Improving Nitrate Fertilization by Encapsulating Zn-Al Layered Double Hydroxides in Alginate Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Zn-Al LDHs Interlayered with 15NO3−
2.2. Synthesis of Zn-Al LDHs with 15NO3− in Alginate Beads
2.3. Characterization of Materials Studied
2.4. Rate of NO3− Release from Zn-Al LDHs and KNO3
2.5. Growth Chamber Experiment
2.5.1. Soil Studied
2.5.2. Procedure
2.6. Data Analyses
3. Results and Discussion
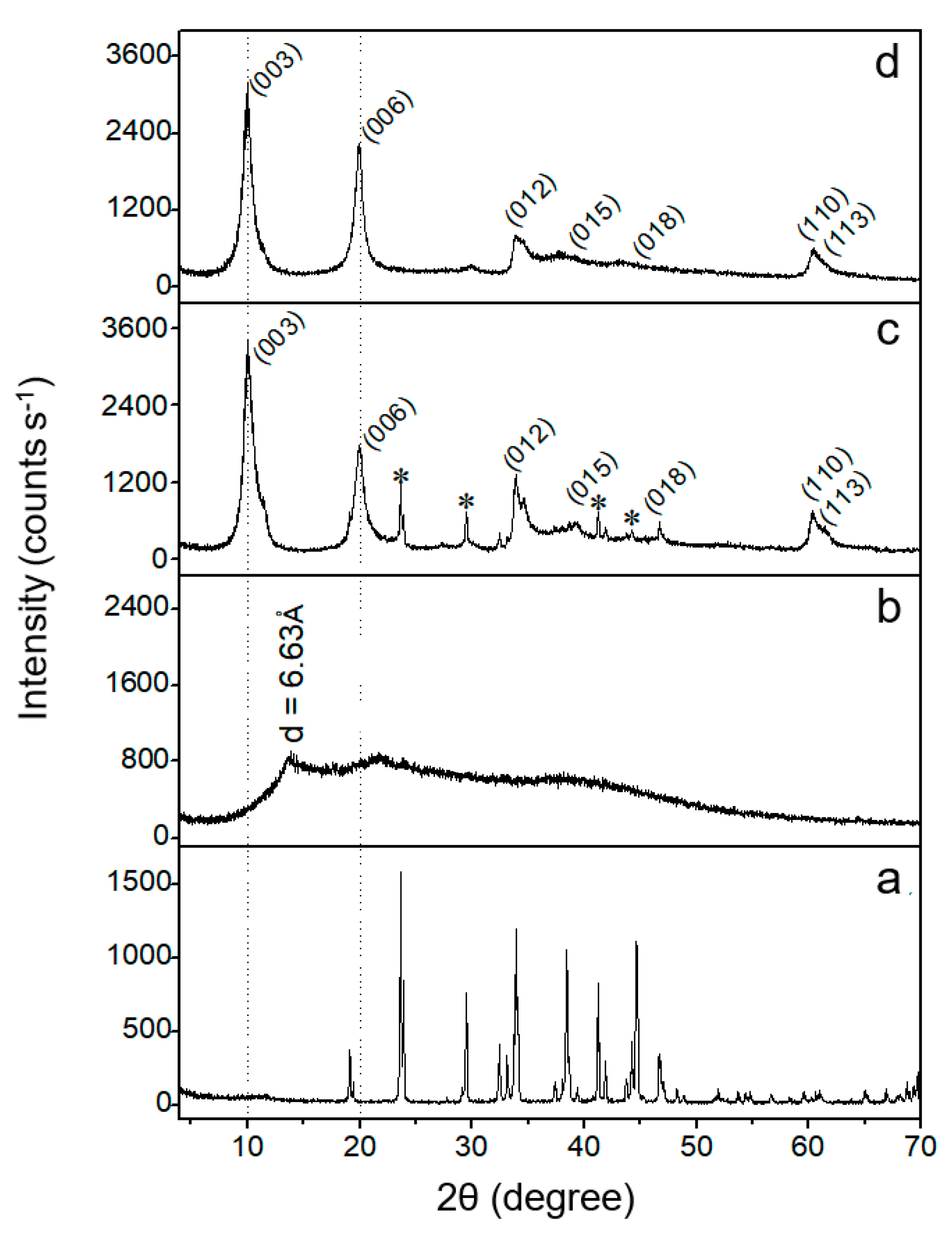
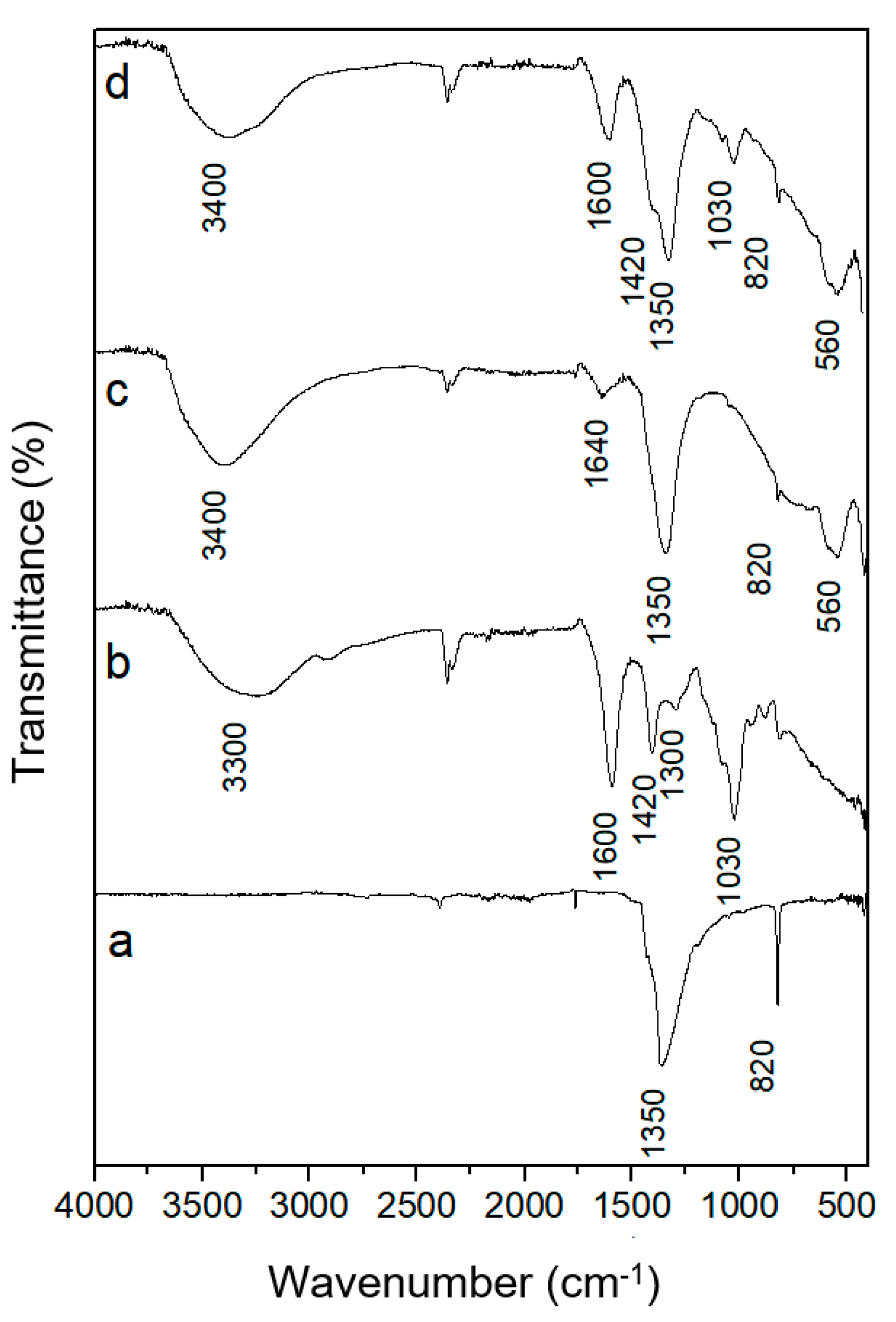
3.1. Characterization
3.2. Kinetic Study
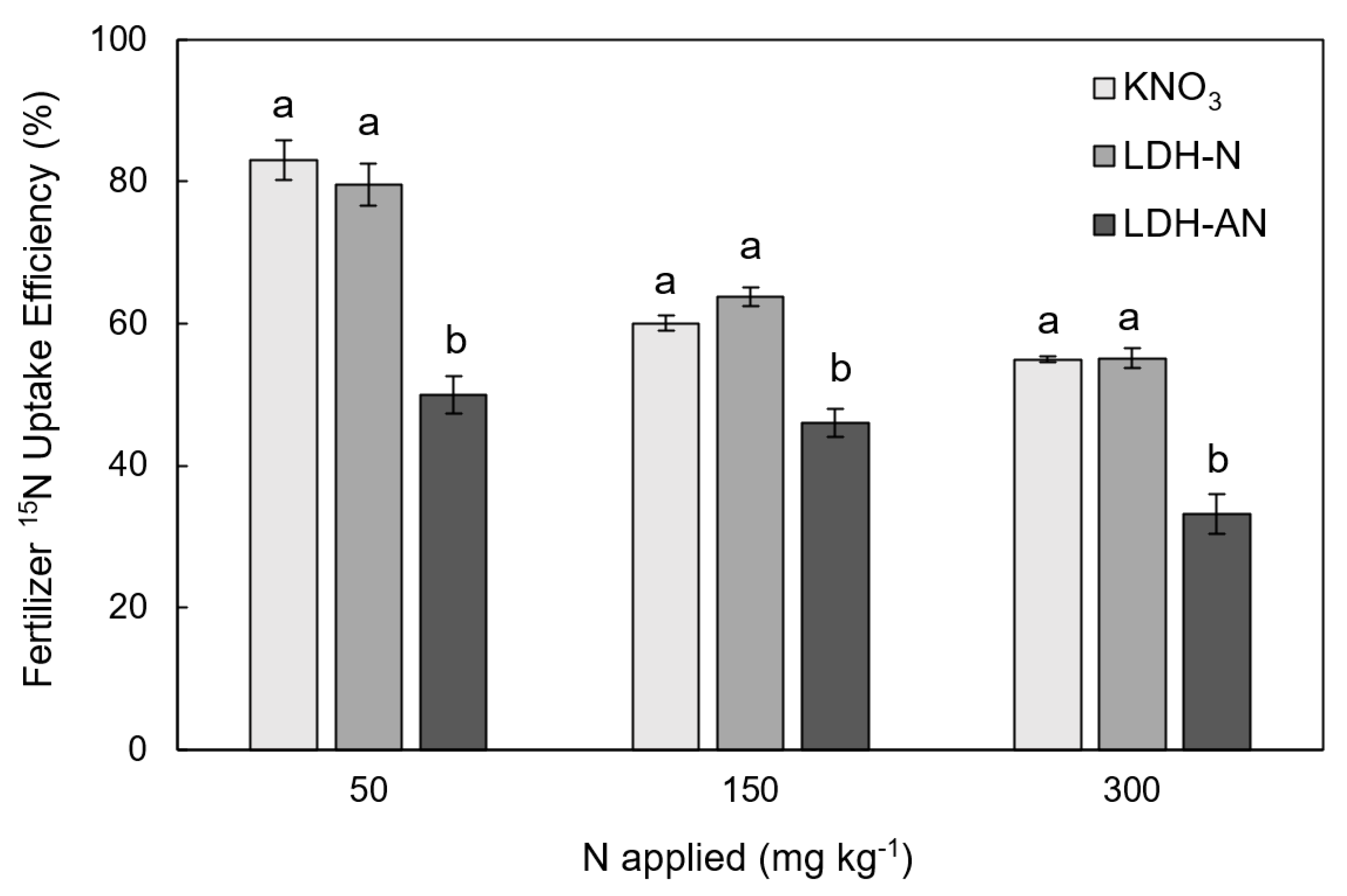
3.3. Growth Chamber Experiment
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smil, V. Enriching the Earth. Fritz Haber, Carl Bosch, and the Transformation of World Food Production; MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Nelson, D.W. Gaseous losses of nitrogen other than through denitrification. In Nitrogen in Agricultural Soils; Agronomy 22; SSSA: Madison, WI, USA, 1982; Volume 22, pp. 327–363. [Google Scholar] [CrossRef]
- Bremner, J.M. Recent research on problems in the use of urea as a nitrogen fertilizer. In Nitrogen Economy in Tropical Soils; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 321–329. [Google Scholar]
- Bouwman, A.F.; Van Vuuren, D.P.; Derwent, R.G.; Posch, M. A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut. 2002, 141, 349–382. [Google Scholar] [CrossRef]
- Jansson, S.L. Tracer studies on nitrogen transformations in soil. Ann. Roy. Agric. Coll. Sweden. 1958, 24, 101–361. [Google Scholar]
