Cytokinin Biosynthesis Is Affected by Selenium and Nitrate Availabilities to Regulate Shoot and Root Growth in Rice Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions and Experimental Design
2.2. Vegetative Growth Assessment
2.3. Measurements of Nitrate, Selenium, and Sugars
2.4. Cytokinin Analysis
2.5. Real-Time Quantitative PCR (RT-qPCR)
2.6. Statistical Analysis
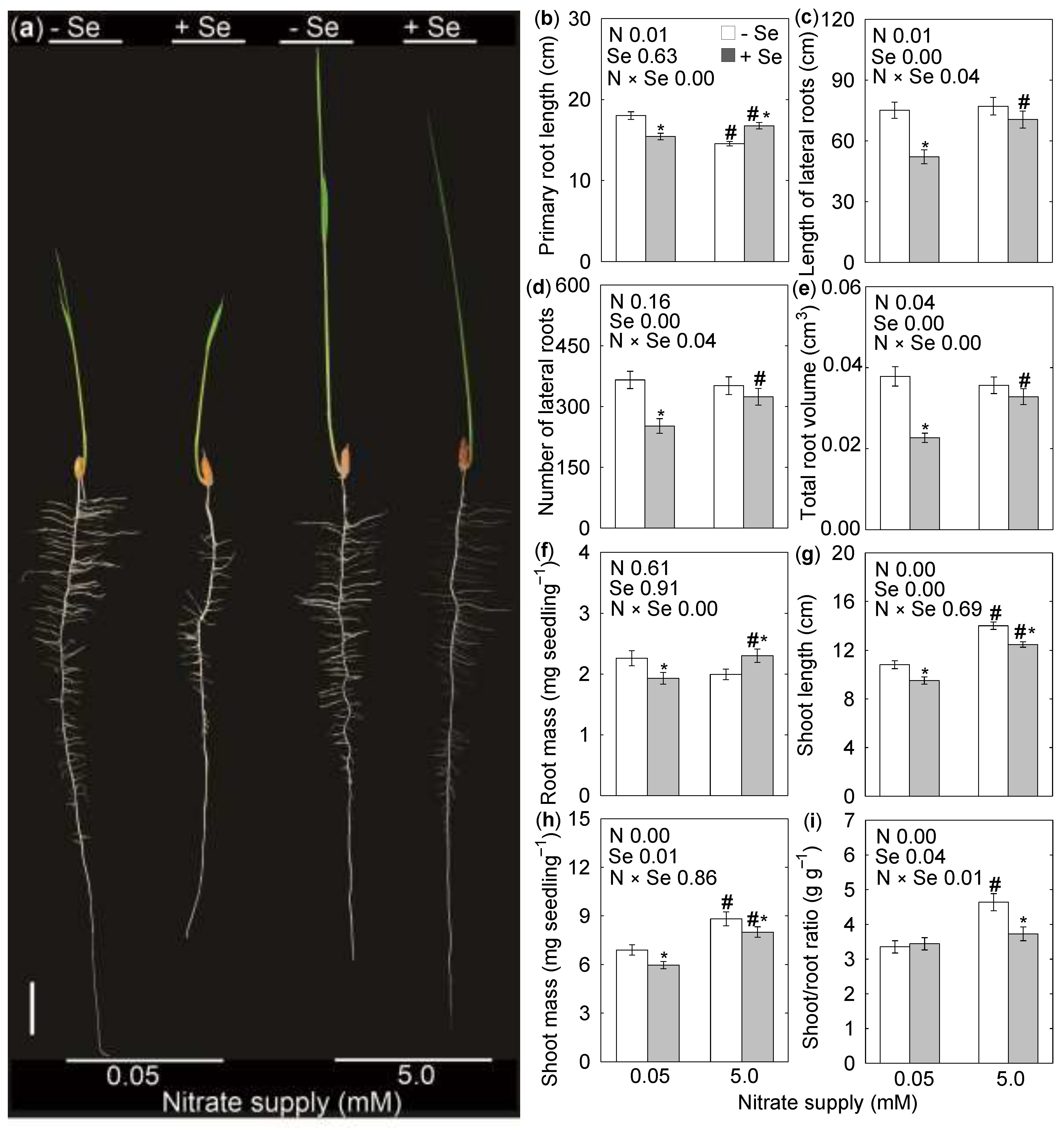
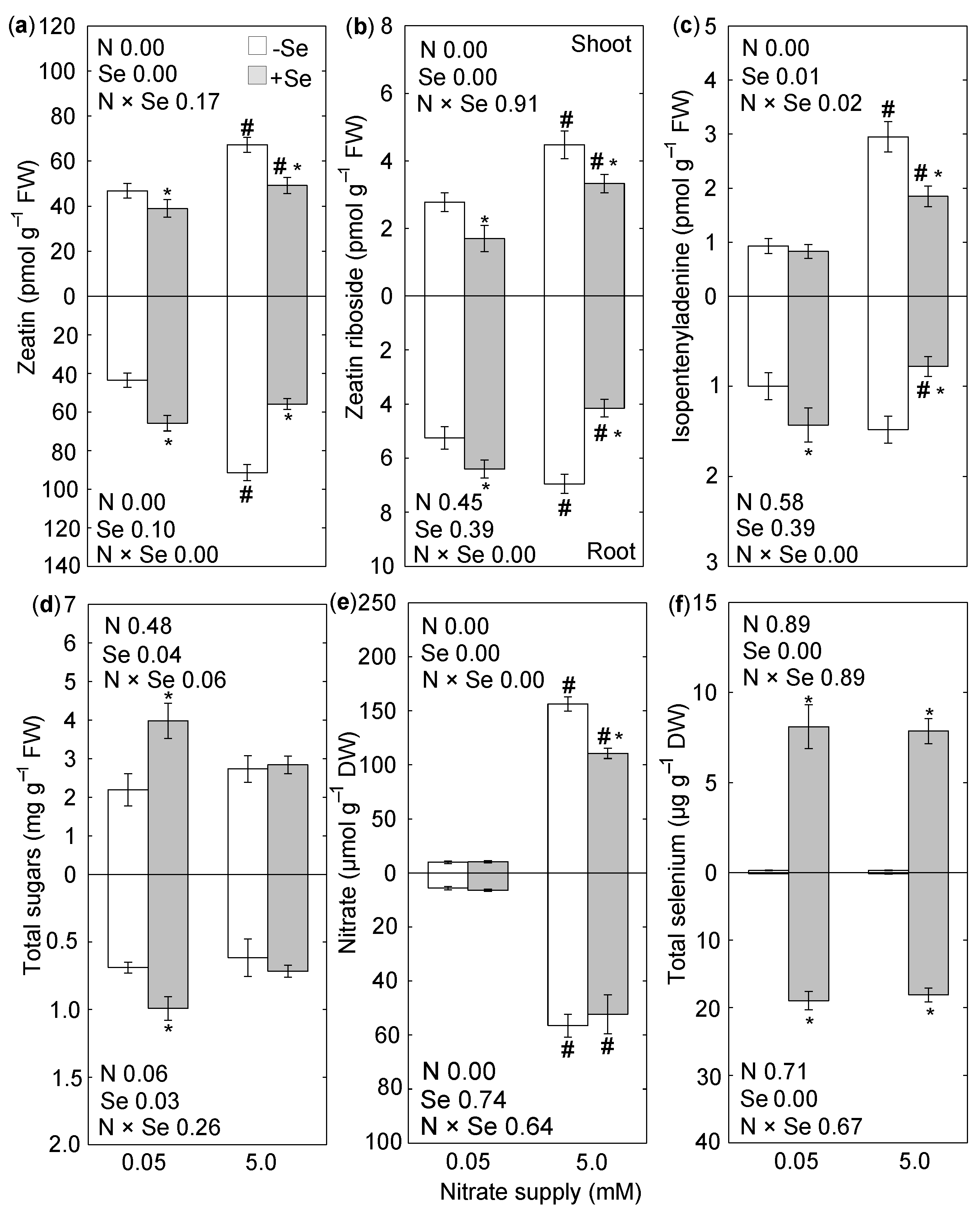
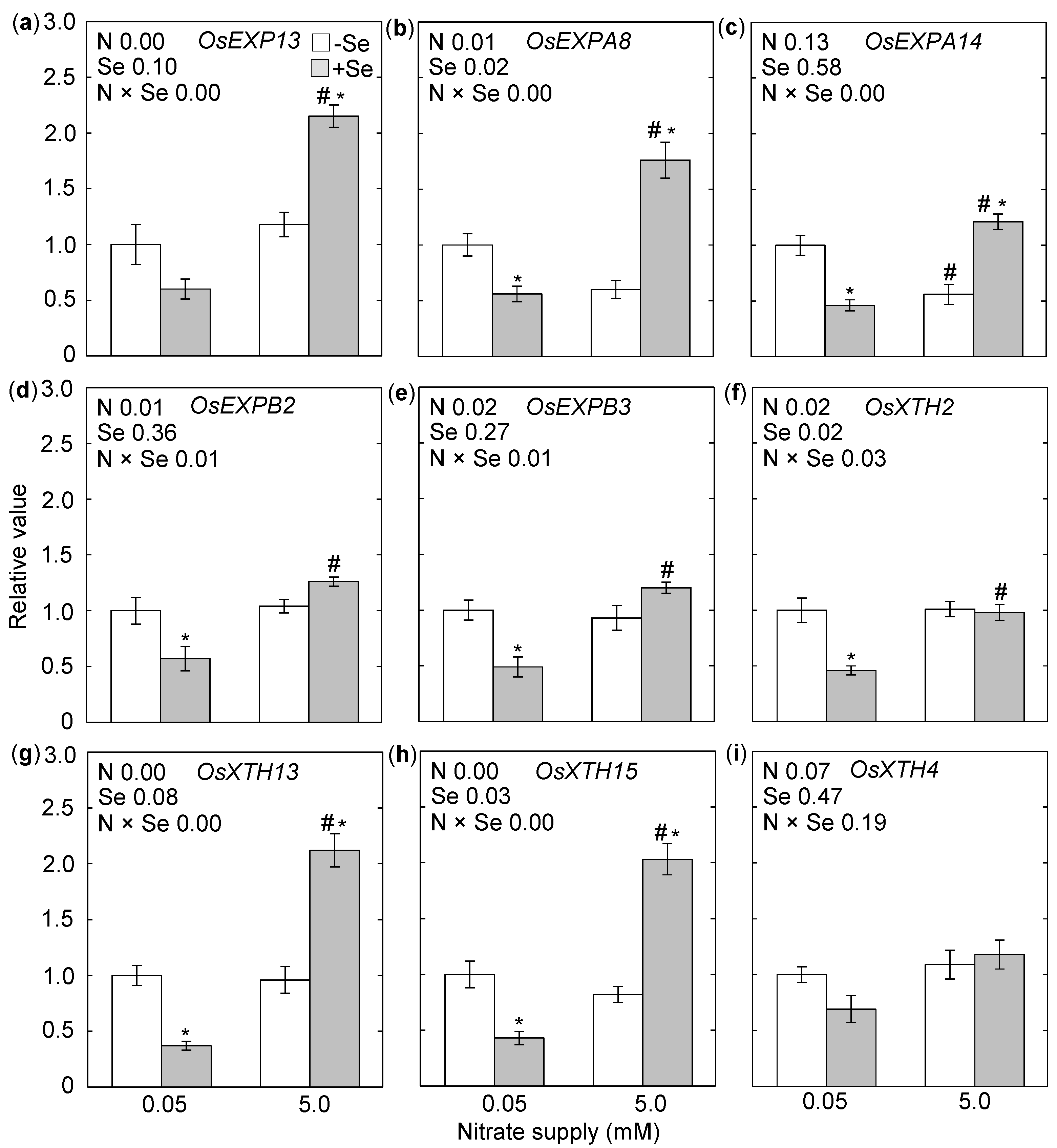
3. Results
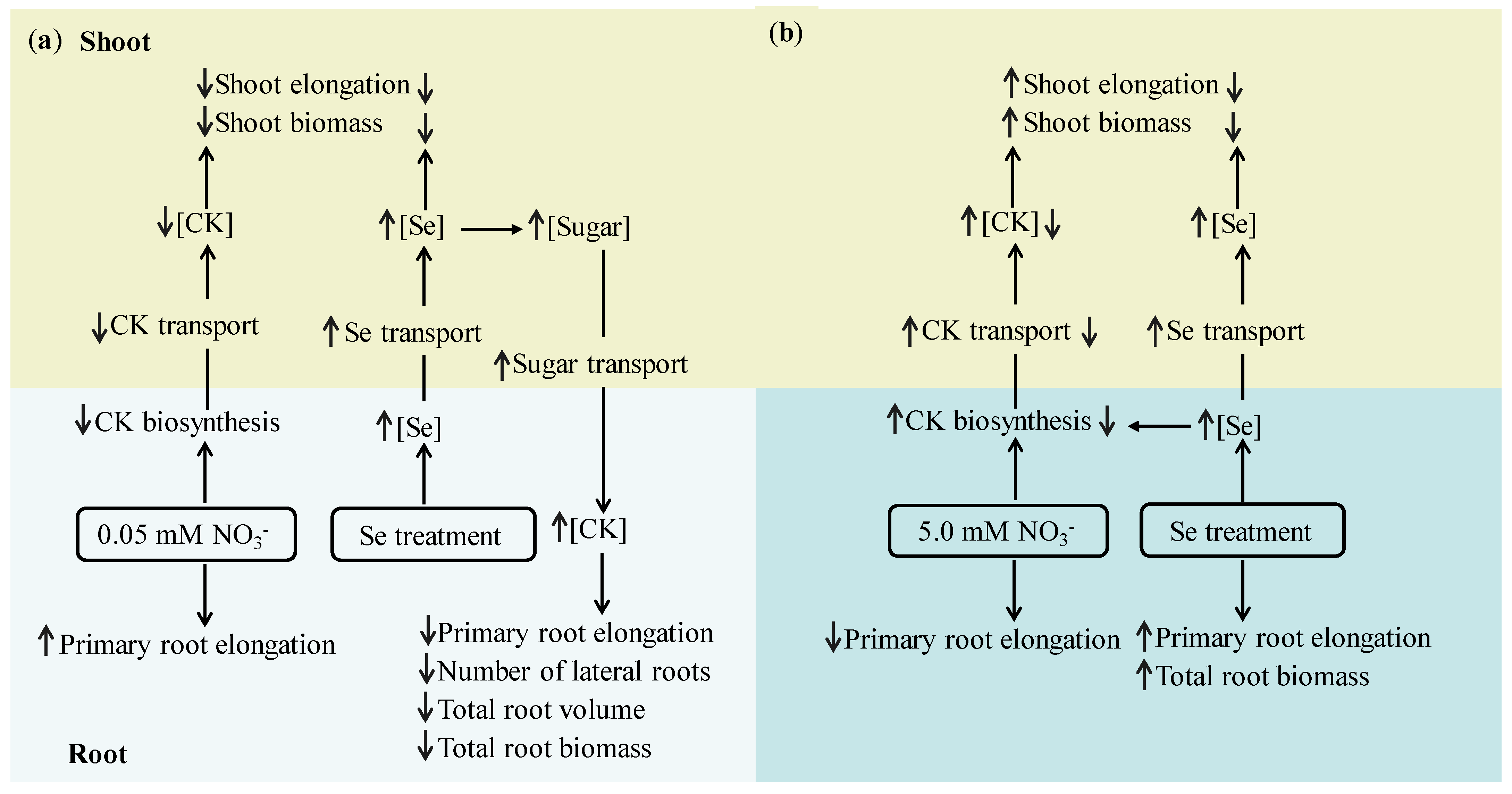
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, Y.; Zou, X.; Li, F.; Zhang, J.; Kang, Z.; Li, X.; Yin, C.; Lin, Y. Nitrogen Deficiency-Induced Decrease in Cytokinins Content Promotes Rice Seminal Root Growth by Promoting Root Meristem Cell Proliferation and Cell Elongation. Cells 2020, 9, 916. [Google Scholar] [CrossRef]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the Crossroads of abiotic stress signalling pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Cai, L.; Li, X.; Fahad, S.; Wang, D. Influence of cultivation practices on the metabolism of cytokinin and its correlation in rice production. Food Energy Secur. 2023, 12, 488. [Google Scholar] [CrossRef]
