Physiological Responses of the Copepods Acartia tonsa and Eurytemora carolleeae to Changes in the Nitrogen:Phosphorus Quality of Their Food
Abstract
1. Introduction
2. Results
2.1. Algal Growth and Cell Composition
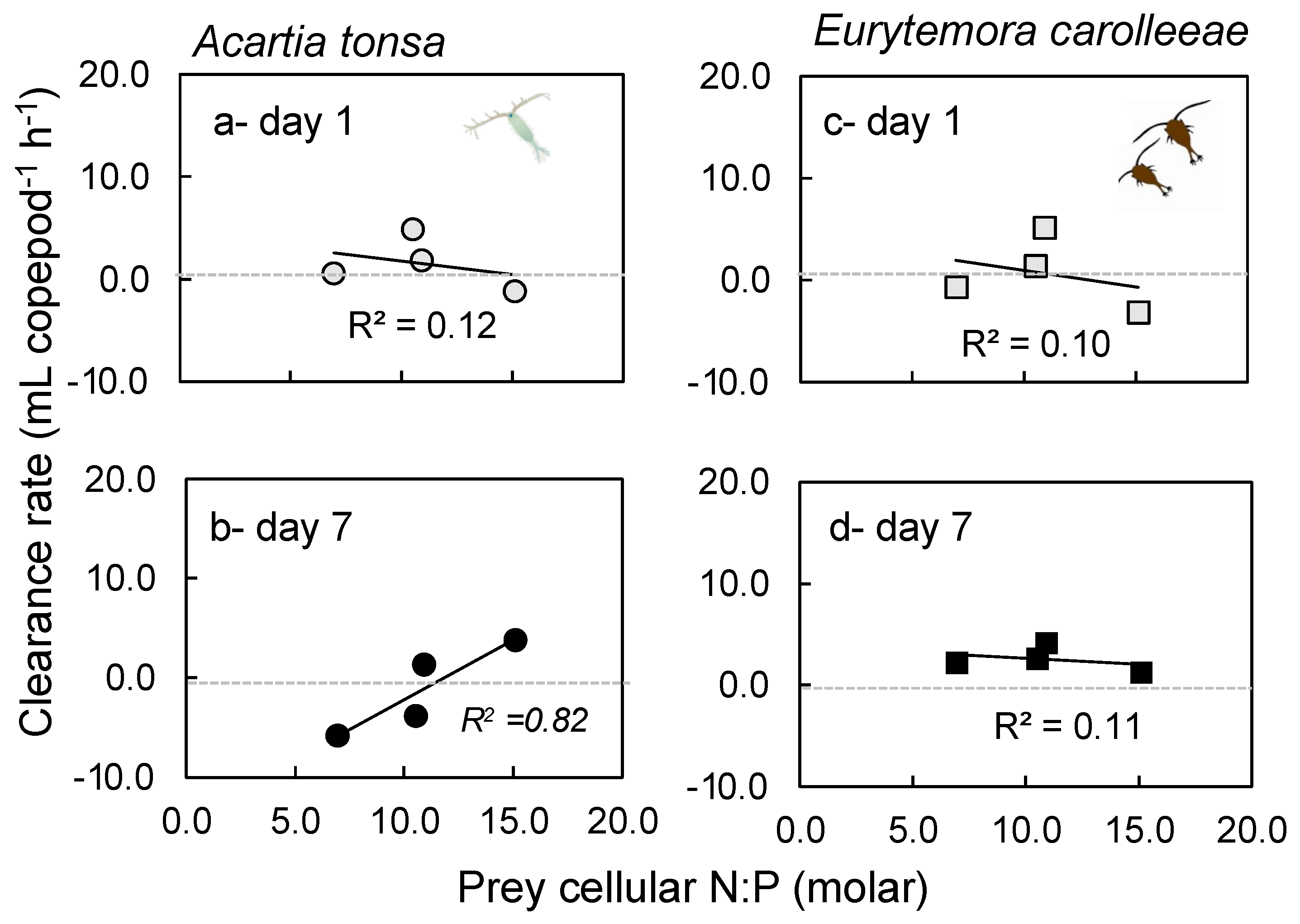
2.2. Grazing Rates
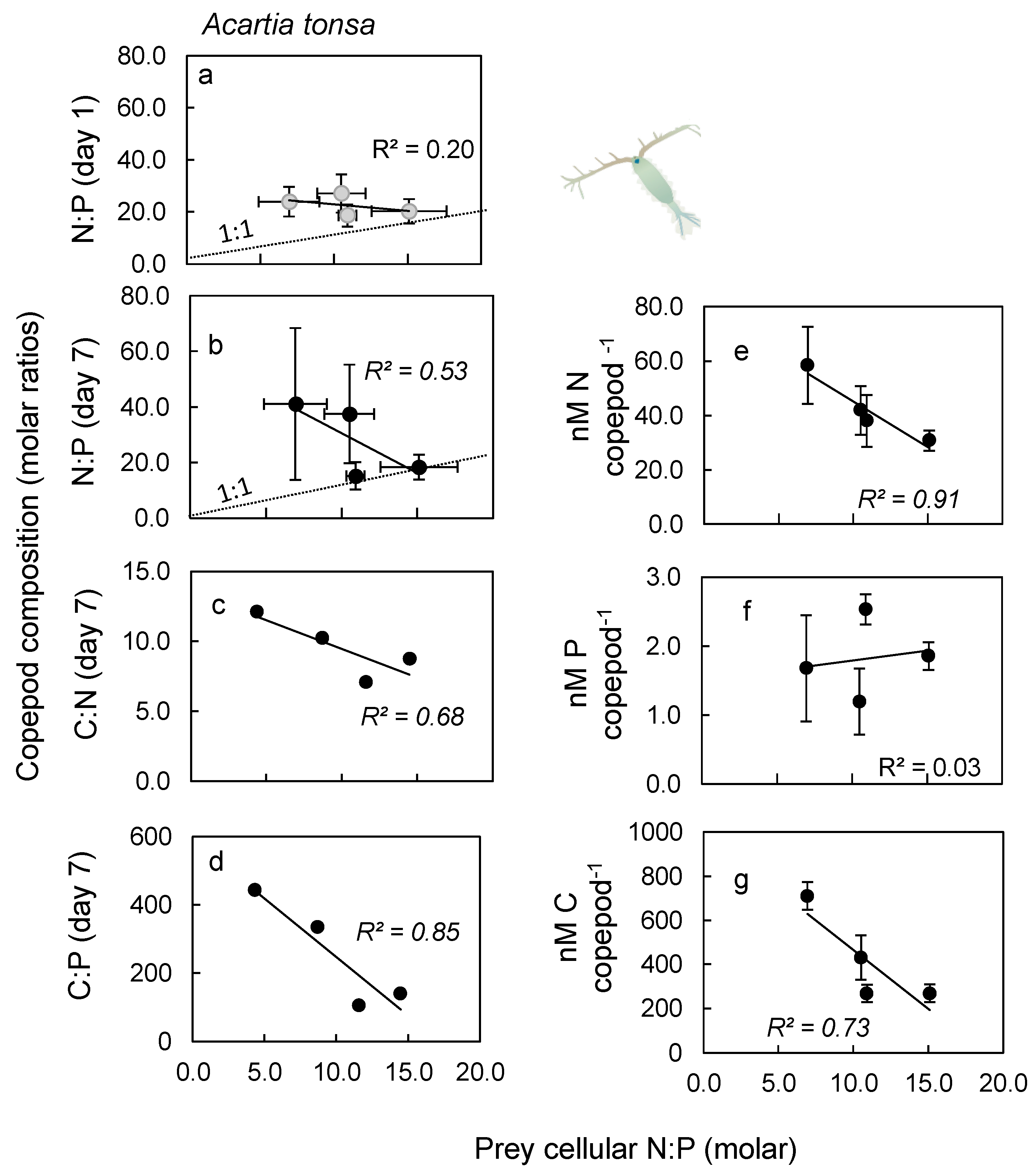
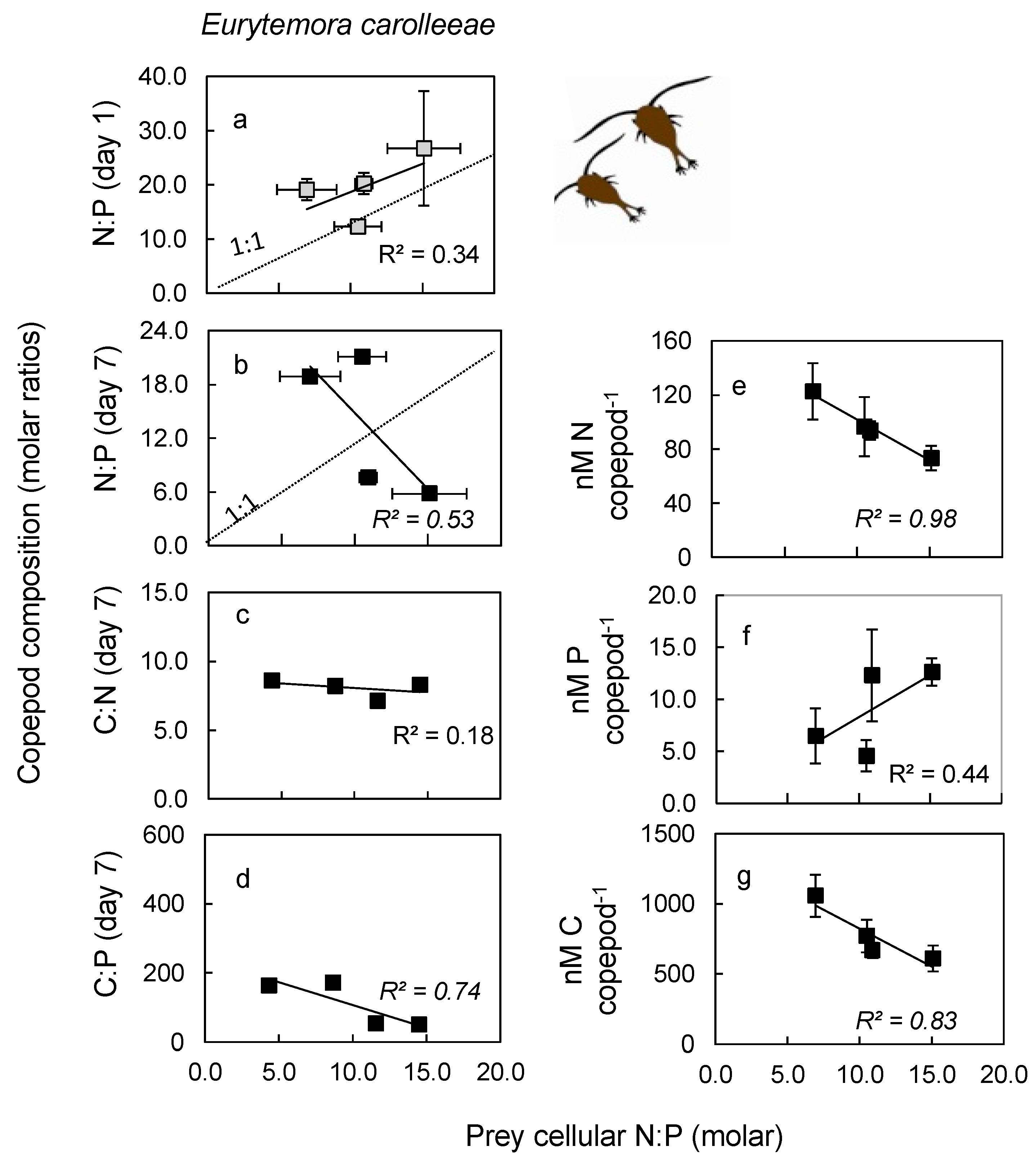
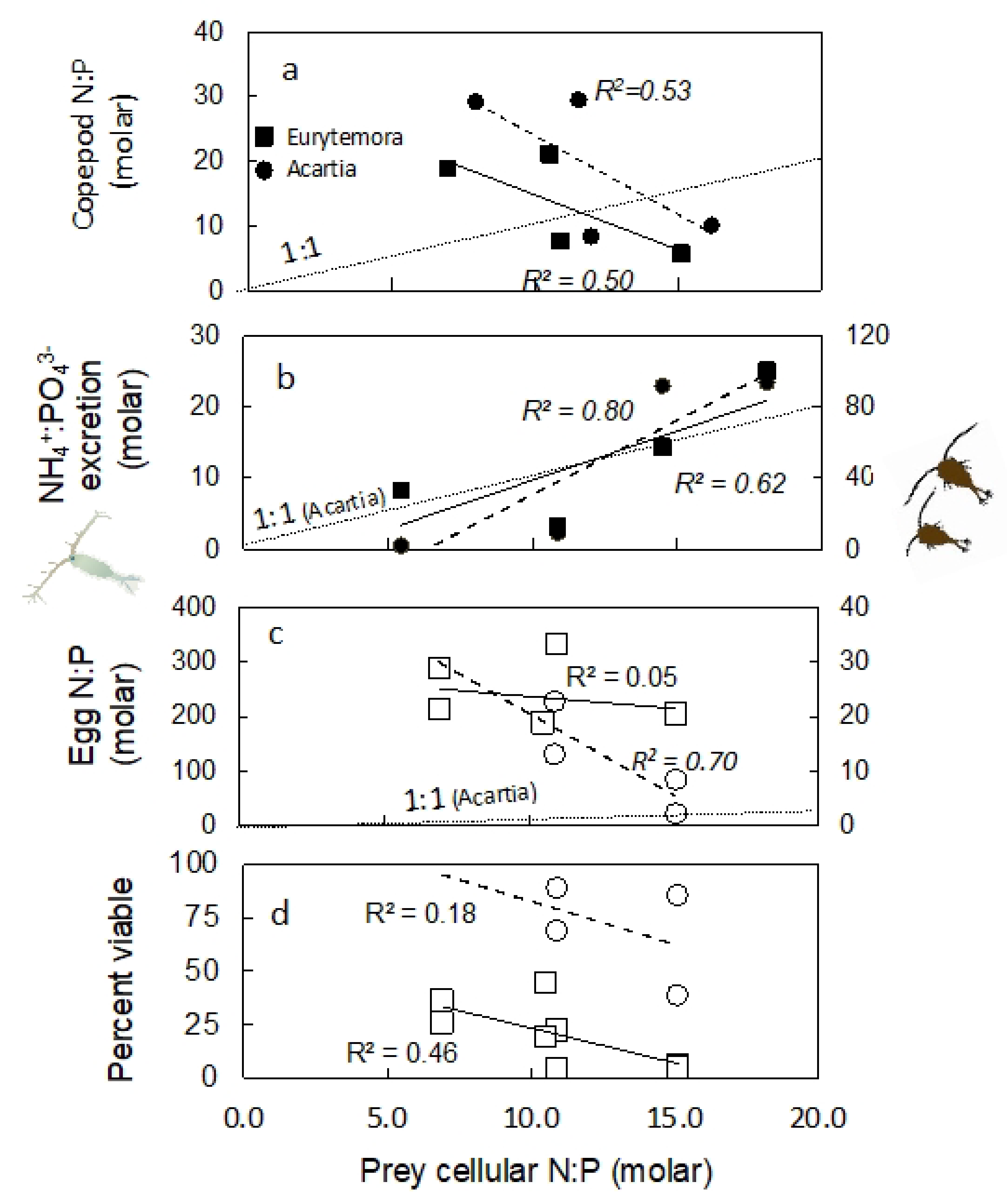
2.3. Nutrient Content in Copepod Tissue
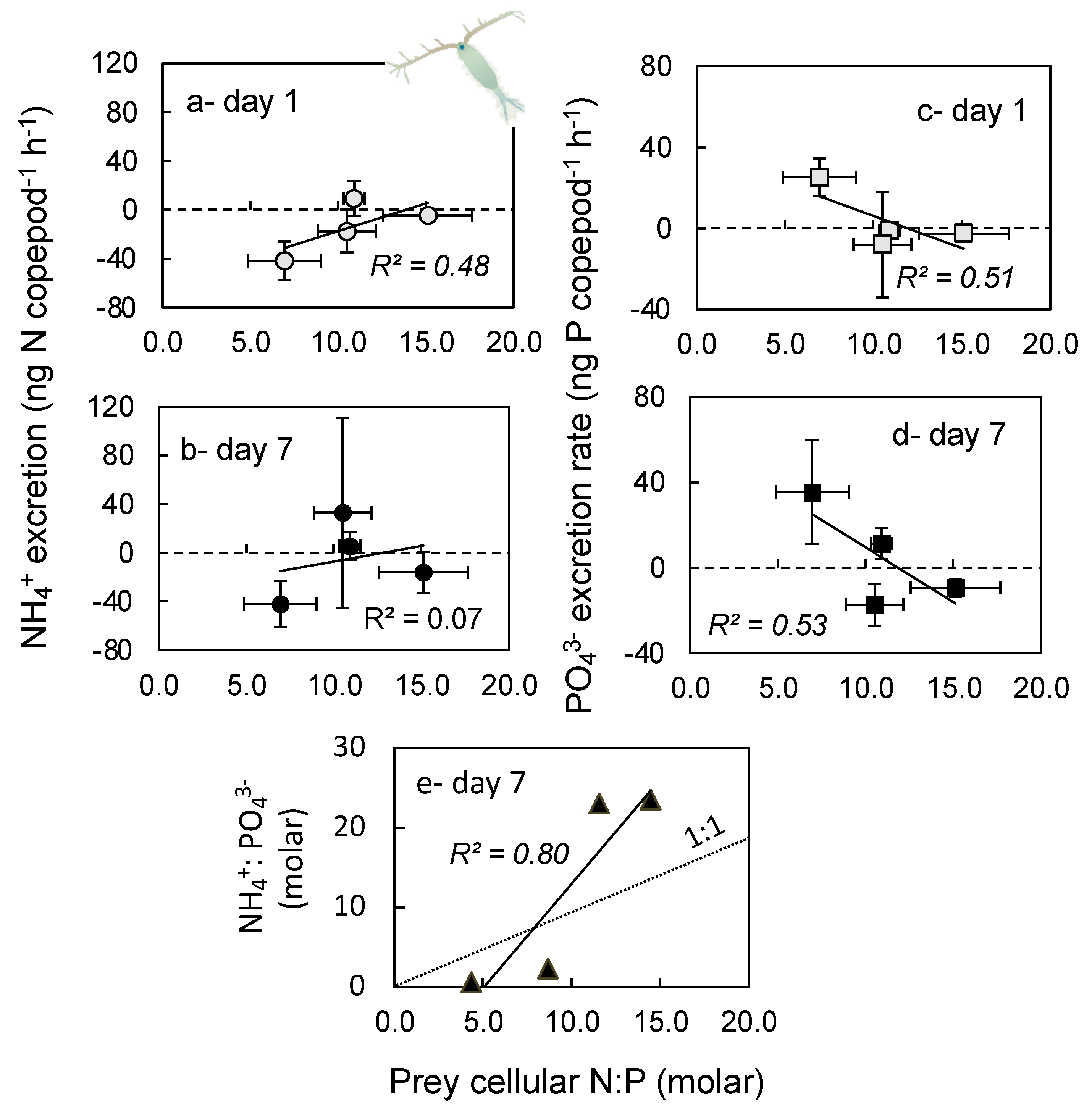
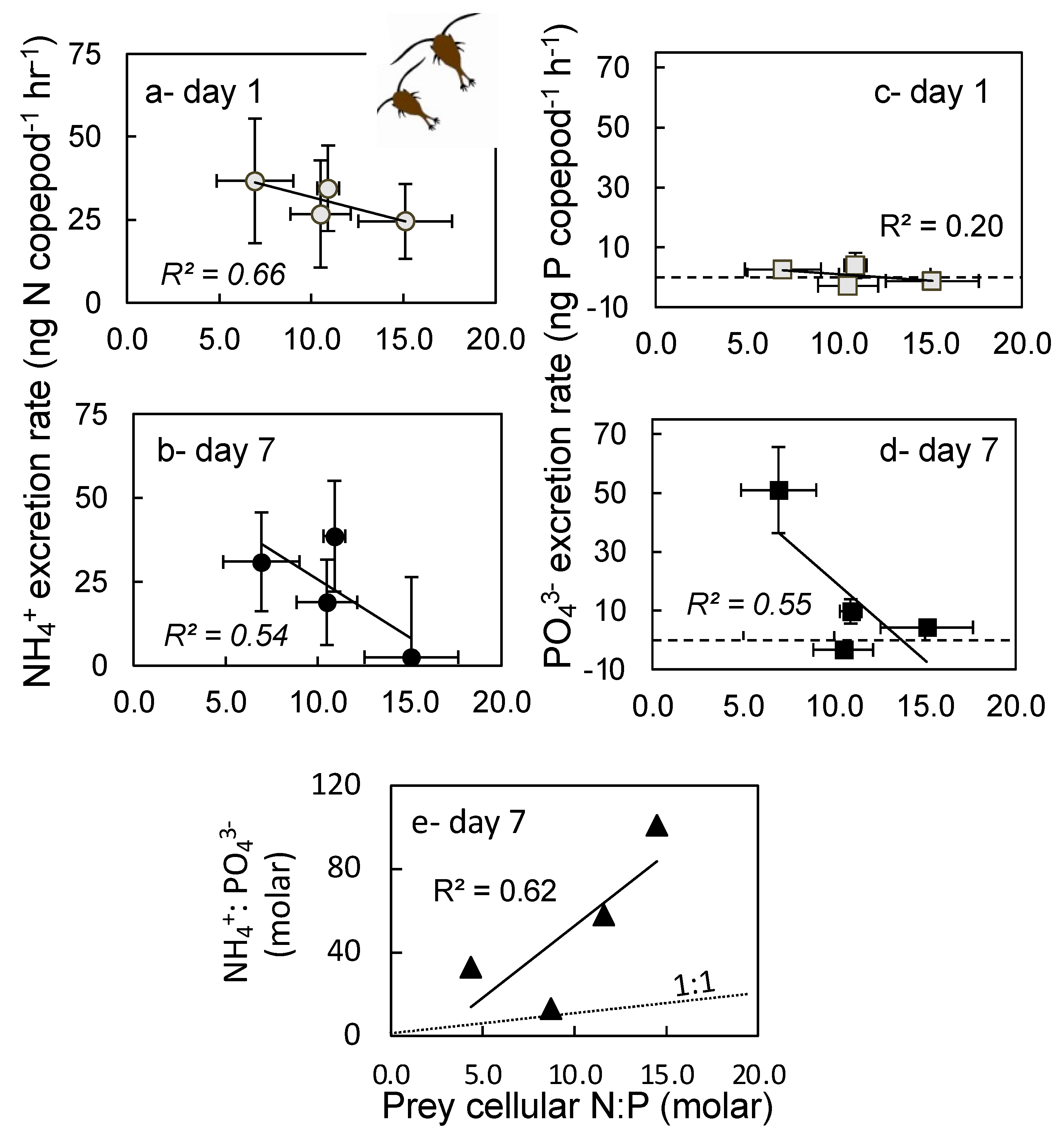
2.4. Excretion Rates
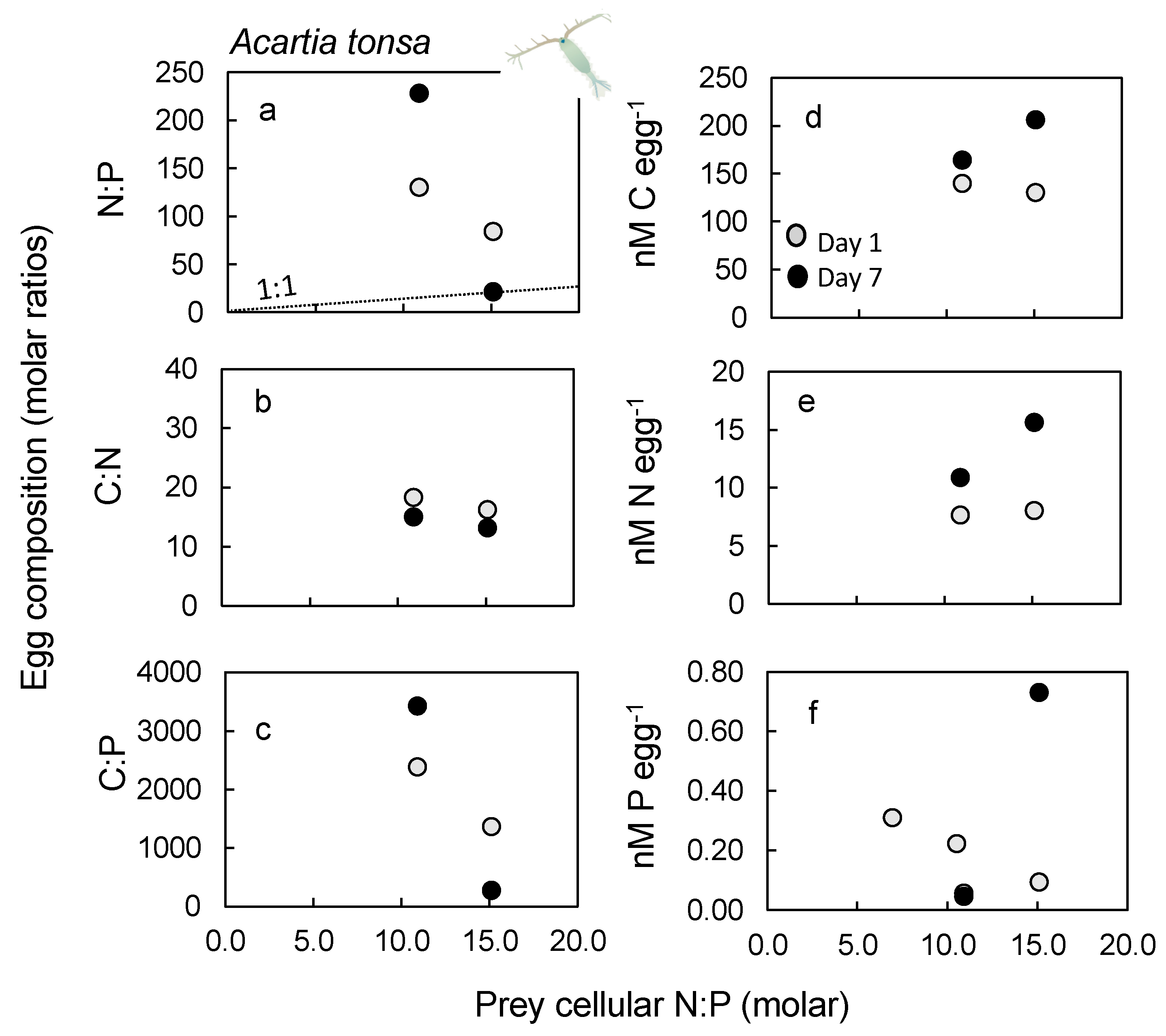
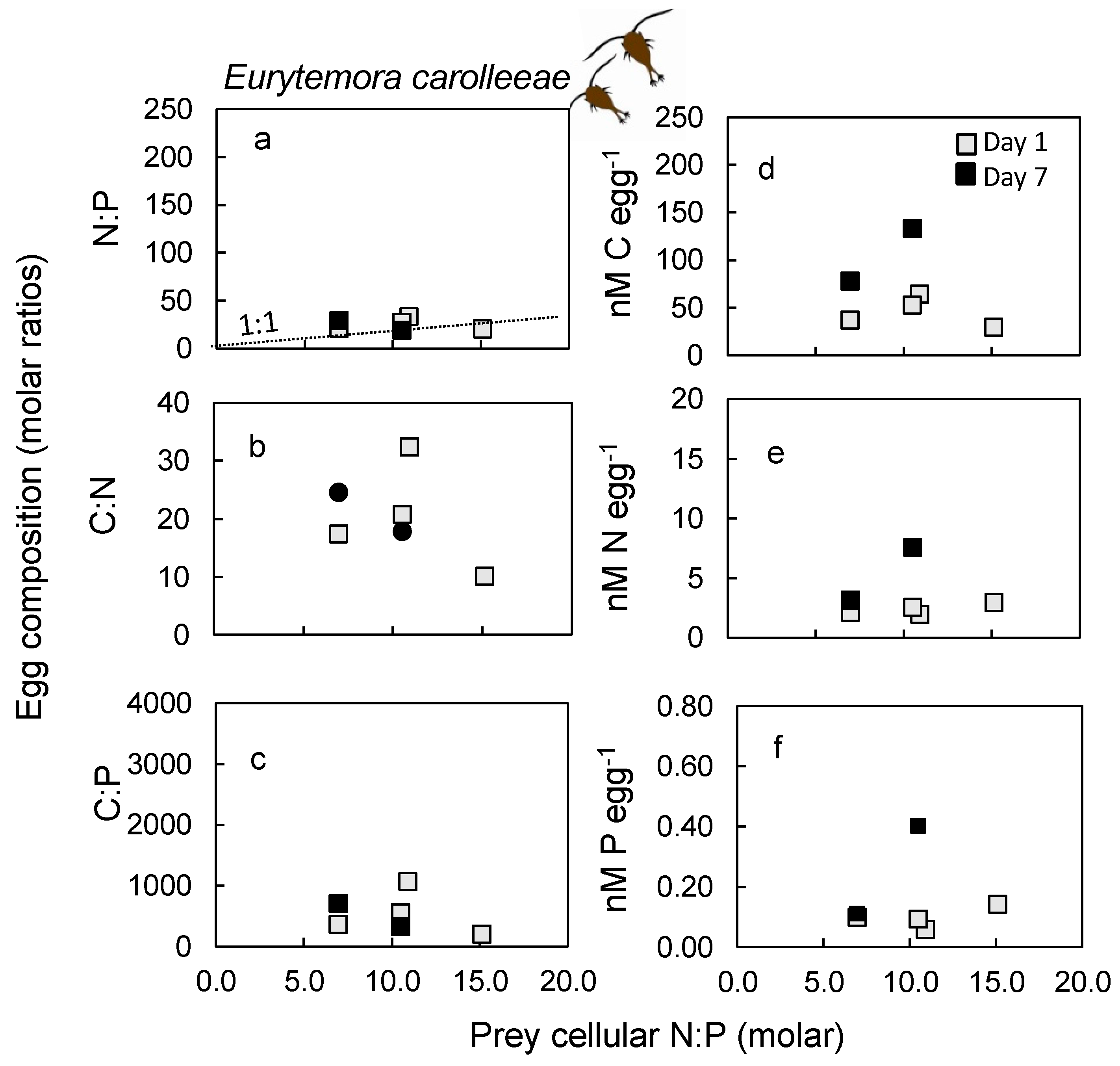
2.5. Nutrient Content in Eggs
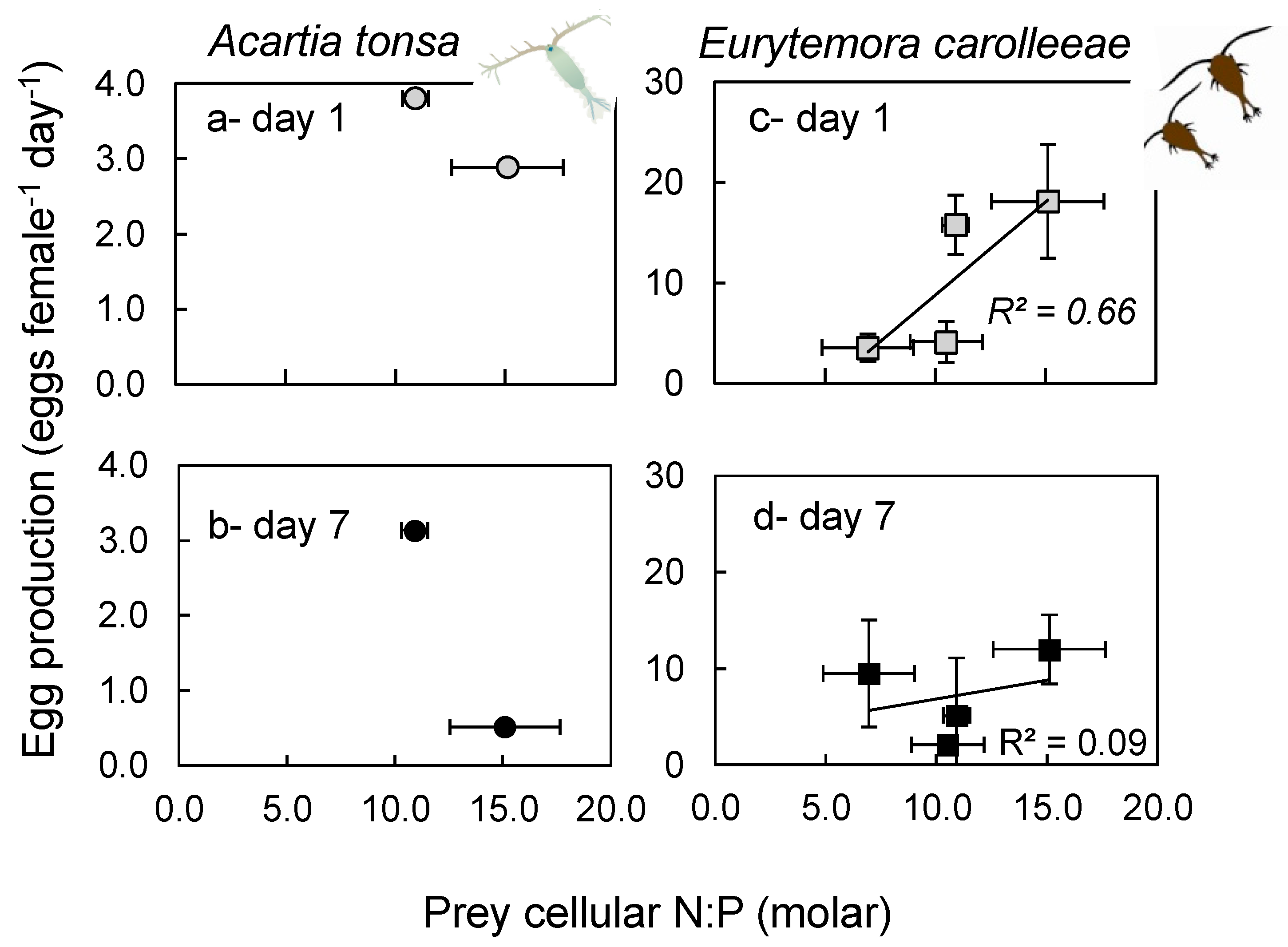
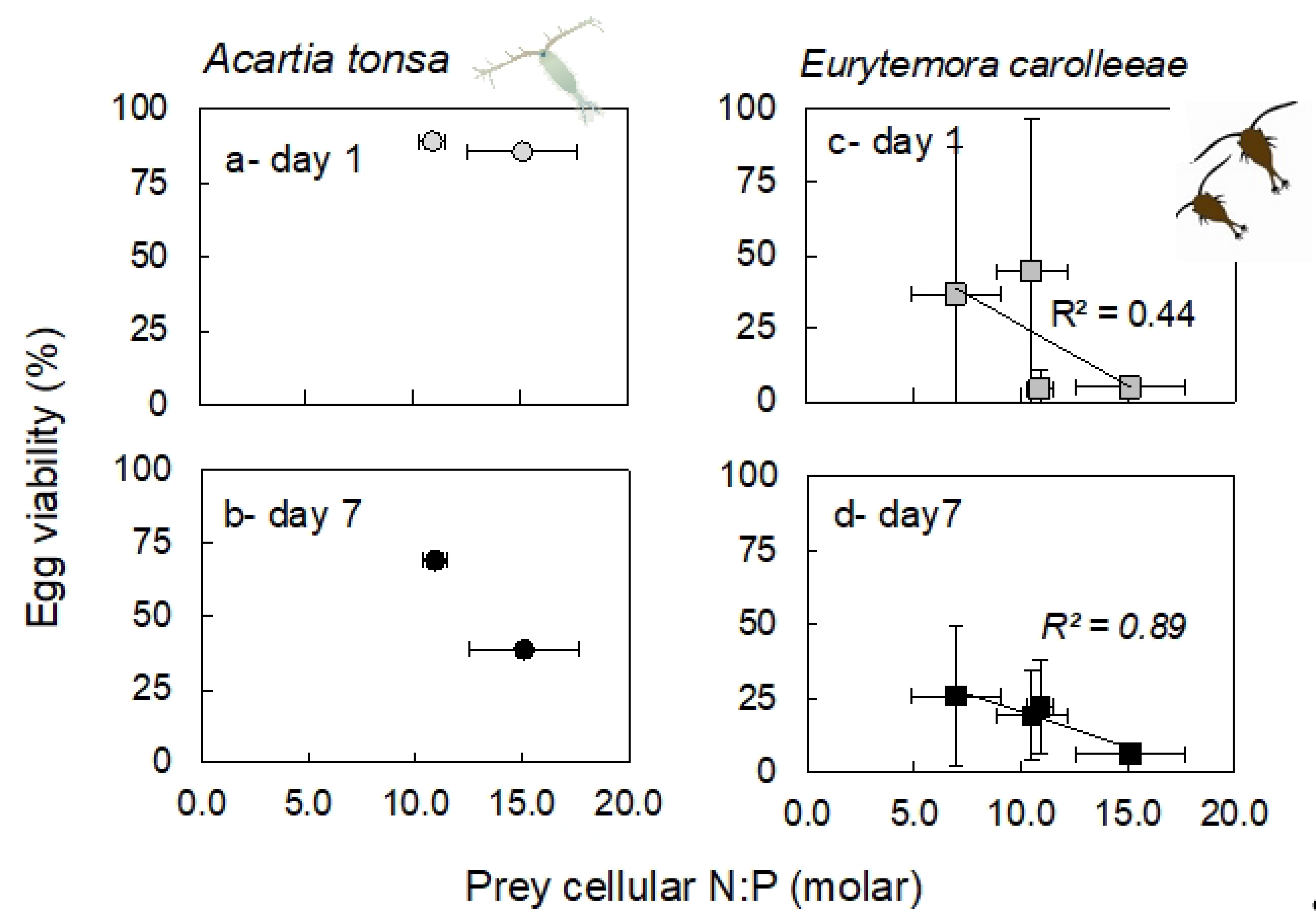
2.6. Egg Production and Viability
3. Discussion
3.1. Evaluating Hypotheses
3.2. Grazing
3.3. Nutrient Content in Copepod Tissue
3.4. Excretion
3.5. Egg Nutrient Stoichiometry
3.6. Egg Production and Viability
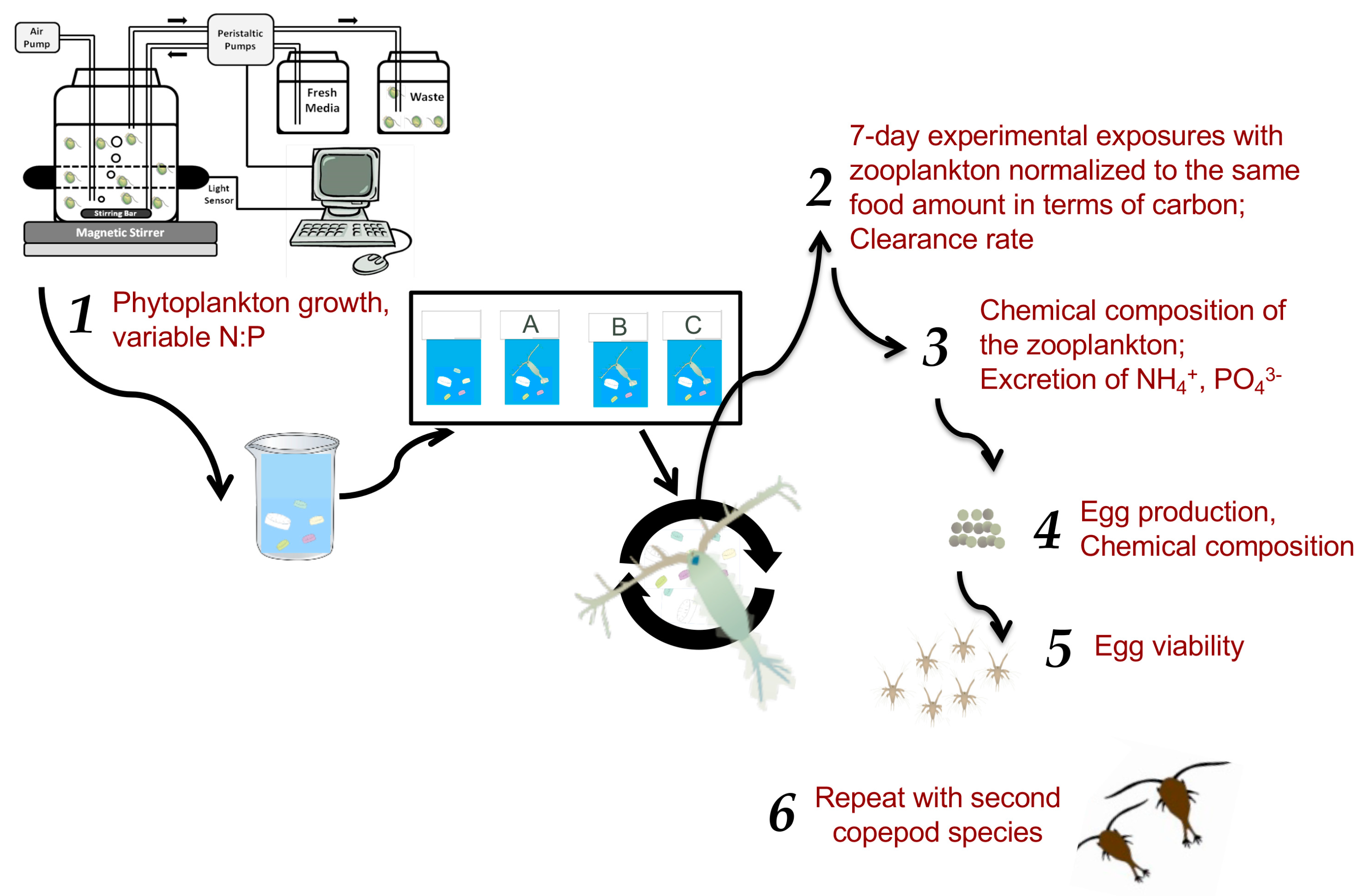
4. Methods
4.1. Overview
4.2. Algal Growth
4.3. Copepod Collection and Growth
4.4. Prey Quality Experiments
4.5. Analytical
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boersma, M.; Kreutzer, C. Life at the edge: Is food quality really of minor importance at low quantities? Ecology 2002, 83, 2552–2561. [Google Scholar] [CrossRef]
- Acheampong, E.; Campbell, R.; Diekmann, A.; St John, M. Food availability effects on reproductive strategy: The case of Acartia tonsa (Copepoda: Calanoida). Mar. Ecol. Prog. Ser. 2011, 428, 151–159. [Google Scholar] [CrossRef]
- Kimmerer, W.; Ignoffo, T.; Slaughter, A.; Gould, A. Food-limited reproduction and growth of three copepod species in the low-salinity zone of the San Francisco Estuary. J. Plankt. Res. 2014, 36, 722–735. [Google Scholar] [CrossRef]
- Jónasdóttir, S. Effects of food quality on the reproductive success of Acartia tonsa and Acartia hudsonica: Laboratory observations. Mar. Biol. 1994, 121, 67–81. [Google Scholar] [CrossRef]
- Jones, R.; Flynn, K.; Anderson, T. Effect of food quality on carbon and nitrogen growth efficiency in the copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 2002, 235, 147–156. [Google Scholar] [CrossRef]
- Malzahn, A.M.; Boersma, M. Effects of poor food quality on copepod growth are dose dependent and non-reversible. Oikos 2012, 121, 1408–1416. [Google Scholar] [CrossRef]
- Nobili, R.; Robinson, C.; Buitenhuis, E.; Castellani, C. Food quality regulates the metabolism and reproduction of Temora longicornis. Biogeosci. Discuss. 2013, 10, 3203–3239. [Google Scholar]
- Kleppel, G.; Burkart, C.; Houchin, L. Nutrition and the regulation of egg production in the calanoid copepod Acartia tonsa. Limnol. Oceanogr. 1998, 43, 1000–1007. [Google Scholar] [CrossRef]
