Nutrient Availability under Lettuce Grown in Rye Mulch in Histosols
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
Experimental Design
- Site description
- Soil solution sampling
- Soil chemical sampling
- Soil solution analysis
- Soil analysis
- Data visualization and statistical analyses
3. Results
3.1. Rye and Lettuce Crop Yields
3.2. Rye and Rhizosphere Influence on Soil Nutrient Concentrations
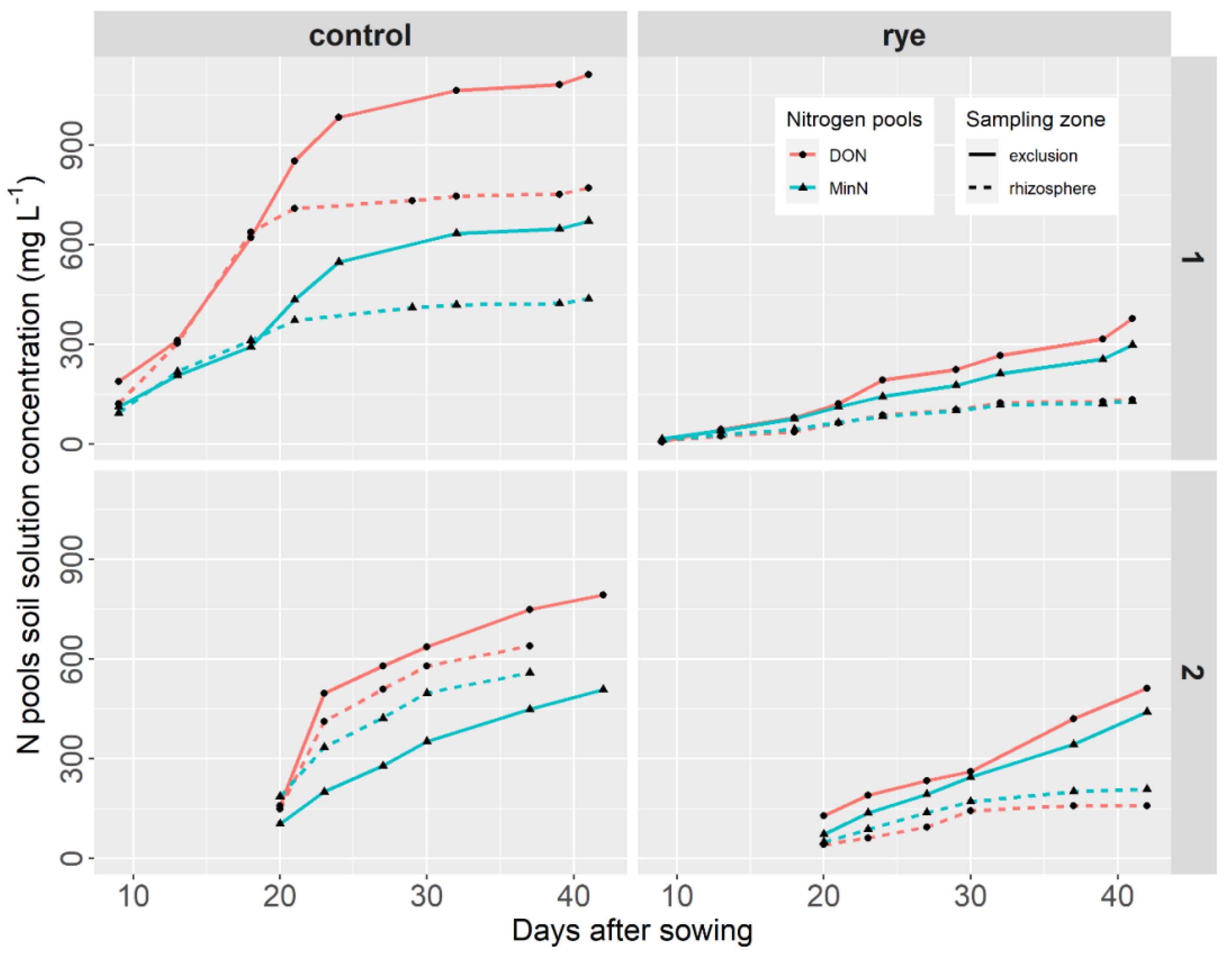
- Nitrogen pools
- b.
