are scarce. In the case of soybean-corn succession, such data apply exclusively to corn, given that N fertilizer is not typically required for soybean crops [
33].
Technical guidelines on good practices for the efficient use of N in second season (off-season) corn in succession to soybeans, recommend an application of between 8 and 10 kg [N] ha
−1 for each Mg ha
−1 of expected productivity of grains produced [
34].
Assuming a constant average value of 9 kg [N] Mg
−1 [dry grains] (
), the annual average values of the amount of N (
, kg [N] ha
−1) applied to corn crops (second harvest) are estimated for Brazil from 2008 to 2020 as shown in
Table 1.
where
refers to the annual average corn productivity in the second harvest (off-season) (kg [moist grain] ha
−1) [
26] and
refers to the grain moisture level, 13% [
32].
2.3.2. Amount of Entering through Atmospheric Deposition
The amount of
entering through atmospheric deposition (
) represents the amount of N (kg [
] ha
−1) by atmospheric deposition in the forms
(
O,
, and
) and
(
and
). We assume for the states of MT and MS the following values
and
[
6,
35]: 175 mg [
] m
−2 and 250 mg [
] m
−2, respectively.
Adding these two values and converting mg [] m−2 to kg [] ha−1, we have the estimation of equal to 4 kg [] ha−1, assumed as constant for the whole period from 2008 to 2020.
2.3.3. Amount of N Added via Animal Excreta
The amount of N added via animal excreta () represents the average annual N input flow into the (kg [N] ha−1) via the addition of organic fertilizer from animal excreta. As soybean-corn succession crops are not used for pasturing, we assume as null.
2.3.4. Amount of Entry N via Biological N Fixation
The biological N fixation (
kg [
] ha
−1) represents the average amount of fixed N
2 by the soybean crop in symbiosis with bradyrhizobia [
36] (
Table 2).
where
refers to the extraction of N by the soybean crop assuming 80 kg [
] Mg
−1 [dry grain]),
to the average annual soybean productivity of moist grain (Mg ha
−1) of the soybean crop [
26],
to the grain water content of 13% [
32], and
to the fraction of N extracted from the BNF (assumed to be 0.8 Mg Mg
−1) [
37].
2.3.5. Mineralization of Organic N from Residues of Previous Crops
The average annual amount of gross mineralized N from organic residues from soybean and corn crops (
NMR, kg [N] ha
−1) left in the soil after grain harvest is given by Equation (4), where
is:
and
is the annual fraction (Mg Mg
−1) of the soybeans organic N (
) mineralized [
38]:
Taking
t as the decomposition time in days as 365 results in
= 0.31. From Equation (8), we have:
Assuming that the soybeans extract is 80 kg [
] Mg
−1 [dry grain] (
) [
39], we can estimate the average annual N extraction (
, kg [
] ha
−1):
where
is in Mg ha
−1 [
26] and
is 13% [
32].
Assuming an average N concentration (
) in the soybean grain equal to 50 kg [
] Mg
−1 [dry grain] [
39], we can estimate the average annual N export (
, kg [
] ha
−1):
Substituting
and
into Equation (8), we obtain
, and substituting
and
into Equation (14), we obtain the average annual values of
(kg [
] ha
−1). Applying the same criteria for the corn crop, we have:
where
is the annual fraction (Mg Mg
−1) of corn organic N (
) mineralized [
38]:
Considering
t (decomposition time in days) equal to 365 days, we have
= 0.33. From Equation (9), we have:
Assuming that the corn extracts 25 kg [
] Mg
−1 [dry grain] (
) [
40], we can estimate average annual values of N extraction (
, kg [
] ha
−1):
where
refers to the average annual productivity of humid grain (Mg ha
−1) of the corn crop [
26], and
is 13% [
32].
