Autumn Tillage Reduces the Effect of Plant Cover on Topsoil Nitrogen Leaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Operations and Sampling
2.2. Sample Handling and Preparation
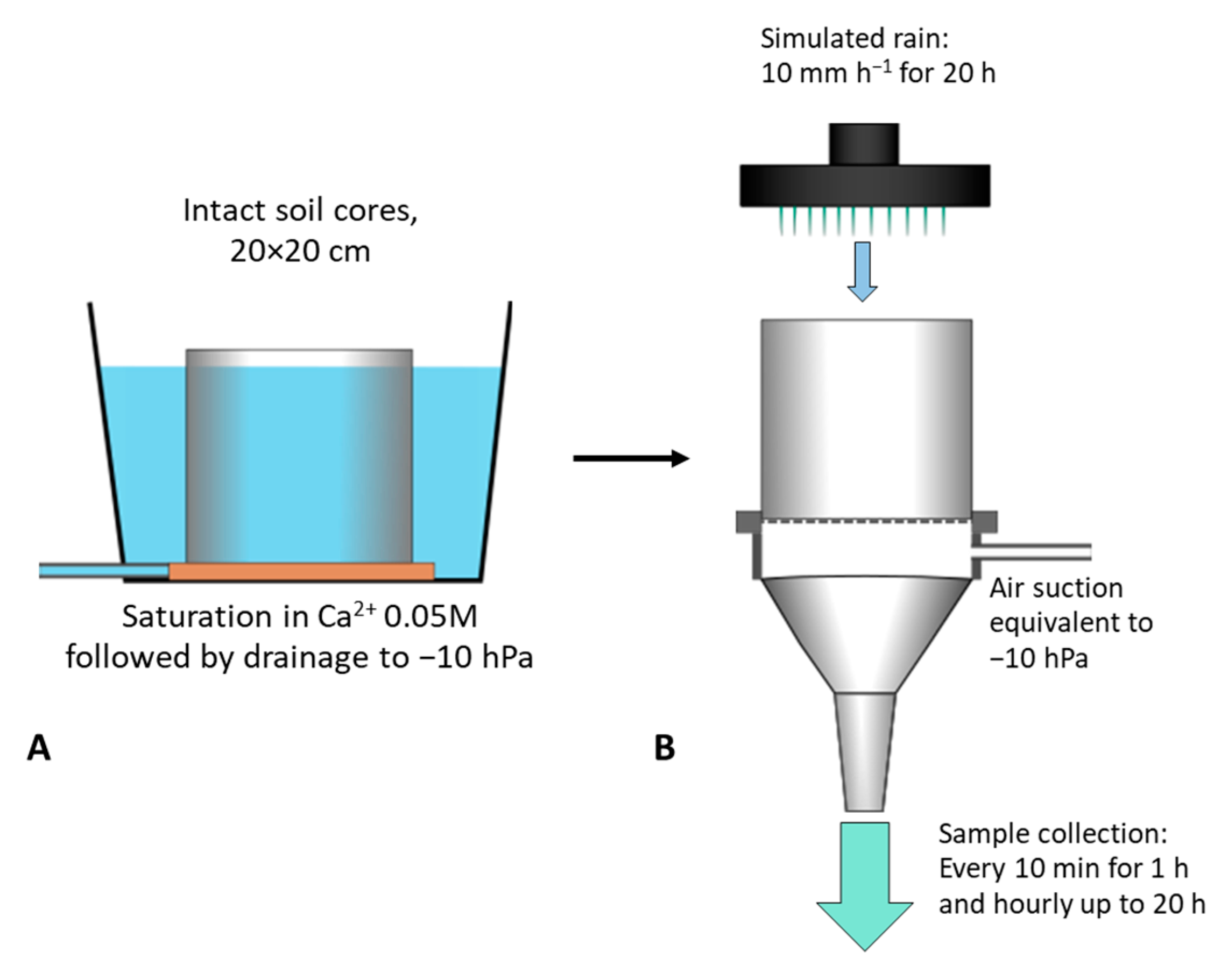
2.3. Leaching Experiment
2.4. Calculations and Statistical Analysis
3. Results and Discussion
3.1. Breakthrough Curves and NNO3 Leaching
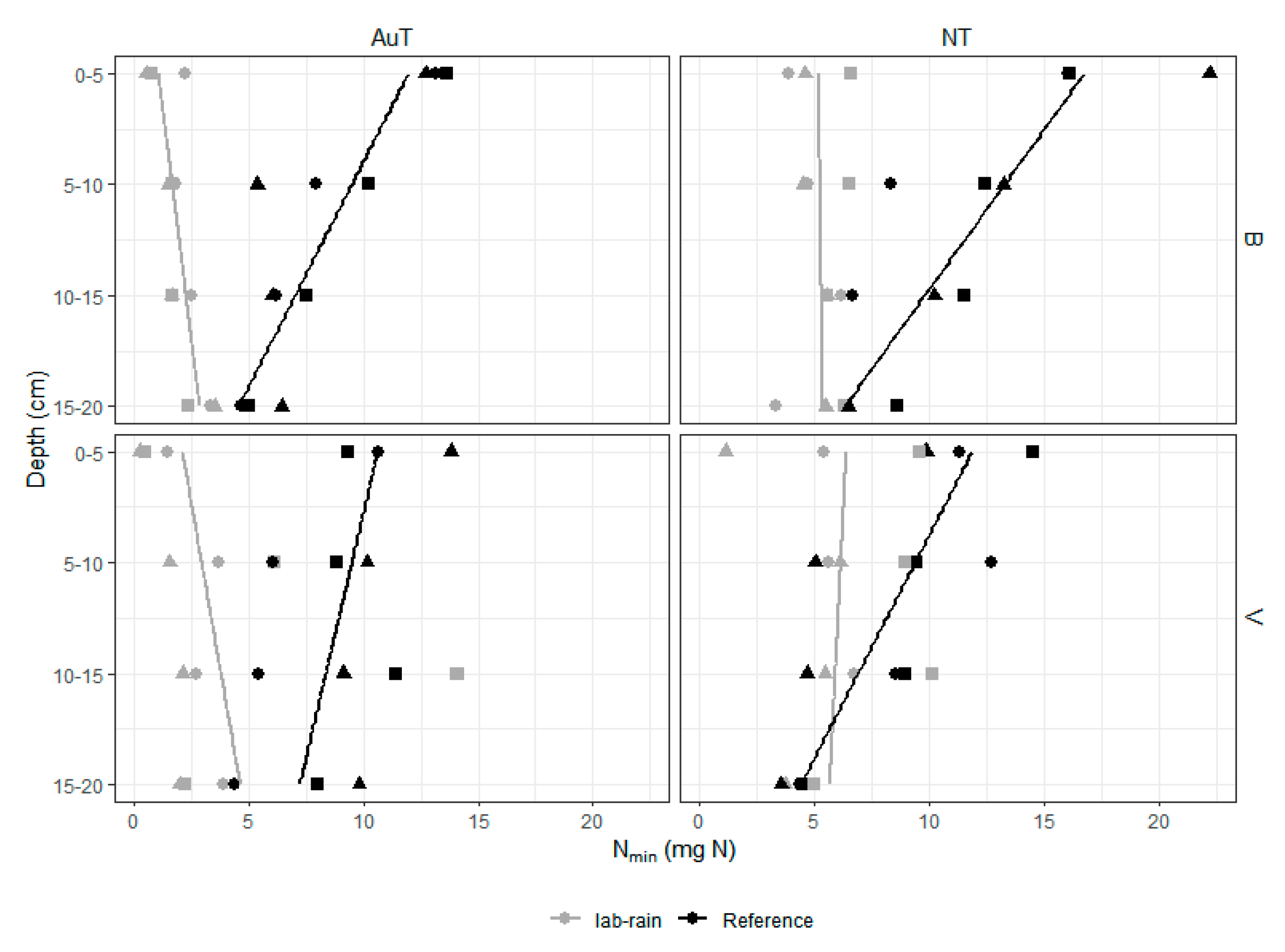
3.2. Soil Nmin Content
3.3. Considerations on N Losses at Field Scale
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creamer, N.G.; Bennett, M.A.; Stinner, B.R.; Cardina, J.; Regnier, E.E. Mechanisms of weed suppression in cover crop-based production systems. HortScience 1996, 31, 410–413. [Google Scholar] [CrossRef]
- Askegaard, M.; Olesen, J.E.; Rasmussen, I.A.; Kristensen, K. Nitrate leaching from organic arable crop rotations is mostly determined by autumn field management. Agric. Ecosyst. Environ. 2011, 142, 149–160. [Google Scholar] [CrossRef]
- Macdonald, A.J.; Poulton, P.R.; Howe, M.T.; Goulding, K.W.T.; Powlson, D.S. The use of cover crops in cereal-based cropping systems to control nitrate leaching in SE England. Plant Soil 2005, 273, 355–373. [Google Scholar] [CrossRef]
- Dabney, S.M.; Delgado, J.A.; Reeves, D.W. Using winter cover crops to improve soil and water quality. Commun. Soil Sci. Plant Anal. 2001, 32, 1221–1250. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. The effect of nitrogen catch crop species on the nitrogen nutrition of succeeding crops. Fertil. Res. 1994, 37, 227–234. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. The Effect of Nitrogen Catch Crops On the Nitrogen Nutrition of A Succeeding Crop: I. Effects Through Mineralization And Pre-Emptive Competition. Acta Agric. Scand. Sect. B Soil Plant Sci. 1993, 43, 74–81. [Google Scholar] [CrossRef]
- Stipešević, B.; Kladivko, E.J. Effects of winter wheat cover crop desiccation times on soil moisture, temperature and early maize growth. Plant Soil Environ. 2005, 51, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Chambers, B.J.; Smith, K.A.; Pain, B.F. Strategies to encourage better use of nitrogen in animal manures. Soil Use Manag. 2000, 16, 157–166. [Google Scholar] [CrossRef]
