Role of Tree Species, the Herb Layer and Watershed Characteristics in Nitrate Assimilation in a Central Appalachian Hardwood Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Sample Collection
2.3. Analytical Methods
2.3.1. Nitrate Reductase Activity
2.3.2. NRAA Calculation
2.4. Statistical Analysis
3. Results
3.1. Summer Overstory Maple Foliage NRA
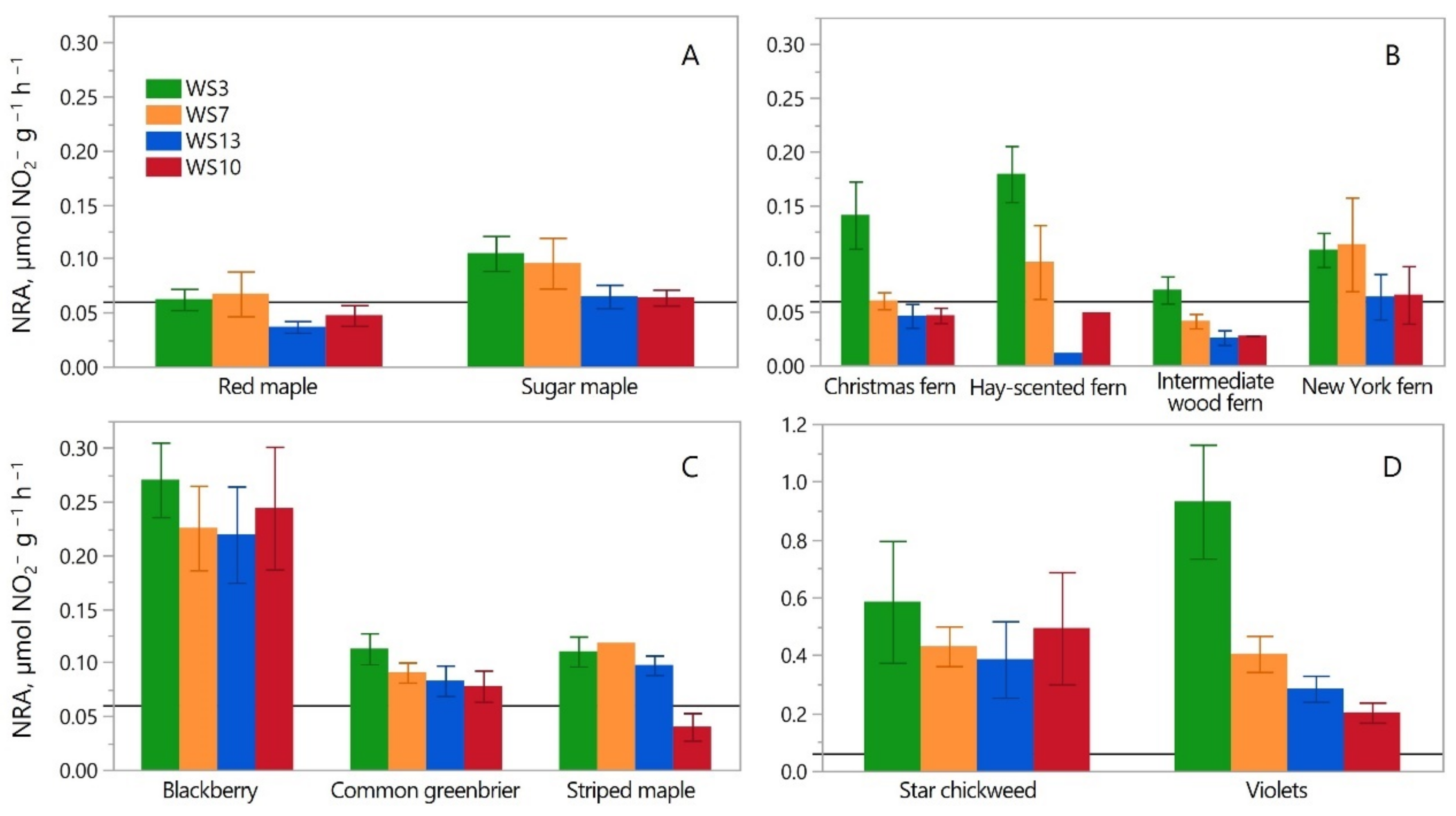
3.2. Summer Herb-Layer Foliage NRA
3.3. Root NRA in Fertilized WS3 and Unfertilized WS7
3.4. Root vs. Foliage NRA in Fertilized WS3 and Unfertilized WS7
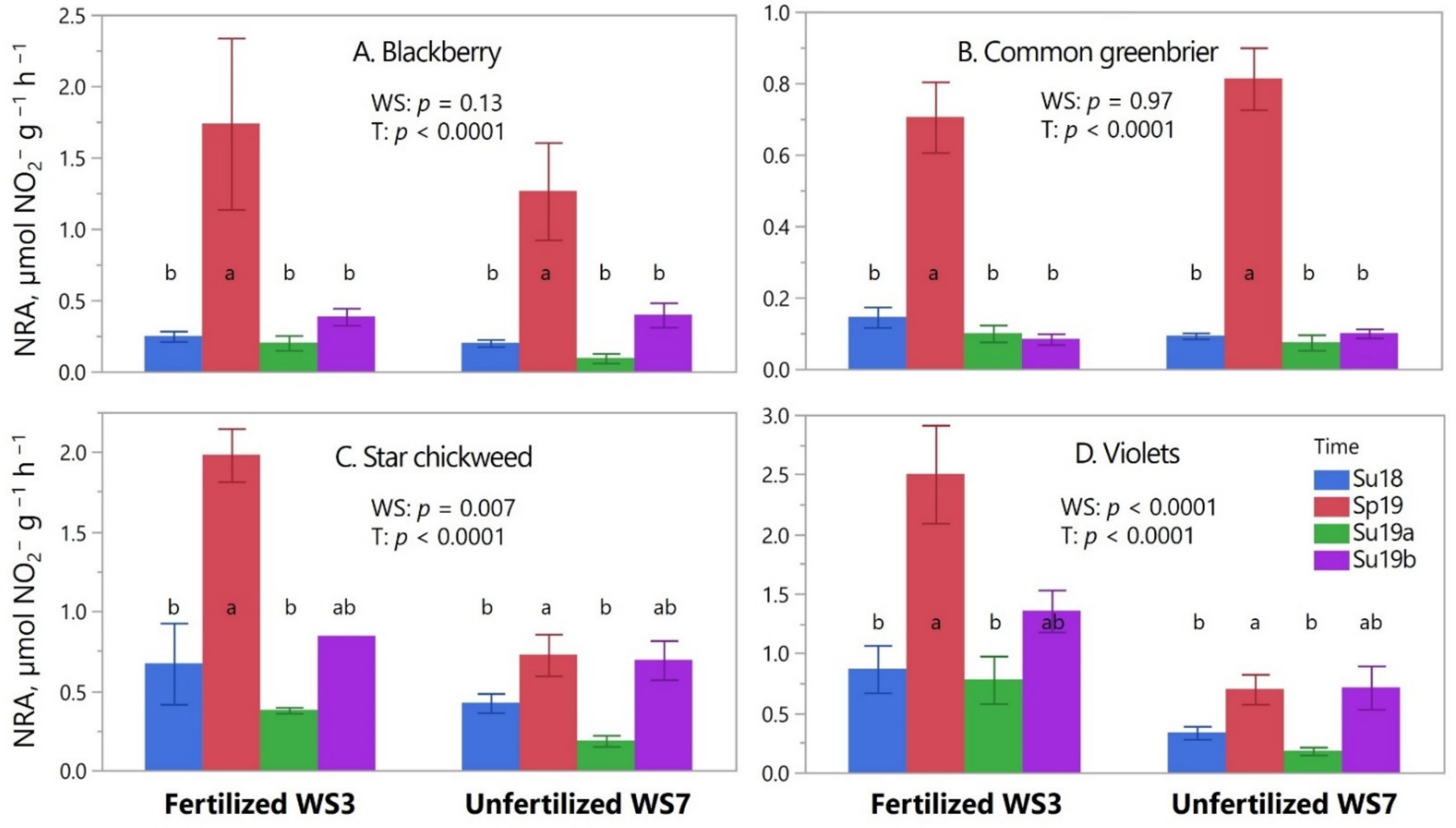
3.5. Spring vs. Summer Foliage NRA of the Herb-Layer in Fertilized WS3 and Unfertilized WS7
3.6. NRAA–Plot-Level Assimilation of Nitrate
3.6.1. Summer Overstory Foliage NRAA
3.6.2. Summer Herb-Layer NRAA
3.6.3. Root vs. Foliar NRAA in WS3 and WS7
3.6.4. Spring vs. Summer Foliage NRAA of the Herb-Layer in WS3 and WS7
4. Discussion
4.1. Nitrate Assimilation and Soil Nitrogen Availability
4.2. Red Maple vs. Sugar Maple
4.3. Root Nitrate Assimilation
4.4. Tree vs. Herb-Layer NRA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothstein, D.E.; Zak, D.R.; Pregitzer, K.S. Nitrate deposition in northern hardwood forests and the nitrogen metabolism of Acer saccharum. Oecologia 1996, 108, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Raven, J.A. Root or shoot nitrate assimilation in terrestrial vascular plants—Does it matter? Plant Soil 2022, 1–32. [Google Scholar] [CrossRef]
- Downs, M.R.; Nadelhoffer, K.J.; Melillo, J.M.; Aber, J.D. Foliar and fine root nitrate reductase activity in seedlings of four forest tree species in relation to nitrogen availability. Trees 1993, 7, 233–236. [Google Scholar] [CrossRef]
- Stadler, J.; Gebauer, G.; Schulze, E.-D. The influence of ammonium on nitrate uptake and assimilation in 2-year-old ash and oak trees—A tracer-study with 15N. Isot. Environ. Health Stud. 1993, 29, 85–92. [Google Scholar] [CrossRef]
- Truax, B.; Gagnon, D.; Chevrier, N. Nitrate reductase activity in relation to growth and soil N forms in red oak and red ash planted in three different environments: Forest, clear-cut and field. For. Ecol. Manag. 1994, 64, 71–82. [Google Scholar] [CrossRef]
- Eddy, W.C.; Zak, D.R.; Holmes, W.E.; Pregitzer, K.S. Chronic atmospheric NO3− deposition does not induce NO3− use by Acer saccharum Marsh. Ecosystems 2008, 11, 469–477. [Google Scholar] [CrossRef]