- Arendt, K.; Jónasdóttir, S.; Hansen, P.; Gartner, S. Effects of dietary fatty acids on the reproductive success of the calanoid copepod Temora longicornis. Mar. Biol. 2005, 146, 513–530. [Google Scholar] [CrossRef]
- Checkley, D.M. Food limitation of egg production by a marine, planktonic copepod in the sea off southern California. Limnol. Oceanogr. 1980, 25, 991–998. [Google Scholar] [CrossRef]
- Guisande, C.; Riveiro, I.; Maneiro, I. Comparisons among the amino acid composition of females, eggs and food to determine the relative importance of food quantity and food quality to copepod reproduction. Mar. Ecol. Prog. Ser. 2000, 202, 135–142. [Google Scholar] [CrossRef]
- Kiørboe, T. Phytoplankton growth rate and nitrogen content: Implications for feeding and fecundity in a herbivorous copepod. Mar. Ecol. Prog. Ser. 1989, 55, 229–234. [Google Scholar] [CrossRef]
- Golz, A.-L.; Burian, A.; Winder, M. Stoichiometric regulation in micro- and mesozooplankton. J. Plankt. Res. 2015, 37, 283–305. [Google Scholar] [CrossRef]
- Lundgren, V.; Glibert, P.M.; Granéli, E.; Vidyarathna, N.K.; Fiori, E.; Ou, L.; Flynn, K.J.; Mitra, A.; Stoecker, D.K.; Hansen, P.J. Metabolic and physiological changes in Prymnesium parvum when grown under, and grazing on, prey of variable nitrogen:phosphorus stoichiometry. Harmful Algae. 2016, 55, 1–12. [Google Scholar] [CrossRef]
- Lin, C.-H.; Accoroni, S.; Glibert, P.M. Mixotrophy in the dinoflagellate Karlodinium veneficum under variable nitrogen:phosphorus stoichiometry: Feeding response and effects on larvae of the eastern oyster (Crassostrea virginica). Aq. Microb. Ecol. 2017, 79, 101–114. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Glibert, P.M.; Guo, C.; Ke, Y. Elemental stoichiometry and nutrient excretion of Noctiluca scintillans in response to prey of different quality. Sci. Rep. 2017, 7, 7622. [Google Scholar] [CrossRef] [PubMed]
- Howarth, R.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 51, 364–376. [Google Scholar] [CrossRef]
- Glibert, P.M.; Maranger, R.; Sobota, D.J.; Bouwman, L. The Haber Bosch—Harmful algal bloom (HB-HAB) link. Environ. Res. Lett. 2014, 9, 105001. [Google Scholar] [CrossRef]
- Galloway, J.N.; Cowling, E.B.; Seitzinger, S.P.; Socolow, R.H. Reactive nitrogen: Too much of a good thing? Ambio 2002, 31, 60–63. [Google Scholar] [CrossRef]
- Galloway, J.N.; Cowling, E.B. Reactive nitrogen and the world: 200 years of change. Ambio 2002, 31, 64–71. [Google Scholar] [CrossRef]
- Howarth, R.; Anderson, D.; Cloern, J.; Elfring, C.; Hopkinson, C.; Lapointe, B.; Malone, T.; Marcus, N.; McGlathery, K.; Sharpley, A.; et al. Nutrient pollution of coastal rivers, bays, and seas. Issues Ecol. 2000, 7, 1–15. [Google Scholar]
