Impact of Plant-Based Amendments on Water-Soluble Nitrogen Release Dynamics in Cultivated Peatlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Amendments
2.3. Experimental Design
2.4. Incubation, Leaching Procedure and Leachate Analyses
2.5. Data Analysis
2.6. Kinetic Model
2.7. Statistical Analysis—ANOVA
3. Results
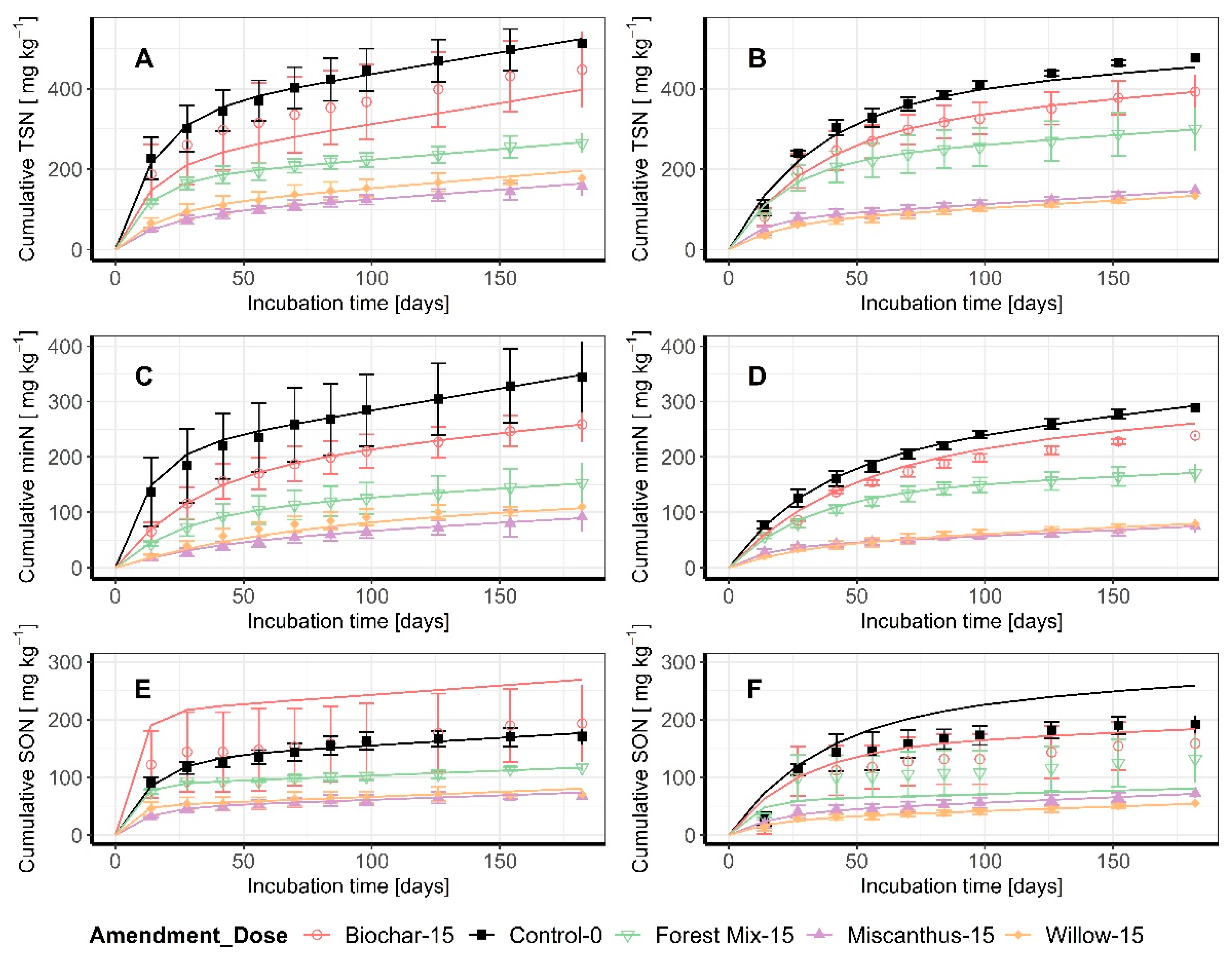
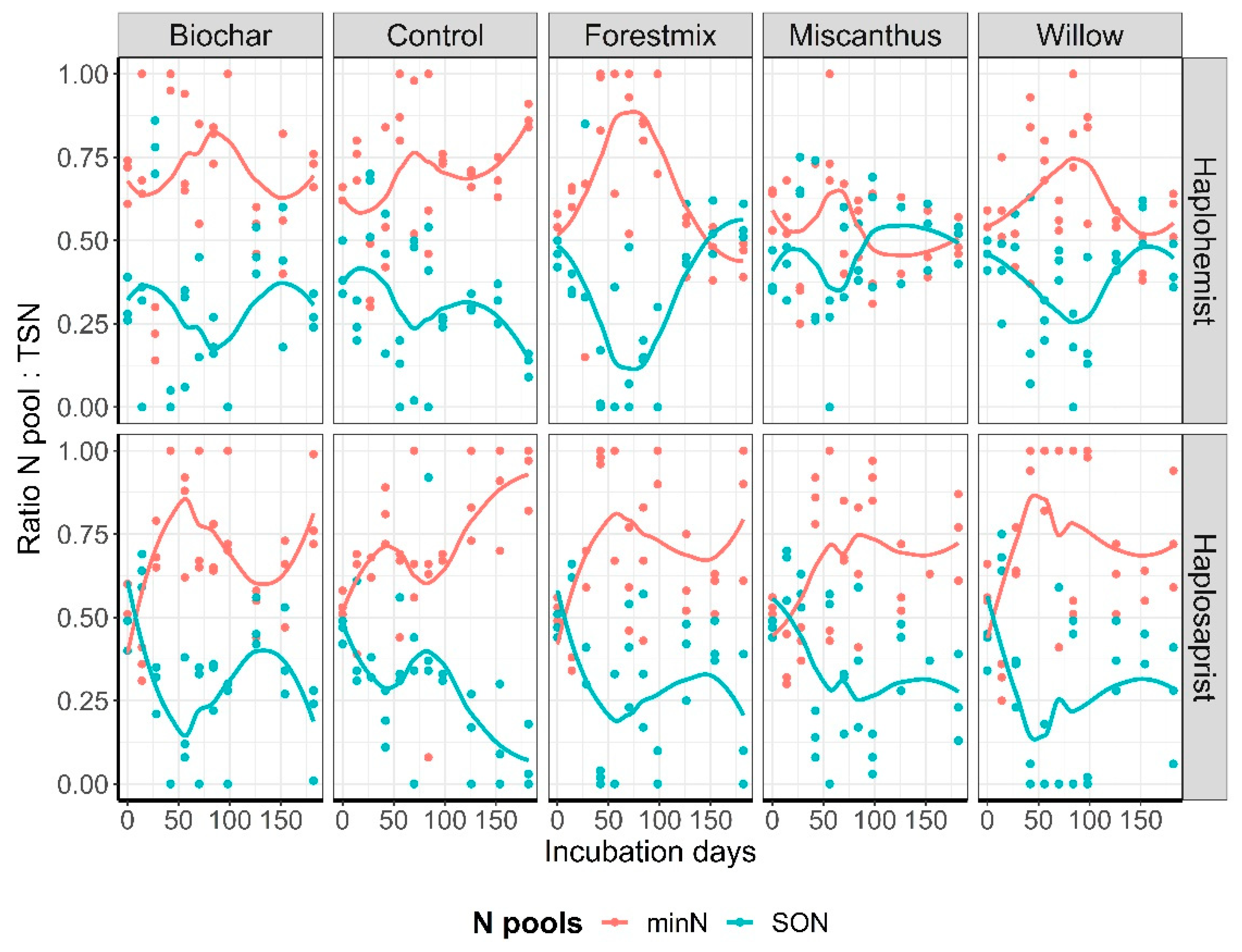
3.1. Release Curves and Kinetic Parameters
3.2. Cumulative Data at the End of the Incubation
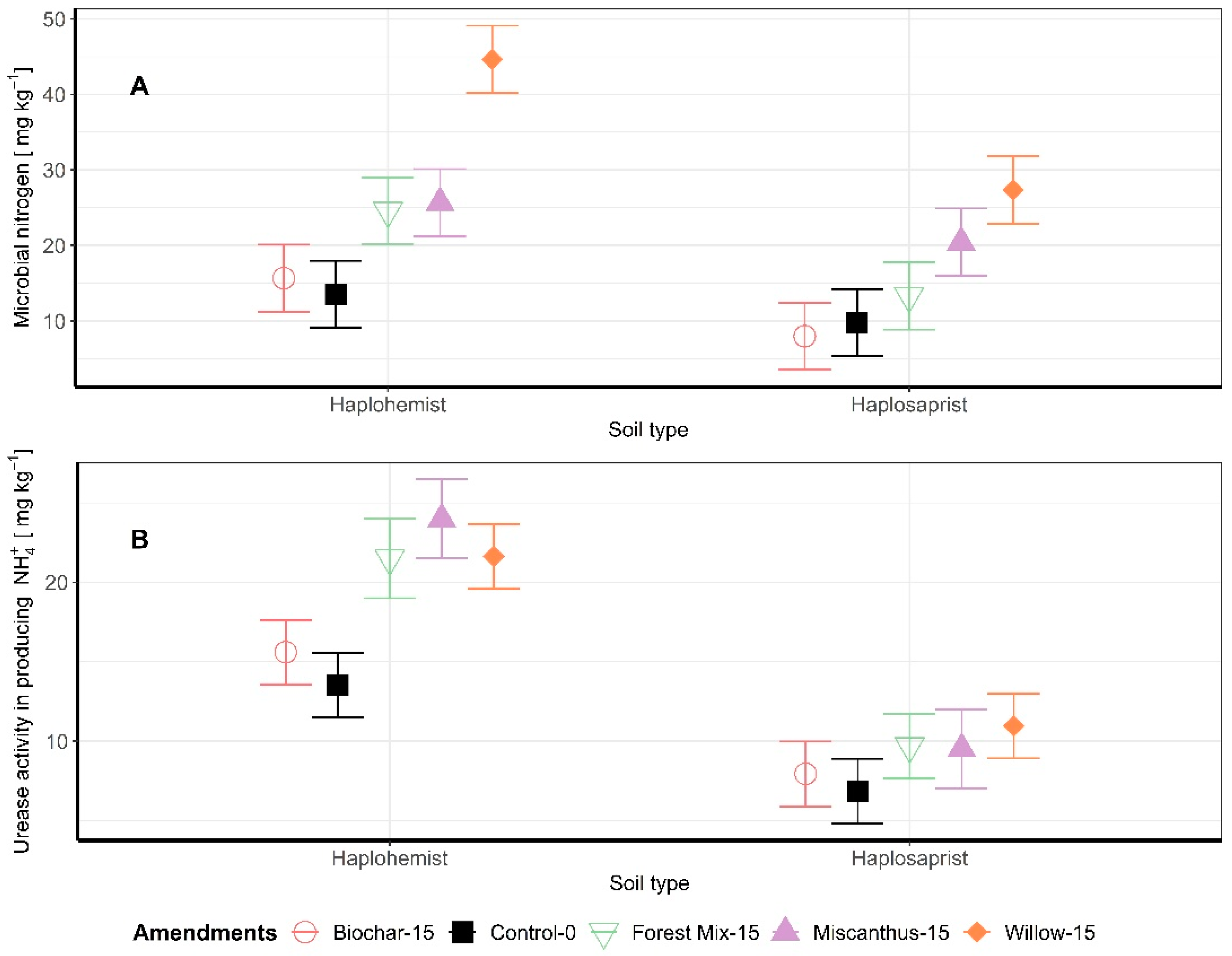
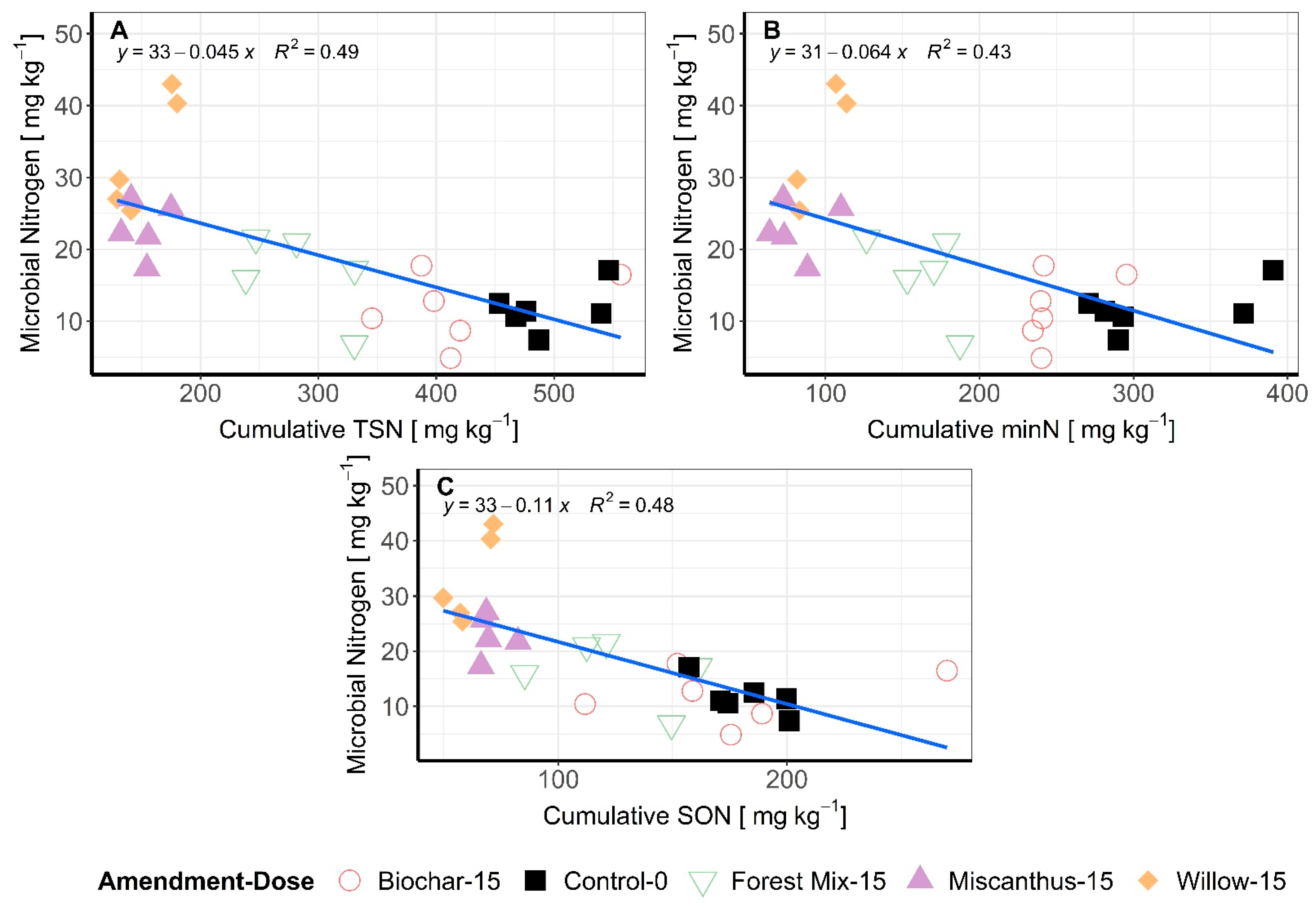
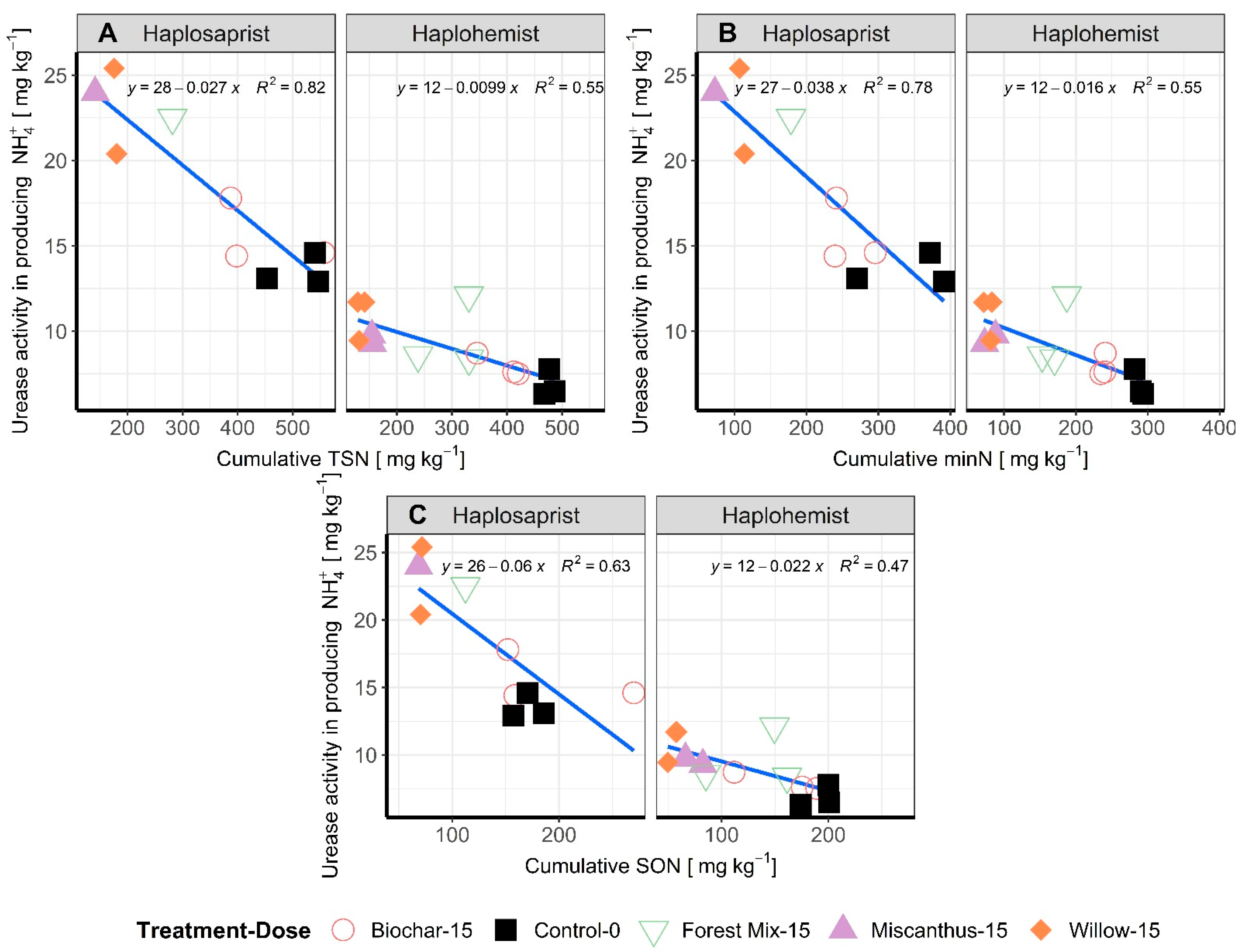