- Tang, M.H.; Porder, S.; Lovett, G.M. Species differences in nitrate reductase activity are unaffected by nitrogen enrichment in northeastern US forests. For. Ecol. Manag. 2012, 275, 52–59. [Google Scholar] [CrossRef]
- Olsson, M.; Falkengren-Grerup, U. Partitioning of nitrate uptake between trees and understory in oak forests. For. Ecol. Manag. 2003, 179, 311–320. [Google Scholar] [CrossRef]
- National Atmospheric Deposition Program (NRSP-3). Available online: https://nadp.slh.wisc.edu/maps-data/animated-maps/ (accessed on 1 April 2021).
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystems. BioScience 1989, 39, 378–386. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, L.A.; Elliott, E.M.; Adams, M.B. Triple nitrate isotopes indicate differing nitrate source contributions to streams across a nitrogen saturation gradient. Ecosystems 2015, 18, 1209–1223. [Google Scholar] [CrossRef]
- Peterjohn, W.T.; Harlacher, M.A.; Christ, M.J.; Adams, M.B. Testing associations between tree species and nitrate availability: Do consistent patterns exist across spatial scales? For. Ecol. Manag. 2015, 358, 335–343. [Google Scholar] [CrossRef]
- Hornbeck, J.W.; Martin, C.W.; Pierce, R.S.; Bormann, F.H.; Likens, G.E.; Eaton, J.S. The Northern Hardwood Forest Ecosystem: Ten Years of Recovery from Clearcutting; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Broomall, PA, USA, 1987. [Google Scholar]
- Muller, R.N.; Bormann, F.H. Role of Erythronium americanum Ker. in energy flow and nutrient dynamics of a northern hardwood forest ecosystem. Science 1976, 193, 1126–1128. [Google Scholar] [CrossRef]
- Blank, J.L.; Olson, R.K.; Vitousek, P.M. Nutrient uptake by a diverse spring ephemeral community. Oecologia 1980, 47, 96–98. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A.; Schultz, J.C. Nitrogen cycling in a northern hardwood forest: Do species matter? Biogeochemistry 2004, 67, 289–308. [Google Scholar] [CrossRef]
- Lovett, G.M.; Mitchell, M.J. Sugar maple and nitrogen cycling in the forests of eastern North America. Front. Ecol. Environ. 2004, 2, 81–88. [Google Scholar] [CrossRef]
- Likens, G.E.; Bormann, F.H.; Johnson, N.M.; Fisher, D.; Pierce, R.S. Effects of forest cutting and herbicide treatment on nutrient budgets in the Hubbard Brook watershed-ecosystem. Ecol. Monogr. 1970, 40, 23–47. [Google Scholar] [CrossRef]