- Poitout, A.; Crabos, A.; Petřík, I.; Novák, O.; Krouk, G.; Lacombe, B.; Ruffel, S. Responses to systemic nitrogen signaling in Arabidopsis roots involve trans-zeatin in shoots. Plant Cell 2018, 30, 1243–1257. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Galindo-Castañeda, T.; Schneider, H.M.; Sidhu, J.S.; Rangarajan, H.; York, L.M. Root phenotypes for improved nitrogen capture. Plant Soil, 2023; Advance online publication. [Google Scholar] [CrossRef]
- Scheible, W.-R.; Lauerer, M.; Schulze, E.-D.; Caboche, M.; Stitt, M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997, 11, 671–691. [Google Scholar] [CrossRef]
- Puig, J.; Pauluzzi, G.; Guiderdoni, E.; Gantet, P. Regulation of shoot and root development through mutual signaling. Mol. Plant 2012, 5, 974–983. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.; Branco, F.J. Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier: London, UK, 2023; pp. 201–281. [Google Scholar] [CrossRef]
- Malheiros, R.S.P.; Costa, L.C.; Ávila, R.T.; Pimenta, T.M.; Teixeira, L.S.; Brito, F.A.L.; Zsögön, A.; Araújo, W.L.; Ribeiro, D.M. Selenium downregulates auxin and ethylene biosynthesis in rice seedlings to modify primary metabolism and root architecture. Planta 2019, 250, 333–345. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Silva Júnior, D.D.; Cardoso, F.B.; Martins, A.O.; Silva, W.A.; Nascimento, V.L.; Araújo, W.L. Growth inhibition by selenium is associated with changes in primary metabolism and nutrient levels in Arabidopsis thaliana. Plant Cell Environ. 2016, 39, 2235–2246. [Google Scholar] [CrossRef]