- Howarth, R. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Glibert, P.M.; Beusen, A.H.W.; Harrison, J.A.; Durr, H.H.; Bouwman, A.F.; Laruelle, G.G. Changing land, sea- and airscapes: Sources of nutrient pollution affecting habitat suitability for harmful algae. In Ecology and Oceanography of Harmful Algal Blooms (GEOHAB); Glibert, P.M., Berdalet, E., Burford, M.A., Pitcher, G.C., Zhou, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 53–76. [Google Scholar]
- Glibert, P.M. From hogs to HABs: Recent changes and current status in fertilizer use and industrial animal farms and their impacts on nitrogen and phosphorus loads and greenhouse gas emissions. Biogeochemistry 2020, 150, 139–180. [Google Scholar] [CrossRef]
- Glibert, P.M. Harmful algal at the complex nexus of eutrophication and climate change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef] [PubMed]
- Boersma, M. The nutritional quality of P-limited algae for Daphina. Limnol. Oceanogr. 2000, 45, 1157–1161. [Google Scholar] [CrossRef]
- Rhee, G.-Y. Effects of N:P atomic ratios and nitrate limitation on algal growth, cell composition, and nitrate uptake. Limnol. Oceanogr. 1978, 23, 10–25. [Google Scholar] [CrossRef]
- Hecky, R.; Kilham, P. Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 1988, 33, 796–822. [Google Scholar] [CrossRef]
- Glibert, P.M.; Fullerton, D.; Burkholder, J.; Cornwell, J.; Kana, T.M. Ecological stoichiometry, biogeochemical cycling, invasive species, and aquatic food webs: San Francisco Estuary and comparative systems. Rev. Fish. Sci. 2011, 19, 358–417. [Google Scholar] [CrossRef]
- Hillebrand, H.; Steinert, G.; Boersma, M.; Malzahn, A.; Meunier, C.L.; Plum, C.; Ptacnik, R. Goldman revisited: Faster-growing phytoplankton has lower N:P and lower stoichiometric flexibility. Limnol. Oceanogr. 2013, 58, 2076–2088. [Google Scholar] [CrossRef]
- Sterner, R.; Elser, J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Shoo, K.L.; Aberle, N.; Malzahn, A.M.; Boersma, M. Food quality affects secondary consumers even at low quantities: An experimental test with larval European lobster. PLoS ONE 2012, 7, e33550. [Google Scholar]
- Hessen, D.; Anderson, T. Excess carbon in aquatic organisms and ecosystems: Physiological, ecological, and evolutionary implications. Limnol. Oceanogr. 2008, 53, 1685–1696. [Google Scholar] [CrossRef]
- Hessen, D.; Elser, J.; Sterner, R.; Urabe, J. Ecological stoichiometry: An elementary approach using basic principles. Limnol. Oceanogr. 2013, 58, 2219–2236. [Google Scholar] [CrossRef]
- Malzahn, A.M.; Aberle, N.; Clemmesen, C.; Boersma, M. Nutrient limitation of primary producers affects planktivorous fish conditions. Limnol. Oceanogr. 2007, 52, 2062–2071. [Google Scholar] [CrossRef]
- Boersma, M.; Becker, C.; Malzahn, A.M.; Vernooij, S. Food chain effects of nutrient limitation in primary producers. Mar. Freshwat. Res. 2009, 60, 983–989. [Google Scholar] [CrossRef]