- Likens, G.; Bormann, F. Effects of forest clearing on the northern hardwood forest ecosystem and its biogeochemistry. In Proceedings of the first International Congress of Ecology, Hague, The Netherlands, 8–14 September 1974; pp. 330–335. [Google Scholar]
- Martin, C.W.; Pierce, R.S.; Likens, G.E.; Bormann, F.H. Clearcutting Affects Stream Chemistry in the White Mountains of New Hampshire; US Department of Agriculture, Forest Service, Northeastern Research Station: Broomall, PA, USA, 1986; Volume 579. [Google Scholar]
- Pardo, L.; Driscoll, C.; Likens, G. Patterns of nitrate loss from a chronosequence of clear-cut watersheds. Water Air Soil Pollut. 1995, 85, 1659–1664. [Google Scholar] [CrossRef]
- Goodale, C.L.; Aber, J.D. The long-term effects of land-use history on nitrogen cycling in northern hardwood forests. Ecol. Appl. 2001, 11, 253–267. [Google Scholar] [CrossRef]
- Ollinger, S.V.; Smith, M.-L.; Martin, M.E.; Hallett, R.A.; Goodale, C.L.; Aber, J.D. Regional variation in foliar chemistry and N cycling among forests of diverse history and composition. Ecology 2002, 83, 339–355. [Google Scholar]
- Binkley, D.; Burnham, H.; Allen, H.L. Water quality impacts of forest fertilization with nitrogen and phosphorus. For. Ecol. Manag. 1999, 121, 191–213. [Google Scholar] [CrossRef]
- Gundersen, P.; Schmidt, I.K.; Raulund-Rasmussen, K. Leaching of nitrate from temperate forests—Effects of air pollution and forest management. Environ. Rev. 2006, 14, 1–57. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Galloway, J.E.; Sarmiento, J.S. Variation with slope aspect in effects of temperature on nitrogen mineralization and nitrification in mineral soil of mixed hardwood forests. Can. J. For. Res. 2015, 45, 958–962. [Google Scholar] [CrossRef]
- Gilliam, F. Spatial variability in soil microbial communities in a nitrogen-saturated hardwood forest watershed. Soil Sci. Soc. America J. 2010, 75, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Fernow Experimental Forest Watersheds LTREB Tree Survey Data. Available online: http://www.as.wvu.edu/fernow/index.html (accessed on 10 June 2021).
- Edwards, P.J.; Wood, F. Fernow Experimental Forest Stream Chemistry; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2011. [Google Scholar] [CrossRef]
- Adams, M.B.; Kochenderfer, J.N.; Edwards, P.J. The Fernow Watershed Acidification Study: Ecosystem Acidification, Nitrogen Saturation and Base Cation Leaching. In Acid Rain-Deposition to Recovery; Springer: Berlin, Germany, 2007; pp. 267–273. [Google Scholar]
