The Pyla-1 Natural Accession of Arabidopsis thaliana Shows Little Nitrate-Induced Plasticity of Root Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Culture
2.3. Two-Dimensional Root Morphology Analysis
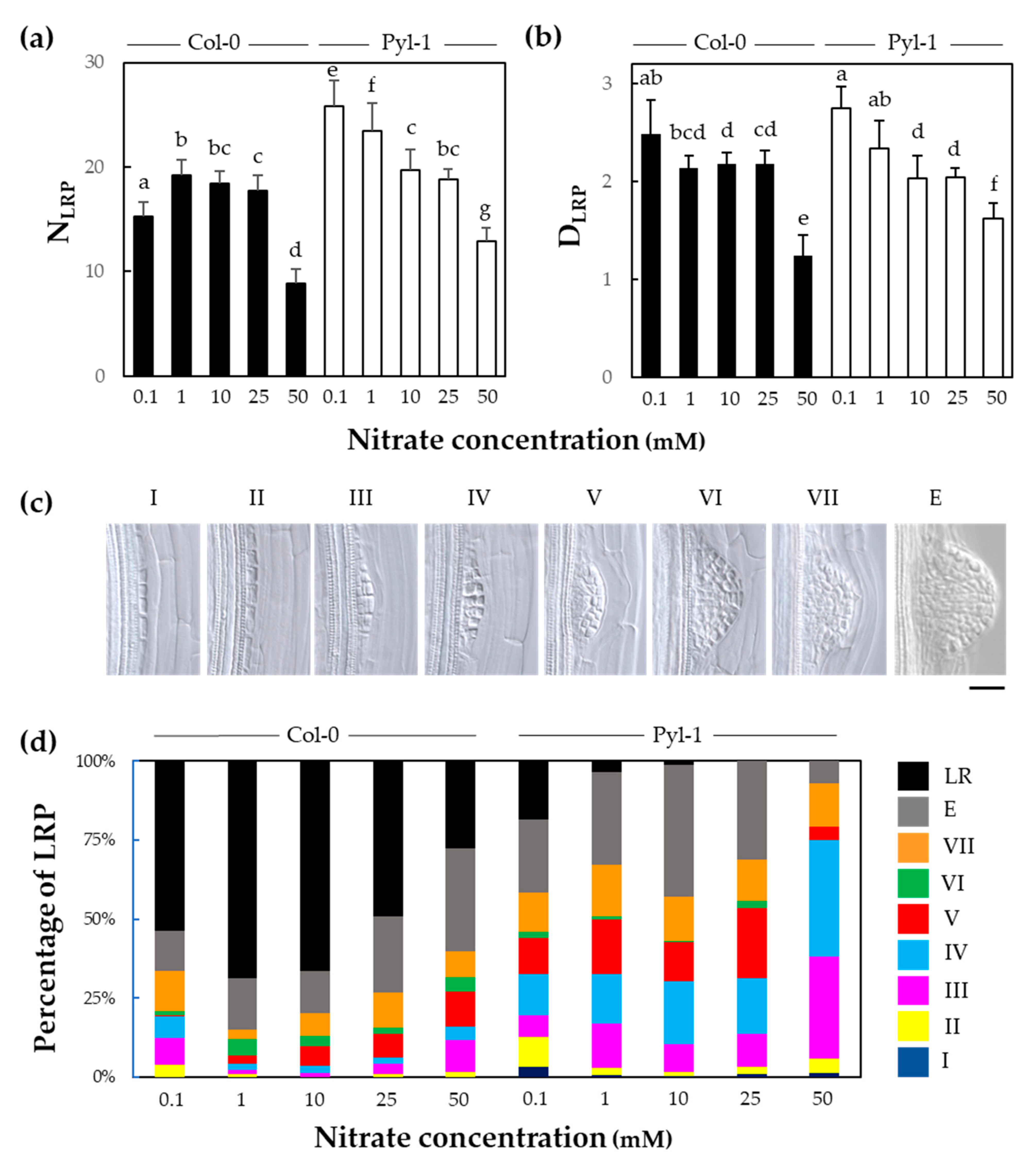
2.4. Quantification of Lateral Root Primordium Developmental Stages
2.5. Lateral Root Bending
2.6. Statistical Treatment
3. Results
3.1. The Pyla-1 Accession Exhibited Little Macroscopic Variation of Root Morphology in Response to Nitrate Supply
3.2. The Initiation of Lateral Root Primordium Was Not Impaired in Pyla-1
3.3. Lateral Root Primordia Exhibit Slower Organ Emergence in Pyla-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walling, E.; Vaneeckhaute, C.J. Greenhouse gas emissions from inorganic and organic fertilizer production and use: A review of emission factors and their variability. J. Environ. Manage. 2020, 276, 111211. [Google Scholar] [CrossRef]
- Picetti, R.; Deeney, M.; Pastorino, S.; Miller, M.R.; Shah, A.; Leon, D.A.; Dangour, A.D.; Green, R. Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ. Res. 2022, 210, 112988. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Popova, A.A.; Shestibratov, K.A. Genetic engineering and genome editing for improving nitrogen use efficiency in plants. Cells 2021, 10, 3303. [Google Scholar] [CrossRef]
- Javed, T.; Indu, I.; Singhal, R.K.; Shabbir, R.; Shah, A.N.; Kumar, P.; Jinger, D.; Dharmappa, P.M.; Shad, M.A.; Saha, D.; et al. Recent advances in agronomic and physio-molecular approaches for improving nitrogen use effiency in crop plants. Front. Plant. Sci. 2022, 13, 877544. [Google Scholar] [CrossRef]
- Whetton, R.L.; Harty, M.A.; Holden, N.M. Communicating nitrogen loss mechanisms for improving nitrogen use efficiency management, focused on global wheat. Nitrogen 2022, 3, 213–246. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef]
- Asim, M.; Ullah, Z.; Xu, F.; An, L.; Aluko, O.O.; Wang, Q.; Liu, H. Nitrate signaling, functions, and regulation of root system architecture: Insights from Arabidopsis thaliana. Genes 2020, 11, 633. [Google Scholar] [CrossRef]
- Louvieaux, J.; Spanoghe, M.; Hermans, C. Root morphological traits of seedlings are predictors of seed yield and quality in winter oilseed rape hybrid cultivars. Front. Plant. Sci. 2020, 11, 568009. [Google Scholar] [CrossRef]
- Kupcsik, L.; Chiodi, C.; Moturu, T.R.; De Gernier, H.; Haelterman, L.; Louvieaux, J.; Tillard, P.; Sturrock, C.J.; Bennett, M.; Nacry, P.; et al. Oilseed rape cultivars show diversity of root morphologies with the potential for better capture of nitrogen. Nitrogen 2021, 2, 491–505. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; An, T.; Chen, Y.J. Lateral root elongation enhances nitrogen-use efficiency in maize genotypes at the seedling stage. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Casimiro, I.; Marchant, A.; Bhalerao, R.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inzé, D.; Sandber, G.; Casero, P.; et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant. Cell 2001, 13, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Dubrovsky, J.G.; Rost, T.L.; Colón-Carmona, A.; Doerner, P. Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta 2001, 214, 30–36. [Google Scholar] [CrossRef]