- Shoo, K.L.; Aberle, N.; Malzahn, A.M.; Boersma, M. Does the nutrient stoichiometry of primary producers affect the secondary consumer Pleurobrachia pileus? Aquat. Ecol. 2010, 44, 233–242. [Google Scholar] [CrossRef]
- Laspoumadres, C.; Modenutti, B.; Elser, J.J.; Balseiro, E. Does the stoichiometric carbon:phosphorus knife edge apply for predaceaous copepods? Oecologia 2015, 178, 557–569. [Google Scholar] [CrossRef]
- Glibert, P.M.; Kana, T.M.; Brown, K. From limitation to excess: The consequences of substrate excess and stoichiometry for phytoplankton physiology, trophodynamics and biogeochemistry, and the implications for modeling. J. Mar. Syst. 2013, 125, 14–28. [Google Scholar] [CrossRef]
- Cushing, D. A difference in structure between ecosystems in strongly stratified waters and in those that are only weakly stratified. J. Plankt. Res. 1989, 11, 1–13. [Google Scholar] [CrossRef]
- Legendre, L. The significance of microalgal blooms for fisheries and for the export of particulate organic carbon in oceans. J. Plankt. Res. 1990, 12, 681–699. [Google Scholar] [CrossRef]
- Schnetzer, A.; Steinberg, D. Natural diets of vertically migrating zooplankton in the Sargasso Sea. Mar. Biol. 2002, 141, 89–99. [Google Scholar]
- Alekseev, V.R.; Souissi, A. A new species within the Eurytemora affinis complex (Copepoda: Calanoida) from the Atlantic Coast of USA, with observations on eight morphologically different European populations. Zootaxa 2011, 2767, 41–56. [Google Scholar] [CrossRef]
- Kimmerer, W.J.; Ferm, N.; Nicolini, M.H.; Penalva, C. Chronic food limitation of egg production in populations of copepods of the genus Acartia in San Francisco estuary. Estuaries 2005, 28, 541–550. [Google Scholar] [CrossRef]
- Kimmel, D.G.; Roman, M.R. Long-term trends in mesozooplankton abundance in Chesapeake Bay, USA: Influence of freshwater input. Mar. Ecol. Prog. Ser. 2004, 267, 71–83. [Google Scholar] [CrossRef]
- Lloyd, S.; Elliott, D.; Roman, M. Egg production by the copepod, Eurytemora affinis in Chesapeake Bay turbidity maximum regions. J. Plankt. Res. 2013, 35, 299–308. [Google Scholar] [CrossRef]
- Mauchline, J. The Biology of Calanoid Copepods; Academic Press Inc.: San Diego, CA, USA; London, UK, 1998; Volume 33, p. 710. [Google Scholar]
- Dagg, M. Some effects of patchy food environments on copepods. Limnol. Oceanogr. 1977, 22, 99–107. [Google Scholar] [CrossRef]
- Sterner, R.; George, N. Carbon, nitrogen, and phosphorus stoichiometry of cyprinid fishes. Ecology 2000, 81, 127–140. [Google Scholar] [CrossRef]
- Malzahn, A.M.; Hantzsche, F.; Schoo, K.L.; Boersma, M.; Aberle, N. Differential effects of nutrient-limited primary production on primary, secondary or tertiary consumers. Oecologia 2010, 162, 35–48. [Google Scholar] [CrossRef]