- Rice, C.W.; Tiedje, J.M. Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biol. Biochem. 1989, 21, 597–602. [Google Scholar] [CrossRef]
- Recous, S.; Mary, B.; Faurie, G. Microbial immobilization of ammonium and nitrate in cultivated soils. Soil Biol. Biochem. 1990, 22, 913–922. [Google Scholar] [CrossRef]
- Jensen, E.S. Nitrogen immobilization and mineralization during initial decomposition of 15N-labelled pea and barley residues. Biol. Fert. Soils 1997, 24, 39–44. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The myth of nitrogen fertilization for soil carbon sequestration. J. Environ. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic nitrogen fertilizers deplete soil nitrogen: A global dilemma for sustainable cereal production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef]
- Powlson, D.S.; Johnston, A.E.; Jenkinson, D.S. The nitrogen cycle in the Broadbalk wheat experiment: Recovery and losses of 15N-labelled fertilizer applied in spring and inputs of nitrogen from the atmosphere. J. Agric. Sci. 1986, 107, 591–609. [Google Scholar] [CrossRef]
- Recous, S.; Machet, J.M.; Mary, B. The fate of labelled 15N urea and ammonium nitrate applied to a winter wheat crop. Plant. Soil 1988, 112, 215–224. [Google Scholar] [CrossRef]
- Martinoia, E.; Heck, U.; Wiemken, A. Vacuoles as storage compartments for nitrate in barley leaves. Nature 1981, 289, 292–294. [Google Scholar] [CrossRef]