- Aronsson, H.; Hansen, E.M.; Thomsen, I.K.; Liu, J.; Øgaard, A.F.; Känkänen, H.; Ulén, B. The ability of cover crops to reduce nitrogen and phosphorus losses from arable land in southern Scandinavia and Finland. J. Soil Water Conserv. 2016, 71, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Tonitto, C.; David, M.B.; Drinkwater, L.E. Replacing bare fallows with cover crops in fertilizer-intensive cropping systems: A meta-analysis of crop yield and N dynamics. Agric. Ecosyst. Environ. 2006, 112, 58–72. [Google Scholar] [CrossRef]
- Valkama, E.; Lemola, R.; Känkänen, H.; Turtola, E. Meta-analysis of the effects of undersown catch crops on nitrogen leaching loss and grain yields in the Nordic countries. Agric. Ecosyst. Environ. 2015, 203, 93–101. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Nielsen, N.E. Modelling and measuring the effect of nitrogen catch crops on the nitrogen supply for succeeding crops. Plant Soil 1998, 203, 79–89. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Dresbøll, D.B. Incorporation time of nitrogen catch crops influences the N effect for the succeeding crop. Soil Use Manag. 2010, 26, 27–35. [Google Scholar] [CrossRef]
- Känkänen, H.; Kangas, A.; Mela, T. Timing incorporation of different green manure crops to minimize the risk of nitrogen leaching. Agric. Food Sci. 1998, 7, 553–567. [Google Scholar] [CrossRef]
- Böldt, M.; Taube, F.; Vogeler, I.; Reinsch, T.; Kluß, C.; Loges, R. Evaluating Different Catch Crop Strategies for Closing the Nitrogen Cycle in Cropping Systems—Field Experiments and Modelling. Sustainability 2021, 13, 394. [Google Scholar] [CrossRef]
- Sieling, K. Improved N transfer by growing catch crops—A challenge. J. Für Kult. 2019, 71, 145–160. [Google Scholar] [CrossRef]
- Hansen, E.M.; Munkholm, L.J.; Olesen, J.E.; Melander, B. Nitrate Leaching, Yields and Carbon Sequestration after Noninversion Tillage, Catch Crops, and Straw Retention. J. Environ. Qual. 2015, 44, 868–881. [Google Scholar] [CrossRef]