- Winder, M.; Jassby, A. Shifts in zooplankton community structure: Implications for food web processes in the upper San Francisco Estuary. Est. Coasts. 2011, 34, 675–690. [Google Scholar] [CrossRef]
- Beaugrand, G.; Brander, K.M.; Lindley, J.A.; Souissa, S.; Reid, P.C. Plankton effect on cod recruitment on the North Sea. Nature 2003, 426, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Houde, S.E.L.; Roman, M.R. Effects of food quality on the functional ingestion responses of the copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 1987, 40, 69–77. [Google Scholar] [CrossRef]
- Richman, S.; Heinle, D.; Huff, R. Grazing by adult estuarine calanoid copepods of the Chesapeake Bay. Mar. Biol. 1977, 42, 69–84. [Google Scholar] [CrossRef]
- Saba, G.K.; Steinberg, D.K.; Bronk, D.A. Effects of diet on release of dissolved organic and inorganic nutrients by the copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 2009, 386, 147–161. [Google Scholar] [CrossRef]
- Thompson, A.; Durbin, E.; Durbin, A. Seasonal changes in maximum ingestion rate of Acartia tonsa in Narragansett Bay, Rhode Island, USA. Mar. Ecol. Prog. Ser. 1994, 108, 91–105. [Google Scholar] [CrossRef]
- Durbin, E.; Durbin, A.; Campbell, R. Body size and egg production in the marine copepod Acartia hudsonica during a winter-spring diatom bloom in Narragansett Bay. Limnol. Oceanogr. 1992, 37, 342–360. [Google Scholar] [CrossRef]
- Miller, C.A.; Roman, M.R. Effects of food nitrogen content and concentration on the forms of nitrogen excreted by the calanoid copepod Acartia tonsa. J. Exp. Mar. Biol. Ecol. 2008, 359, 11–17. [Google Scholar] [CrossRef]
- Uye, S.; Matsuda, O. Phosphorus content of zooplankton from the inland Sea of Japan. J. Oceanogr. Soc. Jpn. 1988, 44, 280–286. [Google Scholar] [CrossRef]
- Meunier, C.L.; Boersma, M.; Wiltshire, K.H.; Malzahn, A.M. Zooplankton eat what they need: Copepod selective feeding and potential consequences for marine systems. Oikos 2016, 125, 50–58. [Google Scholar] [CrossRef]
- Gaudy, R.; Boucher, J. Relation between respiration, excretion (ammonia and inorganic phosphorus) and activity of amylase and trypsin in different species of pelagic copepods from an Indian Ocean equatorial area. Mar. Biol. 1983, 75, 37–45. [Google Scholar] [CrossRef]
- Labuce, A.; Strake, S.; Dimante-Deimantovica, I. Eurtytemora affinis (Poppe, 1880) (Copepoda, Calanoida) in the Gulf of Riga, Baltic Sea—Elemental compositon and diurnal vertical migration. Crustaceana 2019, 3–5, 446. [Google Scholar]
- Koski, M. Carbon:Nitrogen ratios of Baltic Sea copepods—indication of mineral limitation? J. Plankt.Res. 1999, 21, 1565–1573. [Google Scholar] [CrossRef]