- Gilliam, F.S.; Billmyer, J.H.; Walter, C.A.; Peterjohn, W.T. Effects of excess nitrogen on biogeochemistry of a temperate hardwood forest: Evidence of nutrient redistribution by a forest understory species. Atmos. Environ. 2016, 146, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.J.; Stephan, K. Nitrogen fertilization, stand age, and overstory tree species impact the herbaceous layer in a Central Appalachian hardwood forest. Forests 2021, 12, 829. [Google Scholar] [CrossRef]
- Kochenderfer, J.N. Fernow and the Appalachian Hardwood Region. In The Fernow Watershed Acidification Study; Springer: Berlin, Germany, 2006; pp. 17–39. [Google Scholar]
- Trimble, G.R. Age and Stem Origin of Appalachian Hardwood Reproduction Following a Clearcut and Herbicide Treatment; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Newtown Square, PA, USA, 1986; Volume 589. [Google Scholar]
- Aubertin, G.; Patric, J. Water quality after clearcutting a small watershed in West Virginia. J. Environ. Qual. 1974, 3, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Patric, J.H.; Reinhart, K.G. Hydrologic effects of deforesting two mountain watersheds in West Virginia. Water Resour. Res. 1971, 7, 1182–1188. [Google Scholar] [CrossRef]
- Peterjohn, W.T.; Adams, M.B.; Gilliam, F.S. Symptoms of nitrogen saturation in two central Appalachian hardwood forest ecosystems. Biogeochemistry 1996, 35, 507–522. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Welch, N.T.; Phillips, A.H.; Billmyer, J.H.; Peterjohn, W.T.; Fowler, Z.K.; Walter, C.A.; Burnham, M.B.; May, J.D.; Adams, M.B. Twenty-five-year response of the herbaceous layer of a temperate hardwood forest to elevated nitrogen deposition. Ecosphere 2016, 7, e01250. [Google Scholar] [CrossRef] [Green Version]
- DeWalle, D.R.; Kochenderfer, J.N.; Adams, M.B.; Miller, G.W.; Gilliam, F.S.; Wood, F.; Odenwald-Clemens, S.S.; Sharpe, W.E. Vegetation and Acidification. In The Fernow Watershed Acidification Study; Springer: Berlin, Germany, 2006; pp. 137–188. [Google Scholar]
- Gebauer, G.; Melzer, A.; Rehder, H. Nitrate content and nitrate reductase activity in Rumex obtusifolius L. Oecologia 1984, 63, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J. The Impact of Tree Species, Elevated Nitrogen Deposition, Stand Age, and Environmental Factors on Herbaceous Plant Communities in a Central Appalachian Hardwood Forest; West Virginia University: Morgantown, WV, USA, 2019. [Google Scholar]