- Gómez-Muñoz, B.; Jensen, L.S.; Munkholm, L.; Olesen, J.E.; Hansen, E.M.; Bruun, S. Long-term effect of tillage and straw retention in conservation agriculture systems on soil carbon storage. Soil Sci. Soc. Am. J. 2021, 85, 1465–1478. [Google Scholar] [CrossRef]
- Hansen, E.M.; Munkholm, L.J.; Melander, B.; Olesen, J.E. Can non-inversion tillage and straw retainment reduce N leaching in cereal-based crop rotations? Soil Tillage Res. 2010, 109, 1–8. [Google Scholar] [CrossRef]
- Katuwal, S.; Norgaard, T.; Moldrup, P.; Lamandé, M.; Wildenschild, D.; de Jonge, L.W. Linking air and water transport in intact soils to macropore characteristics inferred from X-ray computed tomography. Geoderma 2015, 237–238, 9–20. [Google Scholar] [CrossRef]
- Paradelo, M.; Katuwal, S.; Moldrup, P.; Norgaard, T.; Herath, L.; de Jonge, L.W. X-ray CT-Derived Soil Characteristics Explain Varying Air, Water, and Solute Transport Properties across a Loamy Field. Vadose Zone J. 2016, 15, 1–13. [Google Scholar] [CrossRef]
- Rubek, F. Sammendrag af Vinter 2020–2021. 2021. Available online: https://www.dmi.dk/fileadmin/user_upload/Afrapportering/Seasonsammendrag/Sammendrag_af_vinter_2020-2021.pdf (accessed on 3 March 2022).
- Best, E.K. An Authomated Method for Determining Nitrate-Nitrogen in Soil Extracts. Qld. J. Agric. Anim. Sci. 1976, 33, 161–166. [Google Scholar]
- Crooke, W.M.; Simpson, W.E. Determination of ammonium in Kjeldahl digests of crops by an automated procedure. J. Sci. Food Agric. 1971, 22, 9–10. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.1.0); R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 3 March 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R. Emmeans: Estimated Marginal Means, AKA Least-Squares Means (R Package Version 1.6.2-1). 2021. Available online: https://cran.r-project.org/package=emmeans (accessed on 3 March 2022).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Soto-Gómez, D.; Pérez-Rodríguez, P.; Vázquez-Juiz, L.; López-Periago, J.E.; Paradelo, M. Linking pore network characteristics extracted from CT images to the transport of solute and colloid tracers in soils under different tillage managements. Soil Tillage Res. 2018, 177, 145–154. [Google Scholar] [CrossRef]