- McLaren, I.; Corkett, C. Temperature-dependent growth and production by a marine copepod. Can. J. Fish. Aquat. Sci. 1981, 38, 77–83. [Google Scholar] [CrossRef]
- Miller, C.A.; Glibert, P.M. Nitrogen excretion by the calanoid copepod Acartiatonsa: Results of mesocosm experiments. J. Plankt. Res. 1998, 20, 1767–1780. [Google Scholar] [CrossRef][Green Version]
- Urabe, J. N and P cycling coupled by grazers’ activities: Food quality and nutrient release by zooplankton. Ecology 1993, 74, 2337–2350. [Google Scholar] [CrossRef]
- He, X.; Wang, W. Kinetics of phosphorus in “Daphnia” at different food concentrations and carbon:phosphorus ratios. Limnol. Oceanogr. 2007, 52, 395–406. [Google Scholar] [CrossRef]
- DeMott, W.; Gulati, R.; Siewertsen, K. Effects of phosphorus-deficient diets on the carbon and phosphorus balance of Daphnia magna. Limnol. Oceanogr. 1998, 43, 1147–1161. [Google Scholar] [CrossRef]
- Morales, C. Carbon and nitrogen content of copepod faecal pellets: Effect of food concentration and feeding behavior. Mar. Ecol. Prog. Ser. 1987, 36, 107–114. [Google Scholar] [CrossRef]
- Færøvig, P.; Hessen, D.O. Allocation strategies in crustacean stoichiometry: The potential role of phosphorus in the limitation of reproduction. Freshwat. Biol. 2003, 48, 1782–1792. [Google Scholar] [CrossRef]
- Laspoumaderes, C.; Modenutti, B.; Balseiro, E. Herbivory versus omnivory: Linking homeostasis and elemental imbalance in copepod development. J. Plankt. Res. 2010, 32, 1573–1582. [Google Scholar] [CrossRef]
- Frost, P.C.; Ebert, D.; Larson, J.H.; Marcus, M.A.; Wagner, N.D.; Zalewski, A. Transgenerational effects of poor elemental food quality on Daphnia magna. Oecologia 2010, 164, 865–872. [Google Scholar] [CrossRef]
- Durbin, E.; Durbin, A.; Smayda, T.; Verity, P. Food limitation of production by adult Acartia tonsa in Narragansett Bay, Rhode Island. Limnol. Oceanogr. 1983, 28, 1199–1213. [Google Scholar] [CrossRef]
- Castro-Longoria, E. Egg production and hatching success of four Acartia species under different temperature and salinity regimes. J. Crust. Biol. 2003, 23, 289–299. [Google Scholar] [CrossRef]
- Kleppel, G.; Burkart, C. Egg production and the nutritional environment of Acartia tonsa: The role of food quality in copepod nutrition. ICES J. Mar. Sci. 1975, 52, 297–304. [Google Scholar] [CrossRef]
- Dam, H.; Lopes, R. Omnivory in the calanoid copepod Temora longicornis: Feeding, egg production and egg hatching rates. J. Exp. Mar. Biol. Ecol. 2003, 292, 119–137. [Google Scholar] [CrossRef]
- Ban, S.; Burns, C.; Castel, J.; Chaudron, Y.; Christou, E.; Escribano, R.; Umani, S.; Gasparini, S.; Ruiz, F.; Hoffmeyer, M.; et al. The paradox of diatom-copepod interactions. Mar. Ecol. Prog. Ser. 1997, 157, 287–293. [Google Scholar] [CrossRef]