- Jaworski, E.G. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun. 1971, 43, 1274–1279. [Google Scholar] [CrossRef]
- Stewart, G.; Lee, J.; Orebamjo, T. Nitrogen metabolism of halophytes II. Nitrate availability and utilization. New Phytol. 1973, 72, 539–546. [Google Scholar] [CrossRef]
- Stephan, K.; Kavanagh, K.L.; Koyama, A. Comparing the influence of wildfire and prescribed burns on watershed nitrogen biogeochemistry using 15N natural abundance in terrestrial and aquatic ecosystem components. PLoS ONE 2015, 10, e0119560. [Google Scholar] [CrossRef]
- Eisenhut, S.E.; Stephan, K. Adapting the in-vivo method for determining Nitrate Reductase Activity (NRA) for processing a large sample size in remote locations. Plant Physiol. 2022. in prep. [Google Scholar]
- LP-80; METER Group, Inc.: Pullman, WA, USA, 2020.
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Wu, S.; Guo, Q.; Wang, J.; Cao, C.; Wang, J. Leaf nitrogen assimilation and partitioning differ among subtropical forest plants in response to canopy addition of nitrogen treatments. Sci. Total Environ. 2018, 637, 1026–1034. [Google Scholar] [CrossRef]
- Knops, J.M.; Reinhart, K. Specific leaf area along a nitrogen fertilization gradient. Am. Midl. Nat. 2000, 144, 265–272. [Google Scholar] [CrossRef]
- Vose, J.M.; Allen, H.L. Leaf area, stemwood growth, and nutrition relationships in loblolly pine. For. Sci. 1988, 34, 547–563. [Google Scholar]
- Eastman, B.A.; Adams, M.B.; Brzostek, E.R.; Burnham, M.B.; Carrara, J.E.; Kelly, C.; McNeil, B.E.; Walter, C.A.; Peterjohn, W.T. Altered plant carbon partitioning enhanced forest ecosystem carbon storage after 25 years of nitrogen additions. New Phytol. 2021, 230, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.W.; Bond-Lamberty, B.; Fahey, R.T.; Haber, L.T.; Stuart-Haëntjens, E.; Hardiman, B.S.; LaRue, E.; McNeil, B.E.; Orwig, D.A.; Stovall, A.E. Application of multidimensional structural characterization to detect and describe moderate forest disturbance. Ecosphere 2020, 11, e03156. [Google Scholar] [CrossRef]
- Fernow Experimental Forest Long Term Soil Productivity (LTSP) Experiment—Lysimeter data (1996–2020). Available online: http://www.as.wvu.edu/fernow/data.html (accessed on 19 May 2022).