- Dimkovikj, A.; Van Hoewyk, D. Selenite activates the alternative oxidase pathway and alters primary metabolism in Brassica napus roots: Evidence of a mitochondrial stress response. BMC Plant Biol. 2014, 14, 259. [Google Scholar] [CrossRef]
- Lima, L.W.; Pilon-Smits, E.A.H.; Schiavon, M. Mechanisms of selenium hyperaccumulation in plants: A survey of molecular, biochemical and ecological cues. BBA—Gen. Subj. 2018, 1862, 2343–2353. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Guo, Y.; Li, H. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnology 2020, 18, 103. [Google Scholar] [CrossRef]
- Lehotai, N.; Kolbert, Z.; Peto, A.; Feigl, G.; Ordog, A.; Kumar, D.; Tari, I.; Erdei, L. Selenite-induced hormonal and signalling mechanisms during growth of Arabidopsis thaliana L. J. Exp. Bot. 2012, 63, 5677–5687. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, R.S.P.; Gonçalves, F.C.M.; Brito, F.A.L.; Zsögön, A.; Ribeiro, D.M. Selenomethionine induces oxidative stress and modifies growth in rice (Oryza sativa L.) seedlings through effects on hormone biosynthesis and primary metabolism. Ecotoxicol. Environ. Saf. 2020, 189, 109942. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants Without Soils; California Agricultural Experimental Station: Berkeley, CA, USA, 1950. [Google Scholar]