Assuming an average N concentration
in corn grain equal to 17 kg [
] Mg
−1 [dry grain of second harvest] [
40], we can estimate annual averages of
(kg [
] ha
−1):
Substituting
and
in Equation (9), we obtain
, and substituting
and
in Equation (19), we have
.
ubstituting the values of
and
in Equation (4), we have the average annual estimation of
(
Table 3). During the mineralization of organic residues from soybean and corn crops, a portion of the mineralized N is temporarily immobilized in the soil microbial biomass [
41]. However, when estimating
NMR, immobilized N was disregarded as it undergoes remineralization after the death of microorganisms [
42].
2.3.6. Amount of N Released by Volatilization
represents the
that leaves the system of the soybean-corn succession by volatilization in the molecular forms
,
, and
.
is expressed in N units according to Equation (20) [
31,
35], where:
and
refer to direct emissions of
and
) and
refers to indirect emissions of
.
Direct Emission Estimates of in N Units
Estimates are based on Equation (21)–(23) [
31,
35]:
represents the annual amount of
applied on the soil in the form of synthetic fertilizer (
, kg [
] ha
−1) discounting the volatilized N in the forms
and
, expressed by:
where
refers to the fraction of
applied in the form of urea (equal to 0.5),
to the fraction of
volatilized as
or
(equal to 0.3), and
to the fraction of
applied as other nitrogenous forms (equal to 0.1) [
32].
represents the annual amount of N applied to the soil as animal organic fertilizer (animal manure), discounting the volatilized N as
and
, given by:
Considering that
is null (see
Section 2.3.3), we have
= 0,
where
is the fraction of
volatized as
or
, equal to 0.2 [
31].
By substituting in Equation (26) the values of
,
,
, and
(
Table 1), we obtain the annual estimation of
in kg [
] ha
−1.
represents the amount of N that turns back to the soil as mineralized residues [
31,
35], being equivalent to the annual flux
(
Table 3).
represents the direct emission factor of
2O applied to the amounts of N added annually to the soil, equivalent to 0.01 [
31,
35]. Substituting in Equation (25) the values of
,
,
, and
, we have the estimation of
in kg [
] ha
−1 in the period of 2008 to 2020 (
Table 4).
Estimation of (
Emissions in N Units
Substituting into Equation (28) the values of
,
,
(
Table 1),
,
, and
, we obtain the estimation of
during the period 2008 to 2020 in N units (
Table 4) [
32].
Estimation of
Emissions in N Units
where
represents the
(in N units) stemming from the volatilized
of synthetic fertilizers that is deposited later on the soil [
31,
35].
where
refers to the fraction of
volatilized as
or
in
units, equal to 0.10 [
31], and EF
4 is the factor of direct emission of
, assumed as 0.01 [
31,
35].
Substituting into Equation (26) [
31,
35] the values of
(
Table 1),
,
(null),
, and EF
4, we obtain
~zero (null).
represents the
emmitted from the lixiviated N of
and
[
31,
35].
where
(lixiviated fraction) is equal to 0.30 and EF
5 (emission factor) is equal to 0.025.
Substituting the values of
,
,
, and EF
5 into Equation (31), we find
. Substituting
into Equation (29) and making
equal to zero, we obtain
in N units (
Table 4). Substituting the values of
,
, and
into Equation (24), we obtain the annual estimation of
in kg [
] ha
−1 as well as the annual averages of
emitted by the soybean-corn succession (
+
) in Gg
units (
Table 4).
2.3.7. Amount of N Lost by Lixiviation/Runoff
represents the N that leaves the
by lixiviation/runoff in the form NO
3−, being expressed in kg [
] ha
−1 according to Equation (28) [
31].
where Frac
lix is equal to 0.30 [
32]. Substituting into Equation (32) Frac
lix (0.30), the annual averages of
estimated in
Table 1; and
(null), we obtain the annual results of
during the period from 2008 to 2020, expressed in
Table 5.
2.3.8. Amount of N Output by the Export of Soybean and Corn Grain
The annual average values of N export by soybean and corn (
) can be found in
Section 2.3.5 (
Table 3).