- Vos, J.; van der Putten, P.E.L. Field observations on nitrogen catch crops. I. Potential and actual growth and nitrogen accumulation in relation to sowing date and crop species. Plant Soil 1997, 195, 299–309. [Google Scholar] [CrossRef]
- Gerke, H.H. Preferential flow descriptions for structured soils. J. Plant Nutr. Soil Sci. 2006, 169, 382–400. [Google Scholar] [CrossRef]
- Shipitalo, M.J.; Dick, W.A.; Edwards, W.M. Conservation tillage and macropore factors that affect water movement and the fate of chemicals. Soil Tillage Res. 2000, 53, 167–183. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Hawkesford, M.J.; Whalley, W.R.; Zhou, H.; Mooney, S.J. Soil strength influences wheat root interactions with soil macropores. Plant Cell Environ. 2020, 43, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Vélez, J.F.; Vogeler, I. Exploring Temperature-Related Effects in Catch Crop Net N Mineralization Outside of First-Order Kinetics. Nitrogen 2021, 2, 110–127. [Google Scholar] [CrossRef]
- Thomsen, I.K.; Elsgaard, L.; Olesen, J.E.; Christensen, B.T. Nitrogen release from differently aged Raphanus sativus L. nitrate catch crops during mineralization at autumn temperatures. Soil Use Manag. 2016, 32, 183–191. [Google Scholar] [CrossRef]
- Gan, Y.T.; Liang, B.C.; Liu, L.P.; Wang, X.Y.; McDonald, C.L. C:N ratios and carbon distribution profile across rooting zones in oilseed and pulse crops. Crop Pasture Sci. 2011, 62, 496–503. [Google Scholar] [CrossRef]
- Munkholm, L.J.; Hansen, E.M.; Olesen, J.E. The effect of tillage intensity on soil structure and winter wheat root/shoot growth. Soil Use Manag. 2008, 24, 392–400. [Google Scholar] [CrossRef]
- Smit, A.L.; Groenwold, J. Root characteristics of selected field crops: Data from the Wageningen Rhizolab (1990–2002). Plant Soil 2005, 272, 365–384. [Google Scholar] [CrossRef] [Green Version]
- Kemper, R.; Bublitz, T.A.; Müller, P.; Kautz, T.; Döring, T.F.; Athmann, M. Vertical Root Distribution of Different Cover Crops Determined with the Profile Wall Method. Agriculture 2020, 10, 503. [Google Scholar] [CrossRef]