- Zhu, J.; Kaeppler, S.; Lynch, J.P. Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor. Appl. Genet. 2005, 111, 688–695. [Google Scholar] [CrossRef] [PubMed]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H.; Schiavon, M. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and non-hyperaccumulator Brassicaceae. New Phytol. 2018, 217, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.M.; von Korff, M.; Altmann, T.; Bartzetko, L.; Sulpice, R.; Gibon, Y.; Palacios, N.; Stitt, M. Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol. 2006, 142, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, J.; Sun, H.; Huang, S.; Gong, X.; Ma, Q.; Zhang, Y.; Xu, G. Increased photosynthetic capacity in response to nitrate is correlated with enhanced cytokinin levels in rice cultivar with high responsiveness to nitrogen nutrients. Plant Soil. 2013, 373, 981–993. [Google Scholar] [CrossRef]
- Li, X.; Yang, D.-L.; Sun, L.; Li, Q.; Mao, B.; He, Z. The systemic acquired resistance regulator OsNPR1 attenuates growth by repressing auxin signaling through promoting IAA-Amido synthase expression. Plant Physiol. 2016, 172, 546–558. [Google Scholar] [CrossRef]
- Figueiredo, D.D.; Barros, P.M.; Cordeiro, A.M.; Serra, T.S.; Lourenço, T.; Chander, S.; Oliveira, M.M.; Saibo, N.J. Seven zinc-finger transcription factors are novel regulators of the stress responsive gene OsDREB1B. J. Exp. Bot. 2012, 63, 3643–3656. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kamada-Nobusada, T.; Makita, N.; Kojima, M.; Sakakibara, H. Nitrogen-dependent regulation of de novo cytokinin biosynthesis in rice: The role of glutamine metabolism as an additional signal. Plant Cell Physiol. 2013, 54, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Takebayashi, Y.; Kojima, M.; Sakakibara, H. Sugar-induced de novo cytokinin biosynthesis contributes to Arabidopsis growth under elevated CO2. Sci. Rep. 2019, 9, 7765. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H. Cytokinin biosynthesis and transport for systemic nitrogen signaling. Plant J. 2021, 105, 421–430. [Google Scholar] [CrossRef]
- Sakakibara, H.; Hayakawa, A.; Deji, A.; Gawronski, S.W.; Sugiyama, T. His-Asp phosphotransfer possibly involved in the nitrogen signal transduction mediated by cytokinin in maize: Molecular cloning of cDNAs for two-component regulatory factors and demonstration of phosphotransfer activity in vitro. Plant Mol. Biol. 1999, 41, 563–573. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Pimenta, T.M.; Brito, F.A.L.; Malheiros, R.S.P.; Arruda, R.S.; Araújo, W.L.; Ribeiro, D.M. Selenium uptake and grain nutritional quality are affected by nitrogen fertilization in rice (Oryza sativa L.). Plant Cell Rep. 2021, 40, 871–880. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, L.S.; Mota, T.A.L.; Lopez, D.J.C.; Amorim, V.A.; Almeida, C.S.; Souza, G.A.; Ribeiro, D.M. Cytokinin Biosynthesis Is Affected by Selenium and Nitrate Availabilities to Regulate Shoot and Root Growth in Rice Seedlings. Nitrogen 2024, 5, 191-201. https://doi.org/10.3390/nitrogen5010013
Teixeira LS, Mota TAL, Lopez DJC, Amorim VA, Almeida CS, Souza GA, Ribeiro DM. Cytokinin Biosynthesis Is Affected by Selenium and Nitrate Availabilities to Regulate Shoot and Root Growth in Rice Seedlings. Nitrogen. 2024; 5(1):191-201. https://doi.org/10.3390/nitrogen5010013
Chicago/Turabian StyleTeixeira, Lubia S., Thiago A. L. Mota, Deisy J. C. Lopez, Victor A. Amorim, Carla S. Almeida, Genaina A. Souza, and Dimas M. Ribeiro. 2024. "Cytokinin Biosynthesis Is Affected by Selenium and Nitrate Availabilities to Regulate Shoot and Root Growth in Rice Seedlings" Nitrogen 5, no. 1: 191-201. https://doi.org/10.3390/nitrogen5010013
APA StyleTeixeira, L. S., Mota, T. A. L., Lopez, D. J. C., Amorim, V. A., Almeida, C. S., Souza, G. A., & Ribeiro, D. M. (2024). Cytokinin Biosynthesis Is Affected by Selenium and Nitrate Availabilities to Regulate Shoot and Root Growth in Rice Seedlings. Nitrogen, 5(1), 191-201. https://doi.org/10.3390/nitrogen5010013