- Granstedt, R.C.; Huffaker, R.C. Identification of the leaf vacuole as a major nitrate storage pool. Plant. Physiol. 1982, 70, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Gerendás, J.; Zhu, Z.; Bendixen, R.; Ratcliffe, R.G.; Sattelmacher, B. Physiological and biochemical processes related to ammonium toxicity in higher plants. Z. Pflanzenernähr. Bodenk. 1997, 160, 239–251. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Silva, V.D.; Kamogawa, M.Y.; Marangoni, R.; Mangrich, A.S.; Wypych, F. Layered double hydroxides as matrices for nitrate slow release fertilizers. Rev. Bras. Ciên. Solo 2014, 38, 272–277. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Constantino, V.R.L.; Pinto, F.G.; Vergütz, L.; Tronto, J.; da Costa, L.M. Layered double hydroxides: New technology in phosphate fertilizers based on nanostructured materials. ACS Sustain. Chem. Eng. 2016, 5, 399–409. [Google Scholar] [CrossRef]
- Everaert, M.; Degryse, F.; McLaughlin, M.J.; De Vos, D.; Smolders, E. Agronomic effectiveness of granulated and powdered P-exchanged Mg–Al LDH relative to struvite and MAP. J. Agric. Food Chem. 2017, 65, 6736–6744. [Google Scholar] [CrossRef]
- Castro, G.F.; Ferreira, J.A.; Eulálio, D.; de Souza, S.J.; Novais, N.R.F.; Tronto, J. Layered double hydroxides: Matrices for storage and source of boron for plant growth. Clay Miner. 2018, 53, 79–89. [Google Scholar] [CrossRef]
- Songkhum, P.; Wuttikhun, T.; Chanlek, N.; Khemthong, P.; Laohhasurayotin, K. Controlled release studies of boron and zinc from layered double hydroxides as the micronutrient hosts for agricultural application. Appl. Clay Sci. 2018, 152, 311–322. [Google Scholar] [CrossRef]