3.3. Microbial Biomass N and Enzymatic Activity
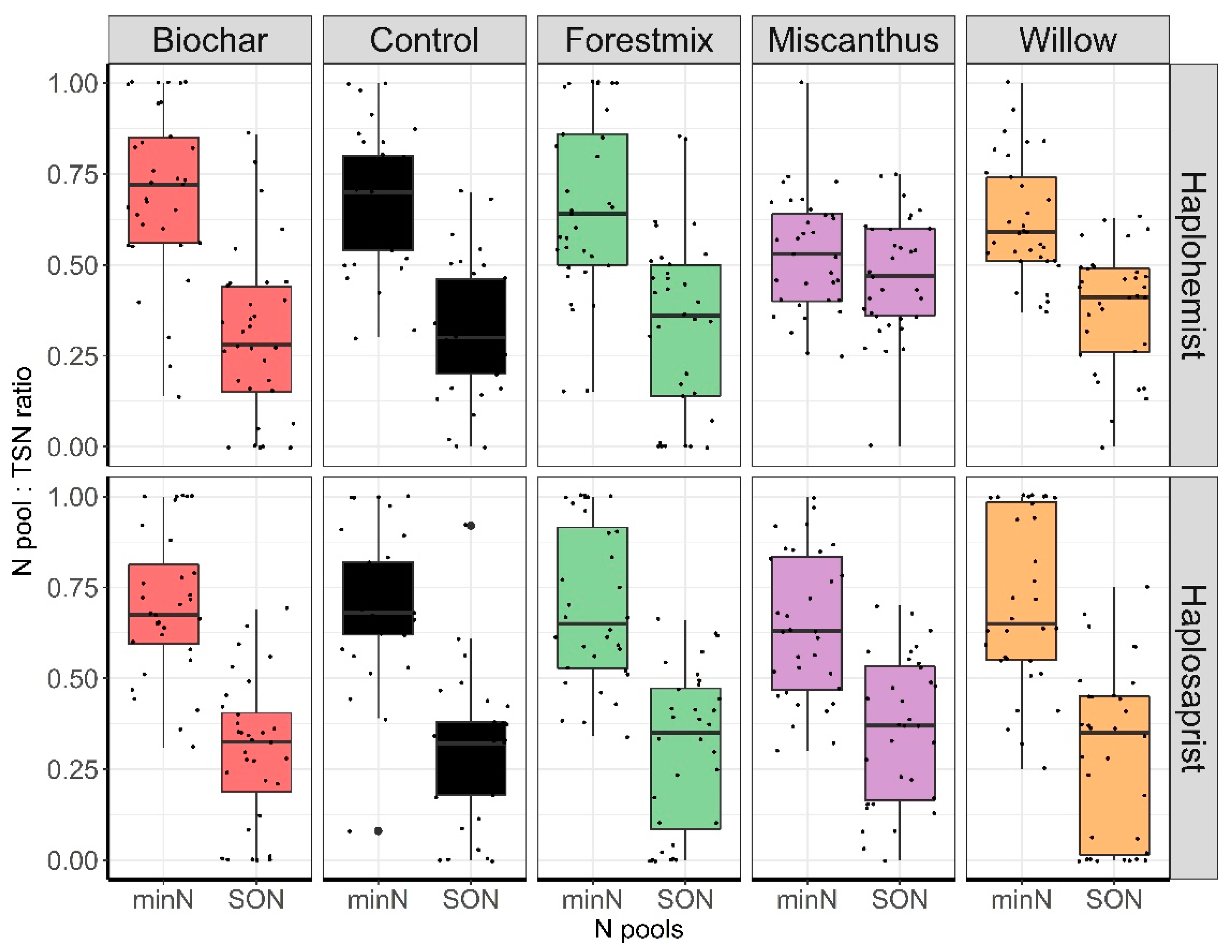
3.4. MinN:TSN Ratios as Influenced by Soil Type and Amendment
4. Discussion
4.1. Plant-Based Amendment Impact on N Pools Release
4.2. Soil N Sink and Microbial Immobilization under Plant-Based Amendement
4.3. N Pools Distribution
4.4. Open Questions and Limitation of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baillie, I. Soil Survey Staff 1999, Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, Agricultural Handbook 436; Wiley Online Library, Ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2001; p. 869.