- Ianora, A.; Poulet, S.; Miralto, A. The effects of diatoms on copepod reproduction: A review. Phycologia 2003, 42, 351–363. [Google Scholar] [CrossRef]
- Dutz, J.; Koski, M.; Jónasdóttir, S. Copepod reproduction is unaffected by diatom aldehydes or lipid composition. Limnol. Oceanogr. 2008, 53, 225–235. [Google Scholar] [CrossRef]
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry 2007, 68, 2059–2067. [Google Scholar] [CrossRef]
- Ribalet, F.; Vidoudez, C.; Cassin, D.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. High plasticity in the production of diatom-derived polyunsaturated aldehydes under nutrient limitation: Physiological and ecological implications. Protist 2009, 160, 444–451. [Google Scholar] [CrossRef]
- Kleppel, G. On the diets of calanoid copepods. Mar. Ecol. Prog. Ser. 1993, 99, 183. [Google Scholar] [CrossRef]
- Colin, S.P.; Dam, H.G. Testing for toxic effects of prey on zooplankton using sole versus mixed diets. Limnol. Oceanogr. 2002, 47, 1430–1437. [Google Scholar] [CrossRef]
- Jónasdóttir, S.; Kiørboe, T. Copepod recruitment and food composition: Do diatoms affect hatching success? Mar. Biol. 1996, 125, 743–750. [Google Scholar] [CrossRef]
- Hirche, H. Egg production of Eurytemora affinis—Effect of k-strategy. Est. Coast. Shelf Sci. 1992, 35, 395–407. [Google Scholar] [CrossRef]
- Bunker, A.; Hirst, A. Fecundity of marine planktonic copepods: Global rates and patterns in relation to chlorophyll a, temperature and body weight. Mar. Ecol. Prog. Ser. 2004, 279, 161–181. [Google Scholar] [CrossRef]
- Ambler, J.W. Seasonal factors affecting egg production and viability of eggs of Acartia tonsa Dana from East Lagoon, Galveston, Texas. Est. Coast. Shelf Sci. 1985, 20, 743–760. [Google Scholar] [CrossRef]
- Guillard, R.; Ryther, J. Studies of marine planktonic diatoms I. Cyclotella nana hustedt and Detonulaconfervacea (Cleve) gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Li, J.; Glibert, P.M.; Alexander, J. Effects of ambient DIN:DIP ratio on the nitrogen uptake of harmful dinoflagellate Prorocentrum minimum and Prorocentrum donghaiense in turbidistat. Chinese J. Oceanol. Limnol. 2011, 29, 746–761. [Google Scholar] [CrossRef]
- Berges, J.; Varla, D.; Harrison, P. Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Mar. Ecol. Prog. Ser. 2002, 225, 139–146. [Google Scholar] [CrossRef]
- Heinle, D.; Flemer, D. Carbon requirements of a population of the estuarine copepod Eurytemora affinis. Mar. Biol. 1975, 31, 235–247. [Google Scholar] [CrossRef]
- Lane, L.; Rhoades, S.; Thomas, C.; Van Heukelem, L. Analytical Services Laboratory-Standard Operating Procedures; Technical Report Number TS-264-00; Horn Point Laboratory, University of Maryland Center for Environmental Science: Cambridge, MD, USA, 2000. [Google Scholar]