- Du, Y.; Scheres, B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot. 2018, 69, 155–167. [Google Scholar] [CrossRef]
- Lucas, M.; Kenobi, K.; von Wangenheim, D.; Voβ, U.; Swarup, K.; De Smet, I.; Van Damme, D.; Lawrence, T.; Péret, B.; Moscardi, E.; et al. Lateral root morphogenesis is dependent on the mechanical properties of the overlaying tissues. Proc. Natl. Acad. Sci. USA 2013, 110, 5229–5234. [Google Scholar] [CrossRef] [Green Version]
- Porco, S.; Larrieu, A.; Du, Y.; Gaudinier, A.; Goh, T.; Swarup, K.; Swarup, R.; Kuempers, B.; Bishopp, A.; Lavenus, J.; et al. Lateral root emergence in Arabidopsis is dependent on transcription factor LBD29 regulation of auxin influx carrier LAX3. Development 2016, 143, 3340–3349. [Google Scholar]
- Malamy, J.E.; Benfey, P.N. Down and out in Arabidopsis: The formation of lateral roots. Trends Plant. Sci. 1997, 2, 390–396. [Google Scholar] [CrossRef]
- Szymanowska-Pulka, J. Form matters: Morphological aspects of lateral root development. Ann. Bot. 2013, 112, 1643–1654. [Google Scholar] [CrossRef]
- Fredes, I.; Moreno, S.; Díaz, F.P.; Gutiérrez, R.A. Nitrate signaling and the control of Arabidopsis growth and development. Curr. Opin. Plant. Biol. 2019, 47, 112–118. [Google Scholar] [CrossRef]
- Pélissier, P.-M.; Motte, M.; Beeckman, T. Lateral root formation and nutrients: Nitrogen in the spotlight. Plant. Physiol. 2021, 187, 1104–1116. [Google Scholar] [CrossRef]
- McKhann, H.I.; Camilleri, C.; Bérard, A.; Bataillon, T.; David, J.L.; Reboud, X.; Le Corre, V.; Caloustian, C.; Gut, I.G.; Brunel, D. Nested core collections maximizing genetic diversity in Arabidopsis thaliana. Plant. J. 2004, 38, 193–202. [Google Scholar] [CrossRef]
- De Pessemier, J.; Chardon, F.; Juraniec, M.; Delaplace, P.; Hermans, C. Natural variation of the root morphological response to nitrate supply in Arabidopsis thaliana. Mech. Dev. 2013, 130, 45–53. [Google Scholar] [CrossRef]
- De Pessemier, J.; Moturu, T.R.; Nacry, P.; Ebert, R.; De Gernier, H.; Tillard, P.; Swarup, K.; Wells, D.M.; Haseloff, J.; Murray, S.C.; et al. Root system size and root hair length are key phenes for nitrate acquisition and biomass production across natural variation in Arabidopsis. J. Exp. Bot. 2022, 73, 3569–3583. [Google Scholar] [CrossRef]
- Pound, M.P.; French, A.P.; Atkinson, J.A.; Wells, D.M.; Bennett, M.J.; Pridmore, T. RootNav: Navigating images of complex root architectures. Plant. Physiol. 2013, 162, 1802–1814. [Google Scholar] [CrossRef] [Green Version]
- Dubrovsky, J.G.; Gambetta, G.A.; Hernández-Barrera, A.; Shishkova, S.; González, I. Lateral root initiation in Arabidopsis: Developmental window, spatial patterning, density and predictability. Ann. Bot. 2006, 97, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Péret, B.; Li, G.; Zhao, J.; Band, L.R.; Voβ, U.; Postaire, O.; Luu, D.-T.; Da Ines, O.; Casimiro, I.; Lucas, M.; et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nature Cell Biol. 2012, 14, 991–998. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 30 June 2022).
- XLSTAT. Statistical Software for Excel. 2007. Available online: https://www.xlstat.com (accessed on 30 June 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. JOSS 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 30 June 2022).
