Nitrogen Pools in Tropical Plantations of N2-Fixing and Non-N2-Fixing Legume Trees under Different Tree Stand Densities
Abstract
1. Introduction
2. Results
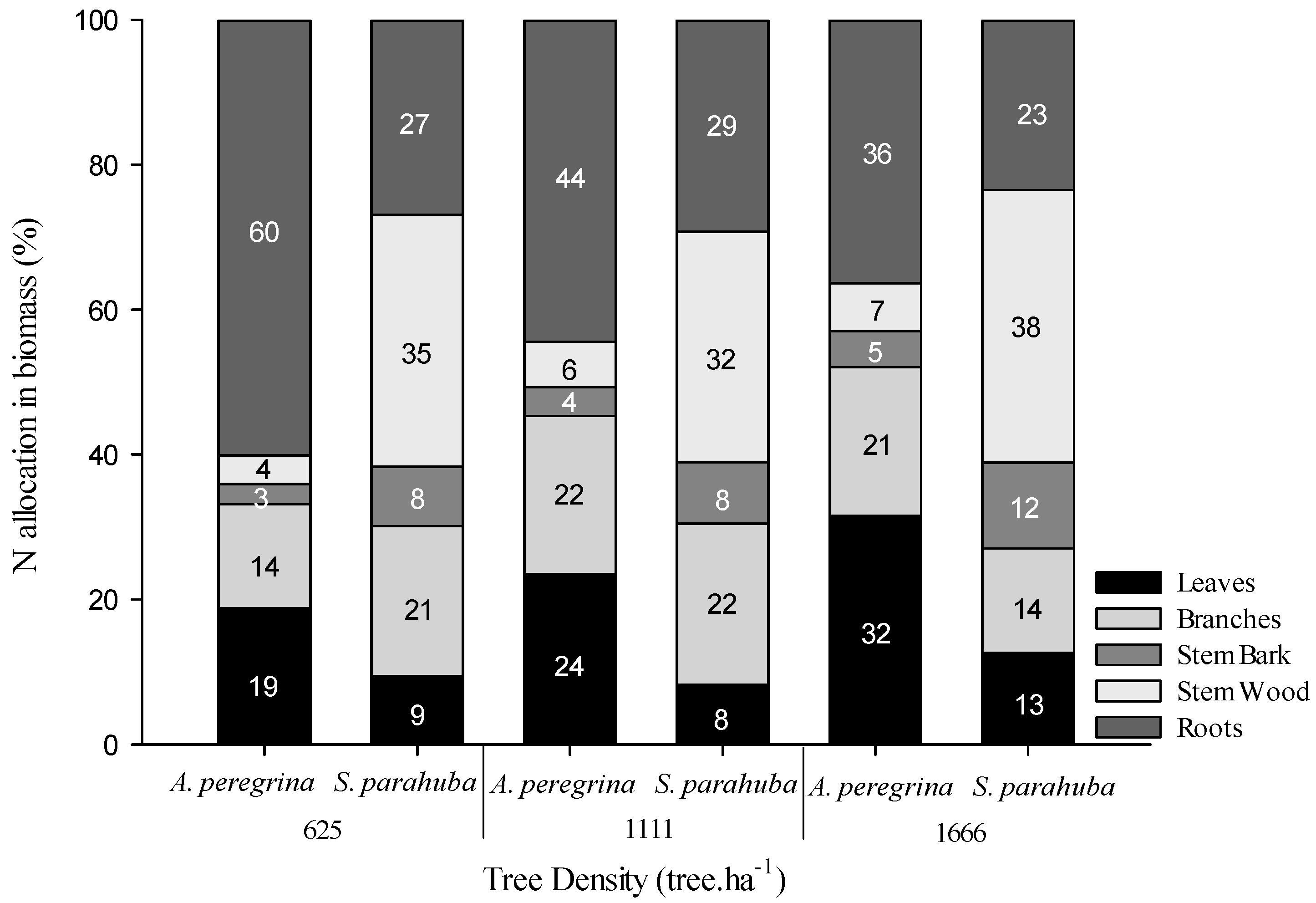
2.1. N Pools in the Biomass and Soil
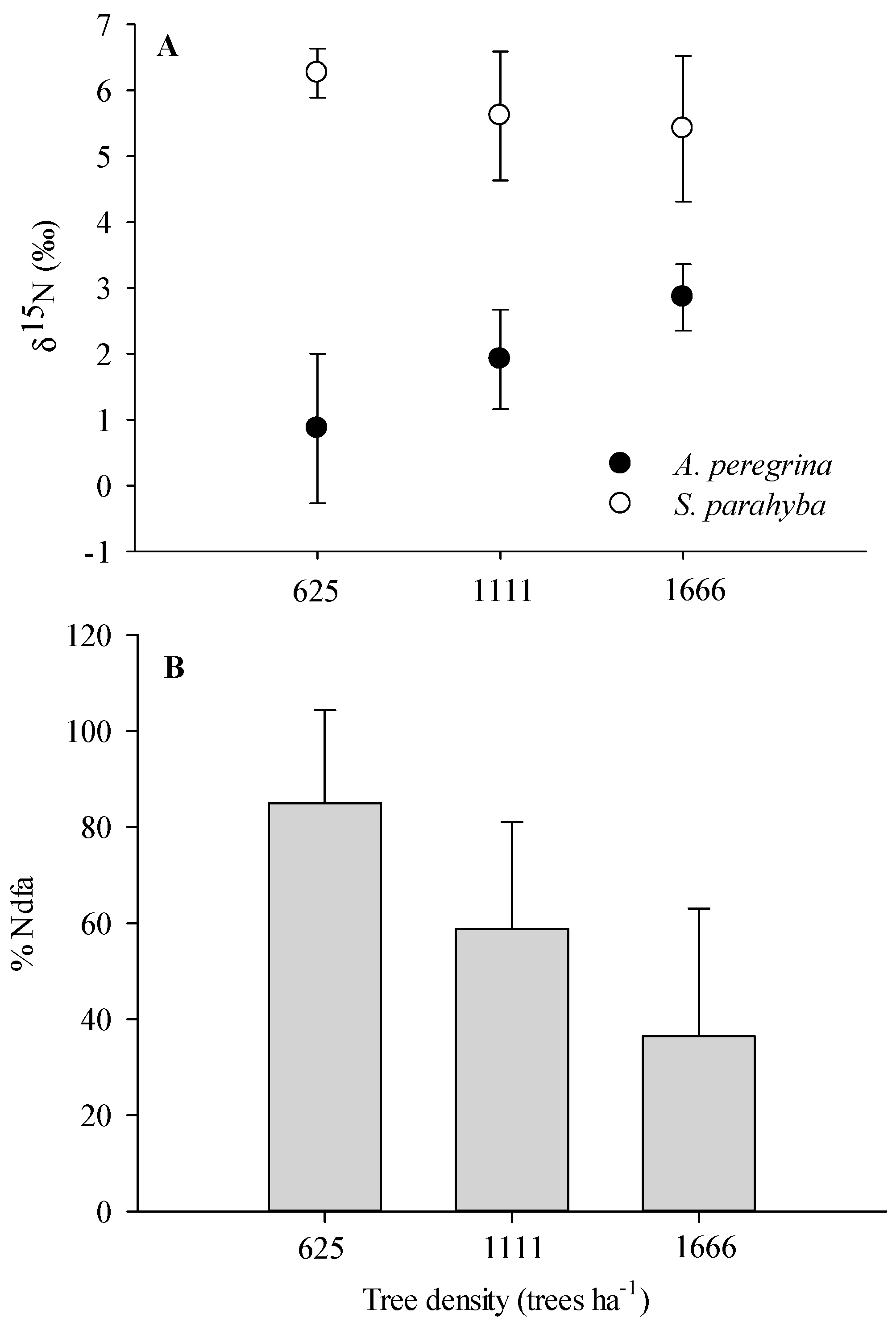
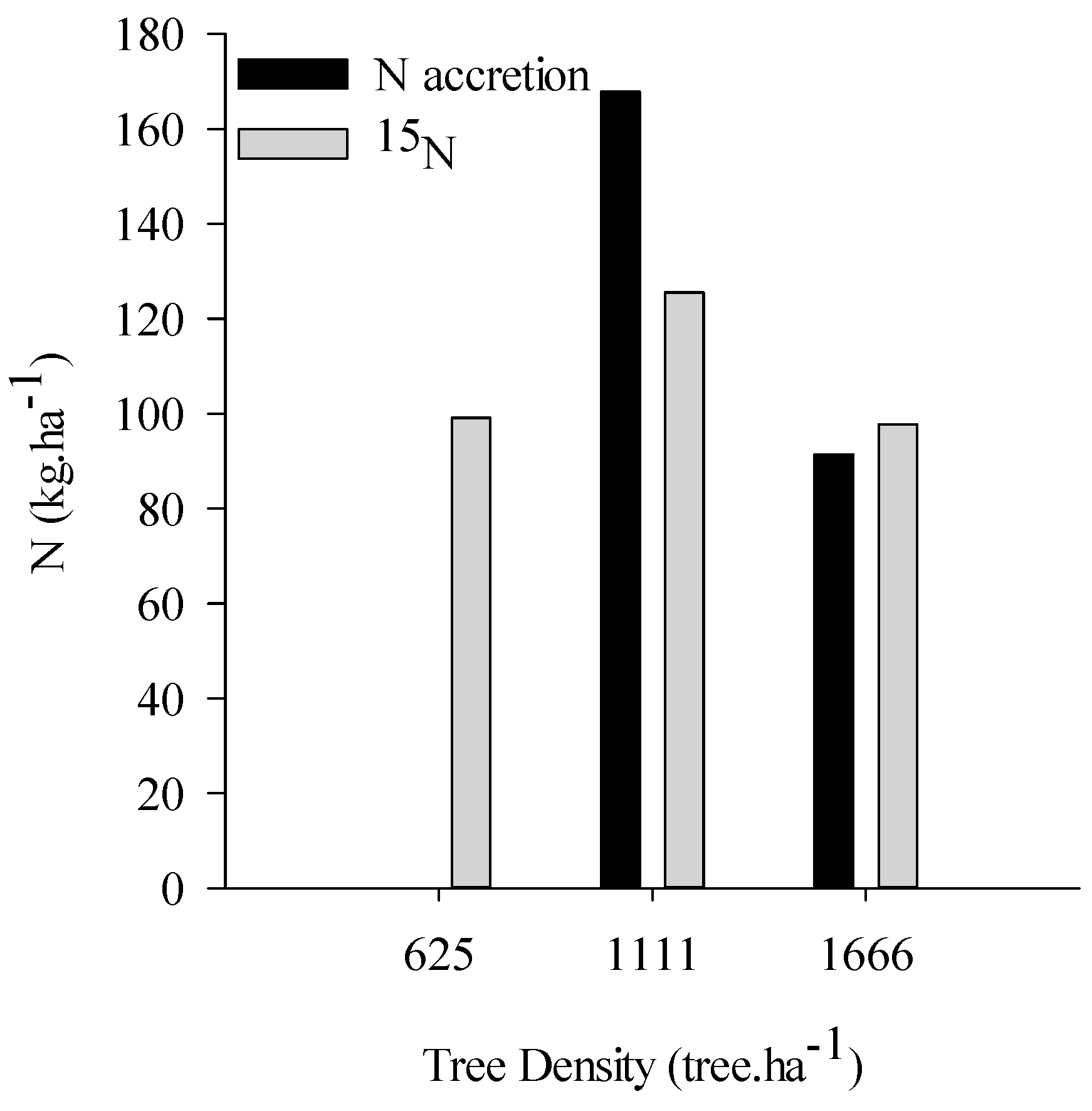
2.2. N Pools via Biological N2 Fixation
3. Discussion
3.1. N Pools in Stands with N2 and Non-N2 Fixing Species
3.2. Biological N Fixation as a Function of Tree Densities and Methods
4. Materials and Methods
4.1. Characterization of the Study Area
4.2. N Pools in Tree Biomass
4.3. N Pools in the Soil
4.4. N Pools via Biological N2 Fixation
4.4.1. 15N Natural Abundance Method
4.4.2. N Accretion Method
4.5. Statistical Analysis of the Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aronson, J.; Goodwin, N.; Orlando, L.; Eisenberg, C.; Cross, A.T. A world of possibilities: Six restoration strategies to support the United Nation’s Decade on Ecosystem Restoration. Restor. Ecol. 2020, 28, 730–736. [Google Scholar] [CrossRef]
- Growing Better: Ten Critical Transitions to Transform Food and Land Use; The Global Consultation Report of the Food and Land Use Coalition. 2019, p. 237. Available online: https://www.foodandlandusecoalition.org/global-report/ (accessed on 5 May 2020).