- Vitousek, P.M.; Gosz, J.R.; Grier, C.C.; Melillo, J.M.; Reiners, W.A. A comparative analysis of potential nitrification and nitrate mobility in forest ecosystems. Ecol. Monogr. 1982, 52, 155–177. [Google Scholar] [CrossRef] [Green Version]
- Finzi, A.C.; Van Breemen, N.; Canham, C.D. Canopy tree–soil interactions within temperate forests: Species effects on soil carbon and nitrogen. Ecol. Appl. 1998, 8, 440–446. [Google Scholar]
- Walter, C.A.; Raiff, D.T.; Burnham, M.B.; Gilliam, F.S.; Adams, M.B.; Peterjohn, W.T. Nitrogen fertilization interacts with light to increase Rubus spp. cover in a temperate forest. Plant Ecol. 2016, 217, 421–430. [Google Scholar] [CrossRef]
- Getzin, S.; Wiegand, T.; Wiegand, K.; He, F. Heterogeneity influences spatial patterns and demographics in forest stands. J. Ecol. 2008, 96, 807–820. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A. Control of nitrogen loss from forested watersheds by soil carbon:nitrogen ratio and tree species composition. Ecosystems 2002, 5, 0712–0718. [Google Scholar] [CrossRef]
- Andrews, M. The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ. 1986, 9, 511–519. [Google Scholar]
- Templer, P.; Dawson, T. Nitrogen uptake by four tree species of the Catskill Mountains, New York: Implications for forest N dynamics. Plant Soil 2004, 262, 251–261. [Google Scholar] [CrossRef]
- Truax, B.; Lambert, F.; Gagnon, D.; Chevrier, N. Nitrate reductase and glutamine synthetase activities in relation to growth and nitrogen assimilation in red oak and red ash seedlings: Effects of N-forms, N concentration and light intensity. Trees 1994, 9, 12–18. [Google Scholar] [CrossRef]
- Sarjala, T.; Raitio, R.; Turkki, E.-M. Nitrate metabolism in Scots pine seedlings during their first growing season. Tree Physiol. 1987, 3, 285–293. [Google Scholar] [CrossRef]
- Black, B.L.; Fuchigami, L.H.; Coleman, G.D. Partitioning of nitrate assimilation among leaves, stems and roots of poplar. Tree Physiol. 2002, 22, 717–724. [Google Scholar] [CrossRef]
- Gebauer, G.; Stadler, J. Nitrate assimilation and nitrate content in different organs of ash trees (Fraxinus excelsior). In Plant Nutrition—Physiology and Applications; Springer: Berlin, Germany, 1990; pp. 101–106. [Google Scholar]
- Högbom, L.; Nilsson, U.; Örlander, G. Nitrate dynamics after clear felling monitored by in vivo nitrate reductase activity (NRA) and natural 15N abundance of Deschampsia flexuosa (L.) Trin. For. Ecol. Manag. 2002, 160, 273–280. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Vernal nitrogen and phosphorus retention by forest understory vegetation and soil microbes. Plant Soil 2003, 256, 443–453. [Google Scholar] [CrossRef]
- Rachmilevitch, S.; Cousins, A.B.; Bloom, A.J. Nitrate assimilation in plant shoots depends on photorespiration. Proc. Natl. Acad. Sci. USA 2004, 101, 11506–11510. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.J. Photorespiration and nitrate assimilation: A major intersection between plant carbon and nitrogen. Photosynth. Res. 2015, 123, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, L.A.; Terai, M.; Tokuchi, N. Nitrate reductase activities in plants from different ecological and taxonomic groups grown in Japan. Ecol. Res. 2020, 35, 708–712. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Burns, D.A.; Driscoll, C.T.; Frey, S.D.; Lovett, G.M.; Watmough, S.A. Decreased atmospheric nitrogen deposition in eastern North America: Predicted responses of forest ecosystems. Environ. Pollut. 2019, 244, 560–574. [Google Scholar] [CrossRef] [PubMed]