- Sun, C.H.; Yu, Q.; Hu, D.G. Nitrate: A crucial signal during lateral roots development. Front. Plant. Sci. 2017, 8, 485. [Google Scholar] [CrossRef]
- Louvieaux, J.; De Gernier, H.; Hermans, C. Exploiting genetic variability of root morphology as a lever to improve nitrogen use efficiency in oilseed rape. In Engineering Nitrogen Utilization in Crop Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 185–201. [Google Scholar]
- Malamy, J.E.; Benfey, P.N. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 1997, 124, 33–44. [Google Scholar] [CrossRef]
- Fernández-Marcos, M.; Desvoyes, B.; Manzano, C.; Liberman, L.M.; Benfey, P.N.; Del Pozo, J.C.; Gutierrez, C. Control of Arabidopsis lateral root primordium boundaries by MYB36. New Phytol. 2017, 213, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Deja-Muylle, A.; Parizot, B.; Motte, H.; Beeckman, T. Exploiting natural variation in root system architecture via genome-wide association studies. J. Exp. Bot. 2020, 71, 2379–2389. [Google Scholar] [CrossRef]
- Deja-Muylle, A.; Opdenacker, D.; Parizot, B.; Motte, H.; Lobe, G.; Storme, V.; Clauw, P.; Njo, M.; Beeckman, T. Genetic variability of Arabidopsis thaliana mature root system architecture and genome-wide association study. Front. Plant. Sci. 2022, 12, 814110. [Google Scholar] [CrossRef]
- North, K.A.; Ehlting, B.; Koprivova, A.; Rennenberg, H.; Kopriva, S. Natural variation in Arabidopsis adaptation to growth at low nitrogen conditions. Plant. Physiol. Biochem. 2009, 47, 912–918. [Google Scholar] [CrossRef]
- Chardon, F.; Barthélémy, J.; Daniel-Vedele, F.; Masclaux-Daubresse, C. Natural variation of nitrate uptake and nitrogen use efficiency in Arabidopsis thaliana cultivated with limiting and ample nitrogen supply. J. Exp. Bot. 2010, 61, 2293–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masclaux-Daubresse, C.; Chardon, F. Exploring nitrogen remobilization for seed filling using natural variation in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 2131–2142. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R.C.; Gryczka, C.; Neitsch, C.; Müller, M.; Bräutigam, A.; Schlereth, A.; Schön, H.; Weigelt-Fischer, K.; Altmann, T. Genetic diversity for nitrogen use efficiency in Arabidopsis thaliana. Planta 2019, 250, 41–57. [Google Scholar] [CrossRef] [Green Version]
- Gifford, M.L.; Banta, J.A.; Katari, M.S.; Hulsmans, J.; Chen, L.; Ristova, D.; Tranchina, D.; Purugganan, M.D.; Coruzzi, G.M.; Birnbaum, K.D. Plasticity regulators modulate specific root traits in discrete nitrogen environments. PLoS Genet. 2013, 9, e1003760. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; von Wirén, N. The root foraging response under low nitrogen depends on DWARF1-mediated brassinosteroid biosynthesis. Plant. Physiol. 2020, 183, 998–1010. [Google Scholar] [CrossRef]
- Satbhai, S.B.; Ristova, D.; Busch, W. Underground tuning: Quantitative regulation of root growth. J. Exp. Bot. 2015, 66, 1099–1112. [Google Scholar] [CrossRef] [Green Version]