- Rezende, C.L.; Scarano, F.R.; Assad, E.D.; Joly, C.A.; Metzger, J.P.; Straussburg, B.B.N.; Tabarelli, M.T.; Fonseca, G.A.; Mittermeier, R.A. From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 2018, 16, 208–214. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Silva, R.F.B.; Batistela, M.; Moran, E.F. Drivers of land change: Human-environment interactions and the Atlantic forest transition in the Paraíba Valley, Brazil. Land Use Policy 2016, 58, 133–144. [Google Scholar] [CrossRef]
- IBÁ (Indústria Brasileira De Árvores). Relatório 2019. p. 80. Available online: https://iba.org/datafiles/publicacoes/relatorios/iba-relatorioanual2019.pdf (accessed on 5 May 2020).
- Gonçalves, J.L.M.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; Ferraz, S.F.B.; Lima, W.P.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Gonçalves, J.L.M.; Alvares, C.A.; Rocha, J.H.T.; Brandani, C.B.; Hakamada, R. Eucalyptus plantation managment in regions with water stress. South. For. 2017, 79, 169–183. [Google Scholar] [CrossRef]
- Rolim, S.G.; Piña-Rodrigues, F.C.M.; Piotto, D.; Batista, A.; Freitas, M.L.M.; Brienza, S., Jr.; Zakia, M.J.B.; Calmon, M. Research Gaps and Priorities in Silviculture of Native Species. In WRI Brasil; Working Paper; São Paulo, Brazil, 2019; Available online: https://wribrasil.org.br/pt/publicacoes (accessed on 5 May 2020).
- Ferreira, T.M.C.; De Carvalho, J.O.P.; Emmert, F.; Ruschel, A.R.; Nascimento, R.G.M. How long does the Amazon rainforest take to grow commercially sized trees? An estimation methodology for Manilkara elata (Allemão ex Miq.) Monach. For. Ecol. Manag. 2020, 473, 118333. [Google Scholar] [CrossRef]
- Gama-Rodrigues, A.C. Multifunctional Mixed-Forest Plantations: The Use of Brazilian Native Leguminous Tree Species for Sustainable Rural Development. In Mixed Plantations of Eucalyptus and Leguminous Trees, 1st ed.; Bran Nogueira Cardoso, E., Gonçalves, J., Balieiro, F., Franco, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 241–256. [Google Scholar] [CrossRef]
- Silva, P.S.L.; Paiva, H.N.D.; Oliveira, V.R.D.; Siqueira, P.L.D.O.F.; Soares, E.B.; Monteiro, A.L.; Tavella, L.B. Biomassas de espécies arbóreas em resposta a densidades de plantio e à competição interespecífica. Rev. Árvore 2014, 38, 319–329. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.H.; Stewart, B.A. Responses of crop plants to ammonium and nitrate N. In Advances in Agronomy; Donald, L.B.T., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 118, pp. 205–397. [Google Scholar] [CrossRef]
- Gehring, C.; Vlek, P.L.G.; Souza, L.A.G.; Denich, M. Biological nitrogen fixation in secondary regrowth and mature rainforest of central Amazonia. Agric. Ecosyst. Environ. 2005, 111, 237–2452. [Google Scholar] [CrossRef]
- Chaer, G.M.; Resende, A.S.; Campello, E.F.C.; De Faria, S.M.; Boddey, R.M. Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol. 2011, 31, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Nardoto, G.B.; Quesada, C.A.; Patiño, S.; Saiz, G.; Baker, T.R.; Schwarz, M.; Schrodt, F.; Feldpausch, T.R.; Domingues, T.F.; Marimon, B.S.; et al. Basin-wide variations in Amazon forest nitrogen-cycling characteristics as inferred from plant and soil 15N:14N measurements. Plant Ecol. Diveristy 2014, 7, 173–187. [Google Scholar] [CrossRef]