- Guo, B.-B.; Liu, B.-C.; He, L.; Wang, Y.-Y.; Feng, W.; Zhu, Y.-J.; Jiao, N.-Y.; Wang, C.-Y.; Guo, T.-C. Root and nitrate-N distribution and optimization of N input in winter wheat. Sci. Rep. 2019, 9, 18018. [Google Scholar] [CrossRef]
- Rubek, F. Efterår 2019. Available online: https://www.dmi.dk/fileadmin/user_upload/Afrapportering/Seasonsammendrag/Sammendrag_2019_efteraar.pdf (accessed on 3 March 2022).
- Ramos, C.; Kücke, M. A Review of Methods for Nitrate Leaching Measurement. Acta Hortic. 2001, 563, 259–266. [Google Scholar] [CrossRef]

| Tillage | Plant Cover | Mean Total Recovered NNO3 (mg N) | 95% CI (mg N) | Group 1 |
|---|---|---|---|---|
| NT | B | 18.4 | 12.09–24.62 | a |
| NT | V | 7.1 | 0.83–13.36 | b |
| AuT | B | 21.1 | 14.86–27.39 | a |
| AuT | V | 19.4 | 13.12–25.64 | a |
| Experimental Set | Tillage | Plant Cover | Total Nmin (mg N) | 95% CI (mg N) | Group 1 |
|---|---|---|---|---|---|
| Reference | NT | B | 46.14 | 35.15–56.1 | a |
| Lab-rain | 21.15 | 11.17–31.1 | b | ||
| Difference | 24.99 | ||||
| Reference | NT | V | 32.55 | 22.57–42.5 | a |
| Lab-rain | 24.16 | 14.17–34.1 | a | ||
| Difference | 8.39 | ||||
| Reference | AuT | B | 33.02 | 23.04–43.0 | a |
| Lab-rain | 7.98 | −2.01–18.0 | b | ||
| Difference | 25.05 | ||||
| Reference | AuT | V | 35.75 | 25.76–45.7 | a |
| Lab-rain | 13.57 | 3.59–23.6 | b | ||
| Difference | 22.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Vélez, J.F.; Vogeler, I. Autumn Tillage Reduces the Effect of Plant Cover on Topsoil Nitrogen Leaching. Nitrogen 2022, 3, 186-196. https://doi.org/10.3390/nitrogen3020014
Miranda-Vélez JF, Vogeler I. Autumn Tillage Reduces the Effect of Plant Cover on Topsoil Nitrogen Leaching. Nitrogen. 2022; 3(2):186-196. https://doi.org/10.3390/nitrogen3020014
Chicago/Turabian StyleMiranda-Vélez, Jorge F., and Iris Vogeler. 2022. "Autumn Tillage Reduces the Effect of Plant Cover on Topsoil Nitrogen Leaching" Nitrogen 3, no. 2: 186-196. https://doi.org/10.3390/nitrogen3020014
APA StyleMiranda-Vélez, J. F., & Vogeler, I. (2022). Autumn Tillage Reduces the Effect of Plant Cover on Topsoil Nitrogen Leaching. Nitrogen, 3(2), 186-196. https://doi.org/10.3390/nitrogen3020014