- Leroux, F.; Besse, J.P. Polymer interleaved layered double hydroxide: A new emerging class of nanocomposites. Chem. Mater. 2001, 13, 3507–3515. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.R.; Lakzian, A. Effects of Mg-Al layered double hydroxide on nitrate leaching and nitrogen uptake by maize in a calcareous soil. Commun. Soil Sci. Plant Anal. 2016, 47, 1162–1175. [Google Scholar] [CrossRef]
- Alcantara, A.C.S.; Aranda, P.; Darder, M.; Ruiz-Hitzky, E. Bionanocomposites based on alginate–zein/layered double hydroxide materials as drug delivery systems. J. Mater. Chem. 2010, 20, 9495–9504. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Ni, B.; Xie, L. κ-Carrageenan–sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind. Eng. Chem. Res. 2012, 51, 1413–1422. [Google Scholar] [CrossRef]
- Shan, L.; Gao, Y.; Zhang, Y.; Yu, W.; Yang, Y.; Shen, S.; Yun, J. Fabrication and use of alginate-based cryogel delivery beads loaded with urea and phosphates as potential carriers for bioremediation. Ind. Eng. Chem. Res. 2016, 55, 7655–7660. [Google Scholar] [CrossRef]
- De Roy, A.; Forano, C.; El Malki, K.; Besse, J.P. Anionic clays: Trends in pillaring chemistry. In Expanded Clays and Other Microporous Solids; Springer: New York, NY, USA, 1992; pp. 108–169. [Google Scholar]
- Fisher Scientific. 2020. Available online: https://www.fishersci.com (accessed on 3 September 2020).
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Natural Resources Conservation Service (USDA, NRCS) Soil Taxonomy. 1999. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051232.pdf (accessed on 21 September 2020).
- Nunes, V.L.N.; Mulvaney, R.L.; Tronto, J.; Cantarutti, R.B. Potential of alginate and mesoporous carbon to improve the efficiency of fertilizer urea. Commun. Soil Sci. Plant. Anal. 2020. [Google Scholar] [CrossRef]