- Santos, F.M.; Chaer, G.M.; Diniz, A.R.; Balieiro, F.C. Nutrient cycling over five years of mixed-species plantations of Eucalyptus and Acacia on a sandy tropical soil. For. Ecol. Manag. 2017, 384, 110–121. [Google Scholar] [CrossRef]
- Voigtlaender, M.; Brandani, C.B.; Caldeira, D.R.M.; Tardy, F.; Bouillet, J.P.; Gonçalves, J.L.M.; Moreira, M.Z.; Leite, F.P.; Brunet, D.; Paula, R.R.; et al. Nitrogen cycling in monospecific and mixed-species plantations of Acacia mangium and Eucalyptus at 4 sites in Brazil. For. Ecol. Manag. 2019, 436, 56–67. [Google Scholar] [CrossRef]
- Munroe, J.W.; Isaac, M.E. N2-fixing trees and the transfer of fixed-N for sustainable agroforestry: A review. Agron. Sustain. Dev. 2014, 34, 417–427. [Google Scholar] [CrossRef]
- Paula, R.R.; Bouillet, J.P.; Trivelin, P.C.O.; Zeller, B.; Gonçalves, J.L.M.; Nouvellon, Y.; Bouvet, J.M.; Plassard, C.; Laclau, J.P. Evidence of short-term belowground transfer of nitrogen from Acacia mangium to Eucalyptus grandis trees in a tropical planted forest. Soil Biol. Biochem. 2015, 91, 99–108. [Google Scholar] [CrossRef]
- Kaye, J.P.; Resh, S.C.; Kaye, M.W.; Chimner, R.A. Nutrient and Carbon Dynamics in a Replacement Series of Eucalyptus and Albizia Trees. Ecology 2000, 81, 3267–3273. [Google Scholar] [CrossRef]
- Resh, S.C.; Binkley, D.; Parrotta, J.A. Greater Soil Carbon Sequestration under Nitrogen-fixing Trees Compared with Eucalyptus Species. Ecosystems 2002, 5, 217–231. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Cassman, K.; Cleveland, C.; Crews, T.; Field, C.B.; Grimm, N.B.; Howarth, R.W.; Marino, R.; Martinelli, L.; Rastetter, E.B.; et al. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 2002, 57, 1–45. [Google Scholar] [CrossRef]
- Paula, R.R.; Bouillet, J.P.; Gonçalves, J.L.M.; Trivelin, P.C.O.; Balieiro, F.C.; Nouvellon, Y.; Oliveira, J.C.; Deus Júnior, J.C.; Bordron, B.; Laclau, J.P. Nitrogen fixation rate of Acacia Mangium wild at mid rotation in Brazil is higher in mixed plantations with Eucalyptus Grandis Hill ex Maiden than in monocultures. Ann. For. Sci. 2018, 75, 14. [Google Scholar] [CrossRef]
- Gourion, B.; Berrabah, F.; Ratet, P.; Stacey, G. Rhizobium–legume symbioses: The crucial role of plant immunity. Trends Plant Sci. 2015, 20, 186–194. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Li, Y.; Chen, D.; Ao, J.; Zhou, W.; Jiang, Y. Influence of nitrogen and phosphorus additions on N2-fixation activity, abundance, and composition of diazotrophic communities in a Chinese fir plantation. Sci. Total Environ. 2018, 619, 1530–1537. [Google Scholar] [CrossRef]
- Chikoti, Y.F.; Duangkhet, M.; Chungopast, S.; Tajima, S.; Ma, J.F.; Nomura, M. Effect of ferritin on nitrogen fixation in Lotus japonicus nodules under various iron concentrations. J. Plant Physiol. 2020, 252, 153247. [Google Scholar] [CrossRef] [PubMed]
- Petit, G.; Savi, T.; Consolini, M.; Anfodillo, T.; Nardini, A. Interplay of growth rate and xylem plasticity for optimal coordination of carbon and hydraulic economies in Fraxinus ornus trees. Tree Physiol. 2016, 36, 1310–1319. [Google Scholar] [CrossRef]