|
Watershed ID | Aspect | Stand Age (yr) in 2020 and History 1 | Treatment | Basal Area (%) of Dominant Tree Species 3 | Streamwater Nitrate (mg L−1) Pre/Post 1990 4 |
|---|---|---|---|---|---|
| WS3 | S | ~50 Partial cuts in 1958, ‘1963,‘1968; clearcut 1969–70; riparian buffer cut in 1970 | Fertilization via aerial applications of 35 kg N ha−1 yr−1 granular ammonium sulfate ((NH4)2SO4) 1989–2019 2 | Black cherry, 51 Red+sugar maple, 12 + 1 Sweet birch, 5 Northern red oak, 5 | 2.8/8.5 |
| WS7 | E | ~50 Clearcut 1963–67; WS main-tained barren by annual herbicide application 1967–69 | No Fertilization | Tulip-poplar, 26 Black cherry, 21 Sweet birch, 21 Red+sugar maple, 8 + 5 | 5.4/4.6 |
| WS10 | S | ~110 | No Fertilization | Chestnut oak, 24 Northern red oak, 22 Red+sugar maple, 19 + 2 Blackgum, 8 | 1.2/0.8 |
| WS13 | N | ~110 | No Fertilization | Northern red oak, 30 Sugar maple, 22 Red maple, 13 Tulip-poplar, 7 | 2.3/2.0 |
| Species | Sample Size | Model Effect p-Values | ||
|---|---|---|---|---|
| n | M | WS | M × WS | |
| Common greenbrier | 67 | 0.78 | 0.27 | 0.87 |
| Blackberry | 57 | 0.76 | 0.78 | 0.34 |
| Striped maple | 20 | 0.39 | 0.05 ** | 0.61 |
| Violets | 58 | 0.63 | <0.0001 ** | 0.83 |
| Star chickweed | 32 | 0.07 * | 0.73 | 0.62 |
| Christmas fern | 40 | 0.60 | 0.001 ** | 0.82 |
| Hay-scented fern | 18 | 0.56 | 0.13 | 0.60 |
| Intermediate Wood fern | 37 | 0.79 | 0.055 * | 0.73 |
| New York fern | 23 | 0.78 | 0.73 | 0.63 |
| Root NRA | Foliage NRA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WS3 | WS7 | WS3 | WS7 | |||||||||
| Mean | SE | n | Mean | SE | n | Mean | SE | n | Mean | SE | n | |
| Red maple | 0.09 | 0.02 | 5 | 0.09 | 0.03 | 5 | 0.04 | 0.01 | 9 | 0.05 | 0.01 | 9 |
| Sugar maple | 0.07 | 0.01 | 4 | 0.05 | 0.03 | 4 | 0.11 | 0.02 | 9 | 0.09 | 0.02 | 9 |
| Blackberry | 0.14 a | 0.02 | 10 | 0.08 b | 0.01 | 10 | 0.38 | 0.06 | 16 | 0.40 | 0.09 | 15 |
| Common greenbrier | 0.07 | 0.01 | 10 | 0.05 | 0.02 | 10 | 0.08 | 0.02 | 14 | 0.10 | 0.01 | 9 |
| Ferns 1 | 0.09 | 0.01 | 10 | 0.04 | 0.01 | 10 | 0.19 | 0.03 | 22 | 0.10 | 0.01 | 27 |
| Violets | 0.29 a | 0.04 | 10 | 0.11 b | 0.04 | 10 | 1.36 | 0.18 | 2 | 0.71 | 0.18 | 16 |
| Model Effect p-Values | |||
|---|---|---|---|
| Species | WS | O | WS × O |
| Red maple | 0.85 | 0.0001 ** | 0.54 |
| Sugar maple | 0.14 | 0.01 ** | 0.47 |
| Blackberry | 0.26 | <0.0001 ** | 0.26 |
| Common greenbrier | 0.95 | 0.03 ** | 0.30 |
| Ferns | 0.001 ** | 0.0008 ** | 0.50 |
| Violets | <0.0001 ** | <0.0001 ** | 0.007 ** |
| WS3 | WS7 | WS13 | WS10 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Overstory | Herb Layer | All | Overstory | Herb Layer | All | Overstory | Herb Layer | All | Overstory | Herb Layer | |||||||||
| Abs | % | Abs | % | Abs | % | Abs | % | Abs | % | Abs | % | Abs | % | Abs | % | |||||
| Summer 2018 | ||||||||||||||||||||
| Red maple | 31.3 | 27.6 (5.1) | 88 | 3.6 (0.6) | 12 | 22.0 | 17 (5.1) | 77 | 5.0 (0.3) | 23 | 9.2 | 6.8 (2.0) | 74.0 | 2.3 (0.4) | 26 | 10.7 | 8.2 (1.5) | 77 | 2.5 (0.3) | 23 |