- Celenza Jr., J. L.; Grisafi, P.L.; Fink, G.R. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995, 9, 2131–2142. [Google Scholar] [CrossRef] [Green Version]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007, 19, 118–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rybel, B.; Vassileva, V.; Parizot, B.; Demeulenaere, M.; Grunewald, W.; Audenaert, D.; Van Campenhout, J.; Overvoorde, P.; Jansen, L.; Vanneste, S.; et al. A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 2010, 20, 697–706. [Google Scholar] [CrossRef]
- Fukaki, H.; Tameda, S.; Masuda, H.; Tasaka, M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant. J. 2022, 29, 153–168. [Google Scholar] [CrossRef]
- Lavenus, J.; Goh, T.; Roberts, I.; Guyomarc'h, S.; Lucas, M.; De Smet, I.; Fukaki, H.; Beeckman, T.; Bennett, M.; Laplaze, L. Lateral root development in Arabidopsis: Fifty shades of auxin. Trends Plant. Sci. 2013, 18, 450–458. [Google Scholar] [CrossRef]
- Swarup, K.; Benková, E.; Swarup, R.; Casimiro, I.; Péret, B.; Yang, Y.; Parry, G.; Nielsen, E.; De Smet, I.; Vanneste, S.; et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 2008, 10, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, D.; Voss, U.; Harding, S.; Fannon, J.; Moody, L.; Yamada, E.; Swarup, K.; Nibau, C.; Bassel, G.; Choudhary, A.; et al. AtMYB93 is a novel negative regulator of lateral root development in Arabidopsis. New Phytol. 2014, 4, 1194–1207. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, D.; Coates, J. AtMYB93 is an endodermis-specific transcriptional regulator of lateral root development in Arabidopsis. Plant. Signal. Behav. 2014, 9, e970406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lev-Yadun, S.; Berleth, T. Expanding ecological and evolutionary insights from wild Arabidopsis thaliana accessions. Plant. Signal. Behav. 2009, 4, 796–797. [Google Scholar] [CrossRef] [Green Version]
- Baxter, I.; Brazelton, J.N.; Yu, D.; Huang, Y.S.; Lahner, B.; Yakubova, E.; Li, Y.; Bergelson, J.; Borevitz, J.O.; Nordborg, M.; et al. A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genet. 2010, 6, e1001193. [Google Scholar] [CrossRef] [Green Version]
- James, G.V.; Patel, V.; Nordström, K.J.; Klasen, J.R.; Salomé, P.A.; Weigel, D.; Schneeberger, K. User guide for mapping-by-sequencing in Arabidopsis. Genome Biol. 2013, 14, R61. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porco, S.; Haelterman, L.; De Pessemier, J.; De Gernier, H.; Reyé, F.; Hermans, C. The Pyla-1 Natural Accession of Arabidopsis thaliana Shows Little Nitrate-Induced Plasticity of Root Development. Nitrogen 2022, 3, 444-454. https://doi.org/10.3390/nitrogen3030029
Porco S, Haelterman L, De Pessemier J, De Gernier H, Reyé F, Hermans C. The Pyla-1 Natural Accession of Arabidopsis thaliana Shows Little Nitrate-Induced Plasticity of Root Development. Nitrogen. 2022; 3(3):444-454. https://doi.org/10.3390/nitrogen3030029
Chicago/Turabian StylePorco, Silvana, Loïc Haelterman, Jérôme De Pessemier, Hugues De Gernier, Florence Reyé, and Christian Hermans. 2022. "The Pyla-1 Natural Accession of Arabidopsis thaliana Shows Little Nitrate-Induced Plasticity of Root Development" Nitrogen 3, no. 3: 444-454. https://doi.org/10.3390/nitrogen3030029
APA StylePorco, S., Haelterman, L., De Pessemier, J., De Gernier, H., Reyé, F., & Hermans, C. (2022). The Pyla-1 Natural Accession of Arabidopsis thaliana Shows Little Nitrate-Induced Plasticity of Root Development. Nitrogen, 3(3), 444-454. https://doi.org/10.3390/nitrogen3030029