- Hoogmoed, M.; Cunningham, S.C.; Baker, P.; Beringer, J.; Cavagnaro, T.R. N-fixing trees in restoration plantings: Effects on nitrogen supply and soil microbial communities. Soil Biol. Biochem. 2014, 77, 203–212. [Google Scholar] [CrossRef]
- Faria, S.M.; Balieiro, F.C.; Paula, R.R.; Santos, F.M.; Zilli, J.L. Biological Nitrogen Fixation (BNF) in Mixed-Forest Plantations. In Mixed Plantations of Eucalyptus and Leguminous Trees: Soil, Microbiology and Ecosystem Services, 1st ed.; Cardoso, E.J.B.N., Gonçalves, J.L.M., Balieiro, F.C., Franco, A.A., Eds.; Springer: Cham, Switzerland, 2020; Volume 1, pp. 103–135. [Google Scholar] [CrossRef]
- Boddey, R.M.; Peoples, M.B.; Palmer, B.; Dart, P.J. Use of the 15N natural abundance technique to quantify biological nitrogen fixation by woody perennials. Nutr. Cycl. Agroecosyst. 2000, 57, 235–270. [Google Scholar] [CrossRef]
- Ruiz-Navarro, A.; Barberá, G.G.; Albaladejo, J.; Querejeta, J.I. Plant δ15N reflects the high landscape-scale heterogeneity of soil fertility and vegetation productivity in a Mediterranean semiarid ecosystem. New Phytol. 2016, 212, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Peoples, M.B.; Faizah, A.W.; Rerkasem, B.; Herridge, D.F. Methods for Evaluating Nitrogen Fixation by Nodulated Legumes in the Field; ACIAR: Sidney, Australia, 1989; p. 76.
- Forrester, D.I.; Schortemeyer, M.; Stock, W.D.; Bauhus, J.; Khanna, P.K.; Cowie, A.L. Assessing nitrogen fixation in mixed-and single-species plantations of Eucalyptus globulus and Acacia mearnsii. Tree Physiol. 2007, 27, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Unkovich, M.J.; Herridge, D.; Peoples, M.B.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; Australian Centre for International Agricultural Research: Canberra, Australia, 2008; p. 258.
- Chalk, P.M.; Inácio, C.T.; Balieiro, F.C.; Rouws, J.R.C. Do techniques based on 15N enrichment and 15N natural abundance give consistent estimates of the symbiotic dependence of N2-fixing plants? Plant Soil 2016, 399, 415–426. [Google Scholar] [CrossRef]
- Zhou, Y.; Mushinski, R.M.; Hyodo, A.; Wu, X.B.; Boutton, T.W. Vegetation change alters soil profile δ15N values at the landscape scale. Soil Biol. Biochem. 2018, 119, 110–120. [Google Scholar] [CrossRef]
- Yu, C.B.; Li, Y.Y.; Li, C.J.; Sun, J.H.; He, X.H.; Zhang, F.S.; Li, L. An improved nitrogen difference method for estimating biological nitrogen fixation in legume-based intercropping systems. Biol. Fertil. Soils 2010, 46, 227–235. [Google Scholar] [CrossRef]
- Cordeiro, I.M.C.C.; Barros, P.L.C.; Lameira, O.A.; Gazel Filho, A.B. Avaliação de plantios de paricá (Schizolobium parahyba var. amazonicum (Huber ex Ducke) Barneby) de diferentes idades e sistemas de cultivo no município de aurora do Pará-PA (BRASIL). Cienc. Florest. 2015, 25, 679–687. [Google Scholar] [CrossRef]
- Barberi, A.; Carneiro, M.A.; Moreira, F.M.; Siqueira, J.O. Nodulação em leguminosas florestais em viveiros no sul de Minas Gerais. Cerne 1998, 4, 145–153. [Google Scholar]