- Bader, C.; Müller, M.; Szidat, S.; Schulin, R.; Leifeld, J. Response of peat decomposition to corn straw addition in managed organic soils. Geoderma 2018, 309, 75–83. [Google Scholar] [CrossRef]
- Bonn, A.; Allott, T.; Evans, M.; Joosten, H.; Stoneman, R. Peatland restoration and ecosystem services: An introduction. In Peatland Restoration and Ecosystem Services: Science, Policyand Practice; Cambridge University Press: Cambridge, UK, 2016; pp. 1–16. [Google Scholar]
- Bader, C.; Müller, M.; Schulin, R.; Leifeld, J. Amount and stability of recent and aged plant residues in degrading peatland soils. Soil Biol. Biochem. 2017, 109, 167–175. [Google Scholar] [CrossRef]
- Shih, S.; Glaz, B.; Barnes, R., Jr. Subsidence of organic soils in the Everglades Agricultural Area during the past 19 years. Proceedings 1998, 57, 20–29. [Google Scholar]
- Stevens, J.; Johnson, L. Subsidence of organic soils in the upper Everglades region of Florida. Soil Sci. Soc. Fla. Proc. 1951, 11, 191–237. [Google Scholar]
- Dessureault-Rompré, J.; Libbrecht, C.; Caron, J. Biomass crops as a soil amendment in cultivated histosols: Can we reach carbon equilibrium? Soil Sci. Soc. Am. J. 2020, 84, 597–608. [Google Scholar] [CrossRef]
- Armentano, T.V.; Menges, E.S. Patterns of change in the carbon balance of organic soil-wetlands of the temperate zone. J. Ecol. 1986, 74, 755–774. [Google Scholar] [CrossRef]
- Castillo, M.S.; Wright, A.L. Soil phosphorus pools for Histosols under sugarcane and pasture in the Everglades, USA. Geoderma 2008, 145, 130–135. [Google Scholar] [CrossRef]
- Millette, J.; Vigier, B.; Broughton, R.S. An evaluation of the drainage and subsidence of some organic soils in Quebec. Can. Agric. Eng. 1982, 24, 5–10. [Google Scholar]
- Parent, L.; Millette, J.; Mehuys, G. Subsidence and erosion of a Histosol. Soil Sci. Soc. Am. J. 1982, 46, 404–408. [Google Scholar] [CrossRef]
- Richardson, S.; Smith, J. Peat wastage in the East Anglian fens. J. Soil Sci. 1977, 28, 485–489. [Google Scholar] [CrossRef]
- Tate, R.L. Microbial oxidation of organic matter of Histosols. In Advances in Microbial Ecology; Springer: New York, NY, USA, 1980; pp. 169–201. [Google Scholar]
- Morris, D.R.; Gilbert, R.A. Inventory, crop use and soil subsidence of Histosols in Florida. Int. J. Food Agric. Environ. 2005, 3, 190–193. [Google Scholar]
- Snyder, G.; Davidson, J. Everglades agriculture: Past, present, and future. In Everglades: The Ecosystem and Its Restoration; CRC Press: Boca Raton, FL, USA, 1994; pp. 85–115. [Google Scholar]
- Höper, H.; Augustin, J.; Cagampan, J.; Drösler, M.; Lundin, L.; Moors, E.; Vasander, H.; Waddington, J.; Wilson, D. Restoration of peatlands and greenhouse gas balances. In Peatlands and Climate Change; International Peat Society: Jyvaskyla, Finland, 2008; pp. 182–210. [Google Scholar]
- Kasimir-Klemedtsson, A.; Klemedtsson, L.; Berglund, K.; Martikainen, P.; Silvola, J.; Oenema, O. Greenhouse gas emissions from farmed organic soils: A review. Soil Use Manag. 1997, 13, 245–250. [Google Scholar] [CrossRef]
- Reiche, M.; Gleixner, G.; Küsel, K. Effect of peat quality on microbial greenhouse gas formation in an acidic fen. Biogeosciences 2010, 7, 187–198. [Google Scholar] [CrossRef]
- Byrne, K.A.; Chojnicki, B.; Christensen, T.R.; Drosler, M.; Frolking, S.; Lindroth, A.; Mailhammer, J.; Malmer, N.; Selin, P.; Turunen, J.; et al. EU peatlands: Current carbon stocks and trace gas fluxes. In CarboEurope-GHG Concerted Action—Synthesis of the European Greenhouse Gas Budget, Report 4/2004, Specific Study, Tipo-Lito Recchioni, Viterbo, October 2004; Geosphere-Biosphere Center: Lund, Sweden, 2004. [Google Scholar]
- Buschmann, C.; Röder, N.; Berglund, K.; Berglund, Ö.; Lærke, P.E.; Maddison, M.; Mander, Ü.; Myllys, M.; Osterburg, B.; van den Akker, J.J. Perspectives on agriculturally used drained peat soils: Comparison of the socioeconomic and ecological business environments of six European regions. Land Use Policy 2020, 90, 104181. [Google Scholar] [CrossRef]
- Buragienė, S.; Šarauskis, E.; Romaneckas, K.; Adamavičienė, A.; Kriaučiūnienė, Z.; Avižienytė, D.; Marozas, V.; Naujokienė, V. Relationship between CO2 emissions and soil properties of differently tilled soils. Sci. Total Environ. 2019, 662, 786–795. [Google Scholar] [CrossRef]
- Rutkowska, B.; Szulc, W.; Sosulski, T.; Skowrońska, M.; Szczepaniak, J. Impact of reduced tillage on CO2 emission from soil under maize cultivation. Soil Tillage Res. 2018, 180, 21–28. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Nogue-Serra, I.; Raffaillac, D.; Cantero-Martínez, C.; Justes, E. Carbon footprint of cropping systems with grain legumes and cover crops: A case-study in SW France. Agric. Syst. 2018, 167, 92–102. [Google Scholar] [CrossRef]
- Ferré, M.; Muller, A.; Leifeld, J.; Bader, C.; Müller, M.; Engel, S.; Wichmann, S. Sustainable management of cultivated peatlands in Switzerland: Insights, challenges, and opportunities. Land Use Policy 2019, 87, 104019. [Google Scholar] [CrossRef]