- Novais, R.F.; Neves, J.C.L.; Barros, N.F. Ensaio em ambiente controlado. In Métodos de Pesquisa em Fertilidade do Solo; EMBRAPA-SEA: Brasília, Brazil, 1991; pp. 189–255. [Google Scholar]
- International Centre for Diffraction Data. 2020. Available online: http://www.icdd.com/ (accessed on 3 September 2020).
- Inayat, A.; Klumpp, M.; Schwieger, W. The urea method for the direct synthesis of ZnAl layered double hydroxides with nitrate as the interlayer anion. Appl. Clay Sci. 2011, 51, 452–459. [Google Scholar] [CrossRef]
- Yasaei, M.; Khakbiz, M.; Ghasemi, E.; Zamanian, A. Synthesis and characterization of ZnAl-NO3(-CO3) layered double hydroxide: A novel structure for intercalation and release of simvastatin. Appl. Surf. Sci. 2019, 467, 782–791. [Google Scholar] [CrossRef]
- Arizaga, G.G.C.; Mangrich, A.S.; da Costa Gardolinski, J.E.F.; Wypych, F. Chemical modification of zinc hydroxide nitrate and Zn–Al-layered double hydroxide with dicarboxylic acids. J. Colloid Interface Sci. 2008, 320, 168–176. [Google Scholar] [CrossRef]
- Li, W.; Liu, A.; Tian, H.; Wang, D. Controlled release of nitrate and molybdate intercalated in Zn-Al-layered double hydroxide nanocontainers towards marine anticorrosion applications. Colloid Interface Sci. Commun. 2018, 24, 18–23. [Google Scholar] [CrossRef]
- Paleontological Statistics Software Package for Education and Data Analysis (PAST). 2020. Available online: https://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 3 September 2020).