- Souza, C.O.; Silva, J.G.M.; Arantes, M.D.C.; Vidaurre, G.B.; Júnior, A.D.; Oliveira, M.P. Pyrolysis of Anadenanthera peregrina wood grown in different spacings from a forest plantation in Brazil aiming at the energy production. Environ. Dev. Sustain. 2019, 22, 5153–5168. [Google Scholar] [CrossRef]
- Gross, E.; Cordeiro, L.; Caetano, F.H. Nodulação e micorrização em Anadenanthera peregrina var. falcata em solo de cerrado autoclavado e não autoclavado. Rev. Bras. Cienc. Solo 2004, 28, 95–101. [Google Scholar] [CrossRef]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.A.M.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2014, 38, 1785–1793. [Google Scholar] [CrossRef]
- Souza, P.H. Biomassa e Estoque de Carbono em Povoamento de Anadenanthera peregrina (l.) Speg Sob Diferentes Espaçamentos. Ph.D. Thesis, Universidade Federal do Espírito Santo, Jerônimo Monteiro, Brazil, 2018. [Google Scholar]
- Delarmelina, W.M. Biomassa e Carbono em Plantios de Schizolobium parahyba var. Amazonicum Sob Diferentes Espaçamentos em Área de Pastagem. Ph.D. Thesis, Universidade Federal do Espírito Santo, Jerônimo Monteiro, Brazil, 2019. [Google Scholar]
- Rondon, E.V. Produção de biomassa e crescimento de árvores de Schizolobium amazonicum (Huber) Ducke sob diferentes espaçamentos na região de mata. Rev. Árvore 2002, 26, 573–576. [Google Scholar] [CrossRef]
- Campanharo, I.F. Mudanças Edáficas após Plantio de Leguminosas Arbóreas em Pastagem no sul do Espírito Santo. Bachelor’s Thesis, Universidade Federal do Espírito Santo, Jerônimo Monteiro, Brazil, 2017. [Google Scholar]
- Feller, C.; Beare, M.H. Physical control of soil organic matter dynamics in the tropics. Geoderma 1997, 79, 69–116. [Google Scholar] [CrossRef]
- Shi, S.; Peng, C.; Wang, M.; Zhu, Q.; Yang, G.; Yang, Y.; Xi, T.; Zhang, T. A global meta-analysis of changes in soil carbon, nitrogen, phosphorus and sulfur, and stoichiometric shifts after forestation. Plant Soil 2016, 407, 323–340. [Google Scholar] [CrossRef]
- Rachid, C.T.C.C.; Balieiro, F.C.; Peixoto, R.S.; Pinheiro, Y.A.S.; Piccolo, M.C.; Chaer, G.M.; Rosado, A.S. Mixed plantations can promote microbial integration and soil nitrate increases with changes in the N cycling genes. Soil Biol. Biochem. 2013, 66, 146–153. [Google Scholar] [CrossRef]
- Sheffer, E.; Batterman, S.A.; Levin, S.A.; Hedin, L.O. Biome-scale nitrogen fixation strategies selected by climatic constraints on nitrogen cycle. Nat. Plants 2015, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2000, 6, 751–765. [Google Scholar] [CrossRef]
- Högberg, P. 15N natural abundance in soil–plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M. Modeling monthly mean air temperature for Brazil. Theor. Appl. Climatol. 2013, 113, 407–427. [Google Scholar] [CrossRef]
- De Faria, S.M.; De Lima, H.C.; Sprent, J.I. Additional studies of the nodulation status of legume species in Brazil. Plant Soil 1998, 200, 185–192. [Google Scholar] [CrossRef]
- Reis Junior, F.B.; Simon, M.F.; Gross, E.; Boddey, R.M.; Elliott, G.N.; Neto, N.E.; De Fatima, L.M.; De Queiroz, L.P.; Scotti, M.R.; Chen, W.-M.; et al. Nodulation and nitrogen fixation by Mimosa spp. in the Cerrado and Caatinga biomes of Brazil. New Phytol. 2010, 186, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, E.S.; Matos, E.S. Matéria Orgânica do Solo: Métodos de Análises; UFV: Viçosa, Brazil, 2005; p. 107. [Google Scholar]