- Joosten, H.; Tapio-Biström, M.-L.; Tol, S. Peatlands: Guidance for Climate Change Mitigation through Conservation, Rehabilitation and Sustainable Use; Food and Agriculture Organization of the United Nations: Roma, Italy, 2012. [Google Scholar]
- Berglund, Ö.; Berglund, K. Influence of water table level and soil properties on emissions of greenhouse gases from cultivated peat soil. Soil Biol. Biochem. 2011, 43, 923–931. [Google Scholar] [CrossRef]
- Regina, K.; Sheehy, J.; Myllys, M. Mitigating greenhouse gas fluxes from cultivated organic soils with raised water table. Mitig. Adapt. Strateg. Glob. Chang. 2015, 20, 1529–1544. [Google Scholar] [CrossRef]
- Taft, H.E.; Cross, P.A.; Jones, D.L. Efficacy of mitigation measures for reducing greenhouse gas emissions from intensively cultivated peatlands. Soil Biol. Biochem. 2018, 127, 10–21. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Wang, Y.; Chen, X.; Whalen, J.K.; Cao, Y.; Quan, Z.; Lu, C.; Shi, Y. Kinetics of inorganic and organic phosphorus release influenced by low molecular weight organic acids in calcareous, neutral and acidic soils. J. Plant Nutr. Soil Sci. 2015, 178, 555–566. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Qiu, C.; Ciais, P.; Zhu, D.; Guenet, B.; Peng, S.; Petrescu, A.M.R.; Lauerwald, R.; Makowski, D.; Gallego-Sala, A.V.; Charman, D.J. Large historical carbon emissions from cultivated northern peatlands. Sci. Adv. 2021, 7, eabf1332. [Google Scholar] [CrossRef]
- Yu, Z. Northern peatland carbon stocks and dynamics: A review. Biogeosciences 2012, 9, 4071–4085. [Google Scholar] [CrossRef]
- Hanlon, E.; Anderson, D.; Diaz, O. Nitrogen mineralization in histosols of the Everglades Agricultural Area. Commun. Soil Sci. Plant Anal. 1997, 28, 73–87. [Google Scholar] [CrossRef]
- Duguet, F. Minéralisation de l’azote et du phosphore dans les sols organiques cultivés du sud-ouest du Québec. Master’s Thesis, Laval University, Québec, QC, Canada, 2005. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Voroney, R.; Brookes, P.; Beyaert, R. Soil microbial biomass C, N, P, and S. Soil Sampl. Methods Anal. 2008, 2, 637–652. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Cordero, I.; Snell, H.; Bardgett, R.D. High throughput method for measuring urease activity in soil. Soil Biol. Biochem. 2019, 134, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Rutherford, P.; McGill, W.; Arocena, J.; Figueiredo, C. Total nitrogen. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Skjemstad, J.; Baldock, J.A. Total and organic carbon. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.E., Gregorich, E.G., Eds.; Soil Science Society of Canada: Boca Raton, FL, USA, 2007; pp. 225–238. [Google Scholar]
- Norme XP U 44-162; Fractionnement biochimique et estimation de la stabilité biologique Association Française de Normalisation. French standardization Association: Paris, France, 2005.
- Robin, D. Intérêt de la caractérisation biochimique pour l’évaluation de la proportion de matière organique stable après décomposition dans le sol et la classification des produits organominéraux. Agronomie 1997, 17, 157–171. [Google Scholar] [CrossRef]
- Stanford, G.; Smith, S. Nitrogen mineralization potentials of soils. Soil Sci. Soc. Am. J. 1972, 36, 465–472. [Google Scholar] [CrossRef]
- Dessureault-Rompré, J.; Burton, D.L.; Zebarth, B. Soluble organic nitrogen in potentially mineralizable N assays: Are we missing an important component? Can. J. Soil Sci. 2018, 98, 570–573. [Google Scholar] [CrossRef]
- Bourdon, K.; Fortin, J.; Dessureault-Rompré, J.; Caron, J. Agricultural peatlands conservation: How does the addition of plant biomass and copper affect soil fertility? Soil Sci. Soc. Am. J. 2021, 85, 1242–1255. [Google Scholar] [CrossRef]
- Marmier, V. Mineralization of Nitrogen and Release of Dissolved Phosphorus and Carbon in Plant-Amended Organic Soils. In Department of Environmental Sciences; ETH Zürich: Zurich, Switzerland, 2021. [Google Scholar]
- Qualls, R. Determination of Total Nitrogen and Phosphorus in Water Using Persulfate Oxidation: A Modification for Small Sample Volumes Using the Method of Koroleff (1983). The Biogeochemical Properties of the Retention of Nitrogen, Phosphorus, and Carbon. Ph.D. Thesis, University of Georgia Institute of Ecology, Athens, GA, USA, 1989. [Google Scholar]
- Team, R. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Dessureault-Rompré, J.; Zebarth, B.J.; Burton, D.L.; Gregorich, E.G.; Goyer, C.; Georgallas, A.; Grant, C.A. Are soil mineralizable nitrogen pools replenished during the growing season in agricultural soils? Soil Sci. Soc. Am. J. 2013, 77, 512–524. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.; Kang, H. An enzymic’latch’on a global carbon store. Nature 2001, 409, 149. [Google Scholar] [CrossRef]
- Zak, D.; Roth, C.; Unger, V.; Goldhammer, T.; Fenner, N.; Freeman, C.; Jurasinski, G. Unraveling the importance of polyphenols for microbial carbon mineralization in rewetted riparian peatlands. Front. Environ. Sci. 2019, 7, 147. [Google Scholar] [CrossRef]