- Solórzano, L.; Sharp, J.H. Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnol. Oceanogr. 1980, 25, 754–758. [Google Scholar] [CrossRef]
- Frost, B. Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol. Oceanogr. 1972, 17, 805–815. [Google Scholar] [CrossRef]
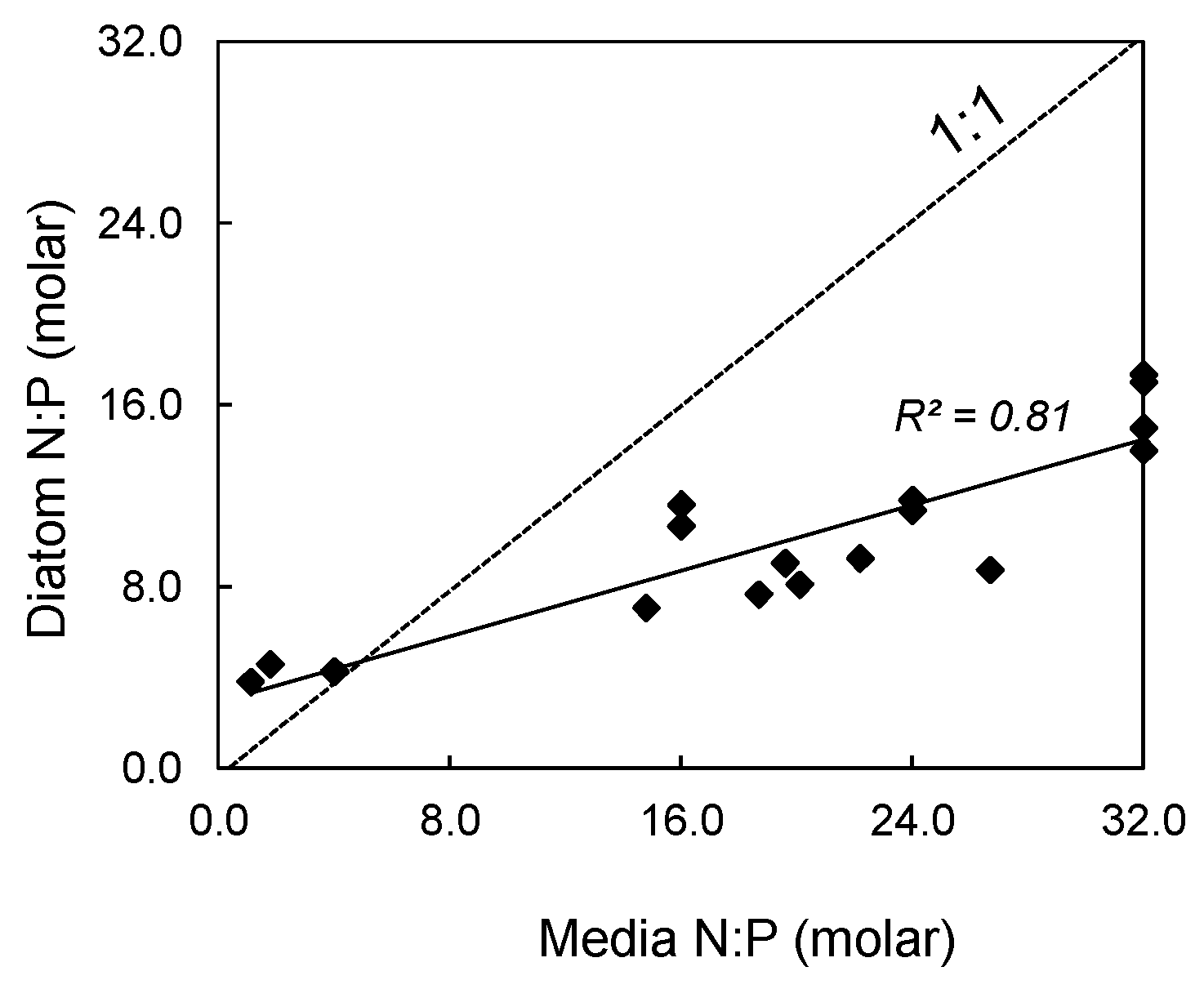
| Media N:P | Diatom N:P | Diatom C | Diatom C | Diatom N | Diatom N | Diatom P | Diatom P |
|---|---|---|---|---|---|---|---|
| (molar) | (molar) | (μM) | (μg C L−1) | (μM) | (μg N L−1) | (μM) | (μg P L−1) |
| 4 | 7.0 ± 2.1 | 393 ± 34 | 4722 ± 403 | 67.89 ± 6.32 | 951 ± 89 | 11.06 ± 5.18 | 343 ± 160 |
| 16 | 10.9 ± 8.8 | 512 ± 62 | 6154 ± 746 | 86.67 ± 10.29 | 1214 ± 144 | 7.68 ± 0.56 | 238 ± 17 |
| 24 | 10.5 ± 1.6 | 486 ± 32 | 5834 ± 384 | 84.41 ± 8.33 | 1182 ± 117 | 8.32 ± 2.24 | 258 ± 69 |
| 32 | 15.1 ± 2.5 | 545 ± 30 | 6552 ± 357 | 89.43 ± 5.38 | 1253 ± 75 | 6.01 ± 0.74 | 186 ± 23 |
| Parameter | Equation | Detail and Definitions | Source |
|---|---|---|---|
| Algal growth rate, k | C2, C1 = algal cell concentrations in control treatments at times t2 and t1 | [95] | |
| Grazing coefficient, g | C2*, C1* = algal cell concentrations in experimental treatments at times t2 and t1 | [95] | |
| Average cell concentration, <C> | as above | [95] | |
| Clearance rate, F | V = volume (mL) in experimental bottle N = number of copepods g as above | [95] | |
| Ingestion rate, I | as above | [95] | |
| Excretion rate, ER | ΔCt, ΔCc = change in concentration of the nutrient (NH4+ or PO43−) in the treatment and control bottles V = volume of the bottle T = duration of incubation N = numbers of grazers | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentley, K.M.; Pierson, J.J.; Glibert, P.M. Physiological Responses of the Copepods Acartia tonsa and Eurytemora carolleeae to Changes in the Nitrogen:Phosphorus Quality of Their Food. Nitrogen 2021, 2, 62-85. https://doi.org/10.3390/nitrogen2010005
Bentley KM, Pierson JJ, Glibert PM. Physiological Responses of the Copepods Acartia tonsa and Eurytemora carolleeae to Changes in the Nitrogen:Phosphorus Quality of Their Food. Nitrogen. 2021; 2(1):62-85. https://doi.org/10.3390/nitrogen2010005
Chicago/Turabian StyleBentley, Katherine M., James J. Pierson, and Patricia M. Glibert. 2021. "Physiological Responses of the Copepods Acartia tonsa and Eurytemora carolleeae to Changes in the Nitrogen:Phosphorus Quality of Their Food" Nitrogen 2, no. 1: 62-85. https://doi.org/10.3390/nitrogen2010005
APA StyleBentley, K. M., Pierson, J. J., & Glibert, P. M. (2021). Physiological Responses of the Copepods Acartia tonsa and Eurytemora carolleeae to Changes in the Nitrogen:Phosphorus Quality of Their Food. Nitrogen, 2(1), 62-85. https://doi.org/10.3390/nitrogen2010005