- Kim, S.G.; Lim, G.T.; Jegal, J.; Lee, K.H. Pervaporation separation of MTBE (methyl tert-butyl ether) and methanol mixtures through polyion complex composite membranes consisting of sodium alginate/chitosan. J. Membr. Sci. 2000, 174, 1–15. [Google Scholar] [CrossRef]
- Ionita, M.; Pandele, M.A.; Iovu, H. Sodium alginate/graphene oxide composite films with enhanced thermal and mechanical properties. Carbohyd. Polym. 2013, 94, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.A.; Wilkins, C.H. Infrared spectra and characteristic frequencies of inorganic ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Kloprogge, J.T. Infrared and Raman spectroscopy of naturally occurring hydrotalcites and their synthetic equivalents. In The Application of Vibrational Spectroscopy to Clay Minerals and Layered Double Hydroxides; The Clay Minerals Society: Aurora, CO, USA, 2005; pp. 203–238. [Google Scholar]
- Larosa, C.; Salerno, M.; de Lima, J.S.; Meri, R.M.; da Silva, M.F.; de Carvalho, L.B.; Converti, A. Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef]
- Legrouri, A.; Badreddine, M.; Barroug, A.; De Roy, A.; Besse, J.P. Influence of pH on the synthesis of the Zn–Al–nitrate layered double hydroxide and the exchange of nitrate by phosphate ions. J. Mater. Sci. Lett. 1999, 18, 1077–1079. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Talib, Z.A.; bin Hussein, M.Z. Thermal, optical, and dielectric properties of Zn–Al layered double hydroxide. Appl. Clay Sci. 2012, 56, 68–76. [Google Scholar] [CrossRef]
- Komarneni, S.; Newalkar, B.L.; Li, D.; Gheyi, T.; Lopano, C.L.; Heaney, P.J.; Post, J.E. Anionic clays as potential slow-release fertilizers: Nitrate ion exchange. J. Porous Mater. 2003, 10, 243–248. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Wang, S.L.; Wang, P.C. In situ XRD and ATR-FTIR study on the molecular orientation of interlayer nitrate in Mg/Al-layered double hydroxides in water. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 131–138. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef] [PubMed]


| Material | Elemental Analysis (g kg −1) | Atom% 15N | |||||
|---|---|---|---|---|---|---|---|
| C | H | O | N | Zn | Al | ||
| KNO3 | 0 | 0 | 475 | 139 | 0 | 0 | 1.844 |
| SA | 333 | 42 | 518 | 0 | 0 | 0 | na |
| LDH-N | 4 | 30 | 499 | 45 | 282 | 138 | 1.064 |
| LDH-AN | 51 | 34 | 458 | 48 | 277 | 132 | 1.022 |
| Property | Value |
|---|---|
| pH (soil:water, 1:1) | 5.6 |
| Organic C (g kg−1) | 18.4 |
| Total N (g kg−1) | 1.26 |
| Available P (mg kg−1) | 3.8 |
| Exchangeable K (mg kg−1) | 39 |
| Exchangeable Ca (cmolc kg−1) | 1.46 |
| Exchangeable Mg (cmolc kg−1) | 0.57 |
| Potential acidity (cmolc kg−1) | 7.5 |
| Sand (%) | 34 |
| Silt (%) | 12 |
| Clay (%) | 54 |
| Water-holding capacity (%) | 32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, V.L.N.; Mulvaney, R.L.; Cantarutti, R.B.; Pinto, F.G.; Tronto, J. Improving Nitrate Fertilization by Encapsulating Zn-Al Layered Double Hydroxides in Alginate Beads. Nitrogen 2020, 1, 125-136. https://doi.org/10.3390/nitrogen1020011
Nunes VLN, Mulvaney RL, Cantarutti RB, Pinto FG, Tronto J. Improving Nitrate Fertilization by Encapsulating Zn-Al Layered Double Hydroxides in Alginate Beads. Nitrogen. 2020; 1(2):125-136. https://doi.org/10.3390/nitrogen1020011
Chicago/Turabian StyleNunes, Vander L. N., Richard L. Mulvaney, Reinaldo B. Cantarutti, Frederico G. Pinto, and Jairo Tronto. 2020. "Improving Nitrate Fertilization by Encapsulating Zn-Al Layered Double Hydroxides in Alginate Beads" Nitrogen 1, no. 2: 125-136. https://doi.org/10.3390/nitrogen1020011
APA StyleNunes, V. L. N., Mulvaney, R. L., Cantarutti, R. B., Pinto, F. G., & Tronto, J. (2020). Improving Nitrate Fertilization by Encapsulating Zn-Al Layered Double Hydroxides in Alginate Beads. Nitrogen, 1(2), 125-136. https://doi.org/10.3390/nitrogen1020011