- Reichel, R.; Wei, J.; Islam, M.S.; Schmid, C.; Wissel, H.; Schröder, P.; Schloter, M.; Brüggemann, N. Potential of wheat straw, spruce sawdust, and lignin as high organic carbon soil amendments to improve agricultural nitrogen retention capacity: An incubation study. Front. Plant Sci. 2018, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, H.; Zhang, J.; Chen, G.; Zhu, H.; Zhou, S.; Xiao, H. Effects of wheat straw addition on dynamics and fate of nitrogen applied to paddy soils. Soil Tillage Res. 2018, 178, 92–98. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, C.; Ma, E.; Tan, H.; Zhu, T.; Müller, C. Biochar stimulates NH4+ turnover while decreasing NO3− production and N2O emissions in soils under long-term vegetable cultivation. Sci. Total Environ. 2020, 737, 140266. [Google Scholar] [CrossRef]
- Plante, A.F.; Parton, W.J. The dynamics of soil organic matter and nutrient cycling. In Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2007; pp. 433–467. [Google Scholar]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar]
- Fontaine, S.; Bardoux, G.; Abbadie, L.; Mariotti, A. Carbon input to soil may decrease soil carbon content. Ecol. Lett. 2004, 7, 314–320. [Google Scholar] [CrossRef]
- van Kuijk, S.J.; Sonnenberg, A.S.; Baars, J.J.; Hendriks, W.H.; José, C.; Rencoret, J.; Gutiérrez, A.; de Ruijter, N.C.; Cone, J.W. Chemical changes and increased degradability of wheat straw and oak wood chips treated with the white rot fungi Ceriporiopsis subvermispora and Lentinula edodes. Biomass Bioenergy 2017, 105, 381–391. [Google Scholar] [CrossRef]
- Fitzhugh, R.D.; Lovett, G.M.; Venterea, R.T. Biotic and abiotic immobilization of ammonium, nitrite, and nitrate in soils developed under different tree species in the Catskill Mountains, New York, USA. Glob. Chang. Biol. 2003, 9, 1591–1601. [Google Scholar] [CrossRef]
- Fricks, B.; Kaye, J.; Seidel, R. Abiotic nitrate retention in agroecosystems and a forest soil. Soil Sci. Soc. Am. J. 2009, 73, 1137–1141. [Google Scholar] [CrossRef]
- Lewis, D.B.; Kaye, J.P. Inorganic nitrogen immobilization in live and sterile soil of old-growth conifer and hardwood forests: Implications for ecosystem nitrogen retention. Biogeochemistry 2012, 111, 169–186. [Google Scholar] [CrossRef]
- Barrett, J.; Burke, I. Potential nitrogen immobilization in grassland soils across a soil organic matter gradient. Soil Biol. Biochem. 2000, 32, 1707–1716. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Johnson, D.; Cheng, W.; Burke, I. Biotic and abiotic nitrogen retention in a variety of forest soils. Soil Sci. Soc. Am. J. 2000, 64, 1503–1514. [Google Scholar] [CrossRef]
- Dail, D.B.; Davidson, E.A.; Chorover, J. Rapid abiotic transformation of nitrate in an acid forest soil. Biogeochemistry 2001, 54, 131–146. [Google Scholar] [CrossRef]
- Davidson, E.A.; Chorover, J.; Dail, D.B. A mechanism of abiotic immobilization of nitrate in forest ecosystems: The ferrous wheel hypothesis. Glob. Chang. Biol. 2003, 9, 228–236. [Google Scholar] [CrossRef]
- Yansheng, C.; Fengliang, Z.; Zhongyi, Z.; Tongbin, Z.; Huayun, X. Biotic and abiotic nitrogen immobilization in soil incorporated with crop residue. Soil Tillage Res. 2020, 202, 104664. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Crecchio, C.; Curci, M.; Pizzigallo, M.D.; Ricciuti, P.; Ruggiero, P. Effects of municipal solid waste compost amendments on soil enzyme activities and bacterial genetic diversity. Soil Biol. Biochem. 2004, 36, 1595–1605. [Google Scholar] [CrossRef]
- Kızılkaya, R.; Bayraklı, B. Effects of N-enriched sewage sludge on soil enzyme activities. Appl. Soil Ecol. 2005, 30, 192–202. [Google Scholar] [CrossRef]
- Meyer, A.H.; Wooldridge, J.; Dames, J.F. Variation in urease and β-glucosidase activities with soil depth and root density in a ‘Cripp’s Pink’/M7 apple orchard under conventional and organic management. South Afr. J. Plant Soil 2015, 32, 227–234. [Google Scholar] [CrossRef]
- Li, Z.; Schneider, R.L.; Morreale, S.J.; Xie, Y.; Li, C.; Li, J. Woody organic amendments for retaining soil water, improving soil properties and enhancing plant growth in desertified soils of Ningxia, China. Geoderma 2018, 310, 143–152. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Otto, R.; Griesheim, K.L.; Su, K.; Trivelin, P.C.O. Leaching methods can underestimate mineralization potential of soils. Commun. Soil Sci. Plant Anal. 2016, 47, 1701–1708. [Google Scholar] [CrossRef]
- Murphy, D.; Recous, S.; Stockdale, E.; Fillery, I.; Jensen, L.; Hatch, D.; Goulding, K. Gross nitrogen fluxes in soil: Theory, measurement and application of^ 1^ 5n pool dilution techniques. Adv. Agron. 2003, 79, e118. [Google Scholar]
- Hashemi, F.; Olesen, J.E.; Dalgaard, T.; Børgesen, C.D. Review of scenario analyses to reduce agricultural nitrogen and phosphorus loading to the aquatic environment. Sci. Total Environ. 2016, 573, 608–626. [Google Scholar] [CrossRef] [PubMed]

| Haplosaprist | Haplohemist | |
|---|---|---|
| Bulk density [g cm3] | 0.68 | 0.24 |
| pH | 7.1 | 6.5 |
| Ctotal [g kg−1] | 2.4 | 3.9 |