| Sugar maple | 51.1 | 46.2 (4.3) | 90 | 4.9 (0.8) | 10 | 31.9 | 27.4 (5.1) | 86 | 4.5 (0.4) | 14 | 21.9 | 19.2 (4.8) | 88.0 | 2.7 (0.5) | 12 | 28.0 | 25.4 (9.6) | 91 | 2.6 (0.5) | 9 |
| Total | 82.4 | 73.9 | 8.5 | 54.0 | 44.4 | 9.5 | 31.0 | 26.0 | 5.0 | 38.7 | 33.6 | 5.1 | ||||||||
| Early Summer 2019 | ||||||||||||||||||||
| Red maple | 22.4 | 19.9 (4.3) | 88 | 2.6 (0.4) | 12 | 10.6 | 7.6 (2.7) | 71 | 3.0 (0.3) | 29 | 23.8 | 21.5 (8.9) | 90 | 2.3 (0.3) | 10 | 15.5 | 13.3 (4.5) | 86 | 2.2 (0.2) | 14 |
| Sugar maple | 50.7 | 46.0 (10.4) | 91 | 4.7 (0.9) | 9 | 18.8 | 16.2 (6.0) | 86 | 2.6 (0.3) | 14 | 18.6 | 15.6 (4.4) | 84 | 3.0 (0.4) | 16 | 17.8 | 15.9 (6.2) | 90 | 1.8 (0.3) | 10 |
| Total | 73.1 | 65.8 | 7.3 | 29.4 | 23.8 | 5.7 | 42.4 | 37.1 | 5.3 | 33.3 | 29.2 | 4.0 | ||||||||
| Midsummer 2019 | ||||||||||||||||||||
| Red maple | 20.5 | 16.1 (2.3) | 78 | 4.5 (0.9) | 22 | 20.7 | 12.2 (1.5) | 59 | 8.5 (0.6) | 41 | 18.2 | 15.6 (2.2) | 86 | 2.6 (0.4) | 14 | 15.3 | 12.8 (2.3) | 84 | 2.5 (0.3) | 16 |
| Sugar maple | 44.3 | 36.3 (7.0) | 82 | 8.0 (1.9) | 18 | 28.1 | 23.0 (3.5) | 82 | 5.1 (0.5) | 18 | 28.0 | 24.6 (2.8) | 88 | 3.4 (0.6) | 12 | 26.6 | 23.8 (3.0) | 90 | 2.7 (0.4) | 10 |
| Total | 64.8 | 52.4 | 12.4 | 48.8 | 35.2 | 13.6 | 46.2 | 40.2 | 6.0 | 41.8 | 36.6 | 5.2 | ||||||||
| Mean | ||||||||||||||||||||
| Red maple | 21.2 | 3.6 | 12.3 | 5.5 | 14.6 | 2.4 | 11.4 | 2.4 | ||||||||||||
| Sugar maple | 42.8 | 5.9 | 22.2 | 4.1 | 19.8 | 3.0 | 21.7 | 2.4 | ||||||||||||
| Total * | 64.0 a | 86 | 9.4 | 14 | 34.5 b | 77 | 9.6 A | 23 | 34.4 | 85 | 5.4 B | 15 | 33.1 | 86 | 4.8 | 14 | ||||
| Overstory Maple NRAA | Herb-Layer NRAA | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Foliage | Roots | F:R | F + R | Foliage | Roots | F:R | F + R | F + R | |
| Fertilized WS3 | |||||||||
| Red maple | 16.1 (2.3) | 8.0 (2.0) | 2.0 | 24.1 | 4.5 (0.9) | 1.3 (0.7) | 3.5 | 5.8 | 29.9 |
| Sugar maple | 36.3 (7.0) | 1.4 (0.3) | 26 | 37.7 | 8.0 (1.9) | 3.0 (0.9) | 2.7 | 11 | 48.7 |
| Mean | 30.9 | 8.4 | 39.3 | ||||||
| Unfertilized WS7 | |||||||||
| Red maple | 12.2 (1.5) | 4.5 (0.9) | 2.7 | 16.7 | 8.5 (0.6) | 2.7 (1.0) | 3.1 | 11.2 | 27.9 |
| Sugar maple | 23 (3.3) | 2.7 (0.8) | 8.5 | 25.7 | 5.1 (0.5) | 1.2 (0.5) | 4.3 | 6.3 | 32 |
| Mean | 21.2 | 8.8 | 30 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenhut, S.E.; Holásková, I.; Stephan, K. Role of Tree Species, the Herb Layer and Watershed Characteristics in Nitrate Assimilation in a Central Appalachian Hardwood Forest. Nitrogen 2022, 3, 333-352. https://doi.org/10.3390/nitrogen3020022
Eisenhut SE, Holásková I, Stephan K. Role of Tree Species, the Herb Layer and Watershed Characteristics in Nitrate Assimilation in a Central Appalachian Hardwood Forest. Nitrogen. 2022; 3(2):333-352. https://doi.org/10.3390/nitrogen3020022
Chicago/Turabian StyleEisenhut, Sian E., Ida Holásková, and Kirsten Stephan. 2022. "Role of Tree Species, the Herb Layer and Watershed Characteristics in Nitrate Assimilation in a Central Appalachian Hardwood Forest" Nitrogen 3, no. 2: 333-352. https://doi.org/10.3390/nitrogen3020022
APA StyleEisenhut, S. E., Holásková, I., & Stephan, K. (2022). Role of Tree Species, the Herb Layer and Watershed Characteristics in Nitrate Assimilation in a Central Appalachian Hardwood Forest. Nitrogen, 3(2), 333-352. https://doi.org/10.3390/nitrogen3020022