2.3.9. Amount of N Output via the Denitrification Process
The amount of N output via the denitrification process (
) represents the average annual volatilized N from
in the form
via denitrification in N units (kg [N] ha
−1). Denitrification is a microbial process that reduces nitrates (NO
3−) and nitrites (NO
2−) to dinitrogen (N
2), with nitrous oxide (N
2O) being a byproduct [
43,
44]. N
2 emission rates in agricultural soils are quite variable, and their quantification is difficult due to the high concentration of this gas in the atmosphere [
45]. Long-term data on N
2 emissions from agricultural soils are scarce [
44]. Another study has shown results of the ratio between N
2 and N
2O volatilized from agricultural soils via denitrification varying between 5.6 and 7.4 [
46]. Assuming that: (a) the ratio N
2/N
2O in
is constant and equal to 6.5 (average value between 5.6 and 7.4); and (b) the average annual volatilized N
2O is also constant and equal to 1.4 kg [N] ha
−1, the value estimated in
Table 4 by adding (N
2O
d + N
2O
i), we obtain
(average annual N
2 released from
by denitrification in N units) equal to 9 kg [N] ha
−1. This value was assumed to be constant throughout the period of 2008–2020.
2.4. Agro-Environmental Indexes of the
The agro-environmental indexes of the were defined as follows.
2.4.1. Mass Balance of N
The mass balance of nitrogen (|
|) is the difference between
(average annual N added to the
) (Equation (5)) and
(average annual N leaving the
(Equation (2)), expressed in kg [N] ha
−1, according to Equation (33):
2.4.2. Gross Anthropogenic Reactive N
The gross anthropogenic reactive N (
) is equal to the average annual rate of the conversion of atmospheric N
2 to Nr in
, given by the sum of N fixed via synthetic fertilizer (
) plus biologically fixed N (
QNFB), expressed in kg [N] ha
−1, according to Equation (34):
2.4.3. Net Anthropogenic Reactive N
Anthropogenic reactive net N (
) is equal to the difference between
(
) and
(average annual rate of reconversion of
r to atmospheric
by denitrification), expressed in kg [N] ha
−1, according to Equation (35):
2.4.4. N Lost
lost (
, kg [
] ha
−1) represents the outlet flux of N from the
by volatilization (
), lixiviation (
), and denitrification (
), defined by:
2.4.5. Agronomic Efficiency of N Use
The agronomic efficiency of N use (
) [
47] represents the ratio between the exported N during grain harvest (soybean and corn) and the N added to the
(
), expressed by:
2.4.6. Efficiency of Retention of N Added
The Efficiency of retention of N added (
) represents the ratio between N retained in the
and N added, expressed by:
2.4.7. Average Annual Productivity of Soybeans
The average annual soybean () and corn () productivity in the is expressed as kg [grains] ha−1.
2.4.8. Amount of Emitted
The Amount of leaving the by denitrification is expressed in Gg [].
The symbols of all equations presented in the manuscript are summarized in
Appendix A.
4. Conclusions
The average annual results of the N balance (||) and N lost () indicators, respectively, +57 kg [N] ha−1 and 30 kg [N] ha−1, point to a persistent condition of accumulation and loss of N in by volatilization, leaching/runoff, and denitrification.
The average annual results of the agronomic efficiency () and N retention () indicators, 0.71 and 0.90, respectively, reflect the negative effects of N accumulation and loss in the .
Despite the results of and representing factors limiting efficiency in the use of N, there are spaces of opportunity in the soybean-corn succession system for the implementation of management strategies aimed at reducing || and with increased efficiencies and .
The use of experiments in control plots in which it is possible to correlate the results of the experiments with gains in efficiency in the use of N can be an effective strategy in the medium and long term.
Two key questions about the environmental sustainability of must be considered in future research: The first concerns the “cascading effect” caused by anthropogenic Nr created in the and added to the global N cycle (Nran), around 204 kg [N] ha−1. This effect tends to increase, given the expectation of expansion of the soybean-corn succession agricultural model, especially in Latin America and other similar tropical environments; The second question refers to the average annual emission of N2O, here estimated at ~11 Gg [N2O] for the total area studied.