| Ntotal [g kg−1] | 0.16 | 0.17 |
| C:N ratio | 15 | 24 |
| Ptotal [g kg−1] | 2.25 | 5.49 |
| Altotal [mg kg−1] | 6.60 | 1.64 |
| Fetotal [mg kg−1] | 11.29 | 2.23 |
| EC (µS cm−1) | 1140 | 432 |
| Miscanthus | Willow | Forest Mix | Biochar † | |
|---|---|---|---|---|
| C [%] | 45.3 | 46.4 | 47.5 | 72 |
| N [%] | 0.20 | 0.34 | 0.31 | 0.68 |
| C:N ratio | 226 | 136 | 153 | 106 |
| Hemicellulose [%] | 29.8 | 10.9 | 15.1 | NA |
| Cellulose [%] | 23.0 | 32.3 | 30.9 | NA |
| Lignin [%] | 34.8 | 35.5 | 38.7 | NA |
| Lignin:N ratio | 174 | 104 | 125 | NA |
| Type | Amendment | M1 † [mg kg−1] ± SE | % Diff | k1‡ [mg (kg day)−1] | % Diff | k2 †† [mg (kg day)−1] | % Diff | |
|---|---|---|---|---|---|---|---|---|
| TSN | Haplosaprist | Biochar | 208.9 ± 22.5 b | −37% *** | 0.072 ± 0.005 ab | 8% | 1.03 ± 0.06 a | −3% |
| Control | 330.1 ± 18.4 a | 0% | 0.067 ± 0.005 ab | 0% | 1.07 ± 0.06 a | 0% | ||
| Forest Mix | 170.8 ± 22.5 b | −48% *** | 0.078 ± 0.006 a | 16% | 0.53 ± 0.08 b | −50% *** | ||
| Miscanthus | 77.9 ± 18.4 bc | −76% *** | 0.058 ± 0.005 b | −13% | 0.48 ± 0.06 b | −55% *** | ||
| Willow | 100.7 bc | −70% *** | 0.057 ± 0.005 ab | −15% | 0.53 ± 0.06 b | −51% *** | ||
| Haplohemist | Biochar | 300.8 ± 18.4 ab | −19% | 0.030 ± 0.005 b | −1% | 0.51 ± 0.06 a | 8% | |
| Control | 370.0 ± 18.4 a | 0% | 0.031 ± 0.005 b | 0% | 0.47 ± 0.08 a | 0% | ||
| Forest Mix | 217.5 ± 18.4 b | −41% *** | 0.047 ± 0.005 b | 54% | 0.45 ± 0.06 a | −5% | ||
| Miscanthus | 72.6 ± 18.4 c | −80% *** | 0.083 ± 0.006 a | 172% *** | 0.40 ± 0.06 a | −14% | ||
| Willow | 65.2 ± 18.4 c | −82% *** | 0.054 ± 0.005 a | 78% ** | 0.38 ± 0.06 a | −19% | ||
| minN | Haplosaprist | Biochar | 171.6 ± 14.0 a | −16% | 0.033 ± 0.005 b | −58% *** | 0.48 ± 0.04 b | −39% *** |
| Control | 205.0 ± 14.0 a | 0% | 0.080 ± 0.007 a | 0% | 0.79 ± 0.04 a | 0% | ||
| Forest Mix | 100.4 ± 17.1 b | −51% *** | 0.038 ± 0.007 b | −52% *** | 0.28 ± 0.05 bc | −64% *** | ||
| Miscanthus | 44.0 ± 14.0 b | −79% *** | 0.028 ± 0.005 b | −65% *** | 0.25 ± 0.04 c | −68% *** | ||
| Willow | 82.3 ± 17.1 b | −60% *** | 0.016 ± 0.005 b | −80% *** | 0.16 ± 0.05 c | −79% *** | ||
| Haplohemist | Biochar | 198.1 ± 14.0 a | 3% | 0.023 ± 0.005 b | −25% | 0.36 ± 0.05 ab | −35% | |
| Control | 192.8 ± 14.0 a | 0% | 0.031 ± 0.005 b | 0% | 0.55 ± 0.04 a | 0% | ||
| Forest Mix | 135.3 ± 14.0 a | −30% | 0.033 ± 0.005 b | 7% | 0.20 ± 0.04 b | −64% *** | ||
| Miscanthus | 35.9 ± 14.0 b | −81% *** | 0.073 ± 0.007 a | 134% *** | 0.21 ± 0.04 b | −61% *** | ||
| Willow | 41.4 ± 14.0 b | −79% *** | 0.034 ± 0.007 b | 9% | 0.21 ± 0.04 b | −62% *** |
| Variable | Soil Type | Amendment | Cumulative T26 [mg kg−1] | % Diff |
|---|---|---|---|---|
| TSN | Haplosaprist | Biochar | 447.4 ± 94.7 a | −13% |
| Control | 513.3 ± 51.9 ab | 0% | ||
| Forest Mix | 264.2 ± 51.9 bc | −41% ** | ||
| Miscanthus | 158.1 ± 24 c | −65% ** | ||
| Willow | 178 ± 3.2 c | −60% ** | ||
| Haplohemist | Biochar | 392.7 ± 41.1 b | −18% * | |
| Control | 477 ± 9.8 a | 0% | ||
| Forest Mix | 299.9 ± 53.2 c | −37% *** | ||
| Miscanthus | 147.6 ± 12.9 d | −69% *** | ||
| Willow | 133.9 ± 6.4 d | −72% *** | ||
| minN | Haplosaprist | Biochar | 259 ± 31.7 ab | −25% |
| Control | 344.4 ± 64.5 a | 0% | ||
| Forest Mix | 152.5 ± 36.6 bc | −56% ** | ||
| Miscanthus | 91.3 ± 26.5 c | −73% ** | ||
| Willow | 110.3 ± 4.8 c | −68% ** | ||
| Haplohemist | Biochar | 238.6 ± 3.4 b | −17% *** | |
| Control | 288.3 ± 6.2 a | 0% | ||
| Forest Mix | 170.3 ± 17.1 c | −41% *** | ||
| Miscanthus | 75.1 ± 12.4 d | −74% *** | ||
| Willow | 78.9 ± 6.1 d | −73% *** | ||
| SON | Haplosaprist | Biochar | 193.6 ± 14.3 a | 13% |
| Control | 171.2 ± 66.2 a | 0% | ||
| Forest Mix | 116.7 ± 0.9 a | −32% | ||
| Miscanthus | 67.9 ± 0.8 a | −60% | ||
| Willow | 71.1 ± 6.1 a | −58% | ||
| Haplohemist | Biochar | 192.7 ± 41.3 a | 21% | |
| Control | 158.8 ± 15.1 a | 0% | ||
| Forest Mix | 132.0 ± 40.9 ab | −17% | ||
| Miscanthus | 72.7 ± 8.6 bc | −54% ** | ||
| Willow | 55.0 ± 4.6 c | −65% *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marmier, V.; Dessureault-Rompré, J.; Frossard, E.; Caron, J. Impact of Plant-Based Amendments on Water-Soluble Nitrogen Release Dynamics in Cultivated Peatlands. Nitrogen 2022, 3, 426-443. https://doi.org/10.3390/nitrogen3030028
Marmier V, Dessureault-Rompré J, Frossard E, Caron J. Impact of Plant-Based Amendments on Water-Soluble Nitrogen Release Dynamics in Cultivated Peatlands. Nitrogen. 2022; 3(3):426-443. https://doi.org/10.3390/nitrogen3030028
Chicago/Turabian StyleMarmier, Vincent, Jacynthe Dessureault-Rompré, Emmanuel Frossard, and Jean Caron. 2022. "Impact of Plant-Based Amendments on Water-Soluble Nitrogen Release Dynamics in Cultivated Peatlands" Nitrogen 3, no. 3: 426-443. https://doi.org/10.3390/nitrogen3030028
APA StyleMarmier, V., Dessureault-Rompré, J., Frossard, E., & Caron, J. (2022). Impact of Plant-Based Amendments on Water-Soluble Nitrogen Release Dynamics in Cultivated Peatlands. Nitrogen, 3(3), 426-443. https://doi.org/10.3390/nitrogen3030028