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef]
- Shearer, G.; Kohl, D.H. N2-fixation in field settings: Estimations based on natural 15N abundance. Aust. J. Plant Physiol. 1986, 13, 699–756. [Google Scholar] [CrossRef]
- Parrotta, J.A.; Baker, D.D.; Fried, M. Changes in dinitrogen fixation in maturing stands of Casuarina equisetifolia and Leucaena leucocephala. Can. J. For. Res. 1996, 26, 1684–1691. [Google Scholar] [CrossRef]
- Evans, J.; Taylor, A. Estimating dinitrogen (N2) fixation and soil accretion of nitrogen by grain legumes. J. Aust. Inst. Agric. Sci. 1987, 53, 78–82. [Google Scholar]
| Species | Density | Soil N | Aboveground N | Belowground N | Total N |
|---|---|---|---|---|---|
| (Trees ha−1) | ---------------------------- (kg ha−1) ---------------------------- | ||||
| 625 | 4902.8 ± 1088 a | 74.7 ± 43.6 b | 43.9 ± 33.2 | 5019.4 | |
| A. peregrina | 1111 | 4997.5 ± 1093 a | 143.1 ± 55.9 a | 71.7 ± 38.3 | 5211.4 |
| 1666 | 4464.7 ± 1113 a | 174.7 ± 52.2 a | 99.7 ± 26.3 | 4741.8 | |
| 625 | 4954.4 ± 1117 a | 87.5 ± 53.4 b | 32.1 ± 14.2 | 5074.8 | |
| S. parahyba | 1111 | 4906.7 ± 1111 a | 98.9 ± 55.1 b | 40.9 ± 17.0 | 5043.5 |
| 1666 | 4499.1 ± 1100 a | 115.8 ± 53.4 a | 35.5 ± 11.17 | 4650.3 | |
| Species | p-Value | Mean per Block (n = 9) | |||||
|---|---|---|---|---|---|---|---|
| Density | Block | D × B | 1 | 2 | 3 | ||
| A. peregrina | Nsoil | 0.507 | 0.01 | 0.963 | 5233.3 a | 5247.5 a | 3884.3 b |
| Nbio | <0.0001 | <0.0001 | 0.827 | 125.1 b | 84.3 b | 183.2 a | |
| S. parahyba | Nsoil | 0.347 | <0.0001 | 0.392 | 3479.8 b | 5298.6 a | 5582.0 a |
| Nbio | <0.0001 | <0.0001 | <0.0001 | 56.6 c | 138.6 a | 109.5 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bighi, K.N.; Paula, R.R.; Caldeira, M.V.W.; Burak, D.L.; Mendonça, E.d.S.; de Souza, P.H.; Delarmelina, W.M.; Balieiro, F.d.C. Nitrogen Pools in Tropical Plantations of N2-Fixing and Non-N2-Fixing Legume Trees under Different Tree Stand Densities. Nitrogen 2021, 2, 86-98. https://doi.org/10.3390/nitrogen2010006
Bighi KN, Paula RR, Caldeira MVW, Burak DL, Mendonça EdS, de Souza PH, Delarmelina WM, Balieiro FdC. Nitrogen Pools in Tropical Plantations of N2-Fixing and Non-N2-Fixing Legume Trees under Different Tree Stand Densities. Nitrogen. 2021; 2(1):86-98. https://doi.org/10.3390/nitrogen2010006
Chicago/Turabian StyleBighi, Kelly Nery, Ranieri Ribeiro Paula, Marcos Vinícius Winckler Caldeira, Diego Lang Burak, Eduardo de Sá Mendonça, Paulo Henrique de Souza, William Macedo Delarmelina, and Fabiano de Carvalho Balieiro. 2021. "Nitrogen Pools in Tropical Plantations of N2-Fixing and Non-N2-Fixing Legume Trees under Different Tree Stand Densities" Nitrogen 2, no. 1: 86-98. https://doi.org/10.3390/nitrogen2010006
APA StyleBighi, K. N., Paula, R. R., Caldeira, M. V. W., Burak, D. L., Mendonça, E. d. S., de Souza, P. H., Delarmelina, W. M., & Balieiro, F. d. C. (2021). Nitrogen Pools in Tropical Plantations of N2-Fixing and Non-N2-Fixing Legume Trees under Different Tree Stand Densities. Nitrogen, 2(1), 86-98. https://doi.org/10.3390/nitrogen2010006