- Phosphorus
3.3. Soil Nutrients and Carbon Status at the End of the Lettuce Growth Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). The Future of Food and Agriculture—Alternative Pathways to 2050; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Ritchie, H.; Reay, D.S.; Higgins, P. The impact of global dietary guidelines on climate change. Glob. Environ. Chang. 2018, 49, 46–55. [Google Scholar] [CrossRef]
- Schiermeier, Q. Eat less meat: UN climate-change report calls for change to human diet. Nature 2019, 572, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Norris, C.E.; Congreves, K.A. Alternative management practices improve soil health indices in intensive vegetable cropping systems: A review. Front. Environ. Sci. 2018, 6, 50. [Google Scholar] [CrossRef]
- Kroetsch, D.J.; Geng, X.; Chang, S.X.; Saurette, D.D. Organic soils of Canada: Part 1. Wetland organic soils. Can. J. Soil Sci. 2011, 91, 807–822. [Google Scholar] [CrossRef]
- MAPAQ. Portrait-Diagnostic Sectoriel des Légumes frais au Québec. 2018. Available online: https://www.mapaq.gouv.qc.ca/fr/Publications/Portraitsectoriellegumesfrais.pdf (accessed on 10 November 2020).
- Lucas, R.E.; RE, L. Organic Soils (Histosols) Formation, Distribution, Physical and Chemical Properties and Management for Crop Production. 1982. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCALAGROLINEINRA83X0159565 (accessed on 1 August 2019).
- Dessureault-Rompré, J.; Libbrecht, C.; Caron, J. Biomass crops as a soil amendment in cultivated histosols: Can we reach carbon equilibrium? Soil Sci. Soc. Am. J. 2020, 84, 597–608. [Google Scholar] [CrossRef]
- Cassidy, E.S.; West, P.C.; Gerber, J.S.; Foley, J.A. Redefining agricultural yields: From tonnes to people nourished per hectare. Environ. Res. Lett. 2013, 8, 034015. [Google Scholar] [CrossRef]
- Pieper, J.R.; Brown, R.N.; Amador, J.A. Effects of three conservation tillage strategies on yields and soil health in a mixed vegetable production system. HortScience 2015, 50, 1770–1776. [Google Scholar] [CrossRef]
- Saini, R.; Singh, S. Contribution of Cover Crops and Reduced Tillage Systems for Weed Management in Organic Vegetable Production. Agric. Res. 2019, 4, 24. [Google Scholar]
- Siller, A.R.; Albrecht, K.A.; Jokela, W.E. Soil erosion and nutrient runoff in corn silage production with Kura clover living mulch and winter rye. Agron. J. 2016, 108, 989–999. [Google Scholar] [CrossRef]
- Ding, G.; Liu, X.; Herbert, S.; Novak, J.; Amarasiriwardena, D.; Xing, B. Effect of cover crop management on soil organic matter. Geoderma 2006, 130, 229–239. [Google Scholar] [CrossRef]
- Forcella, F.; Eklund, J.; Peterson, D. Rolled–crimped winter rye cover effects on hand-weeding times and fruit yield and quality of cucurbits. Int. J. Veg. Sci. 2015, 21, 386–396. [Google Scholar] [CrossRef]
- Singh, H.; Batish, D.R.; Kohli, R. Allelopathic interactions and allelochemicals: New possibilities for sustainable weed management. Crit. Rev. Plant Sci. 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Barnes, J.P.; Putnam, A.R. Evidence for allelopathy by residues and aqueous extracts of rye (Secale cereale). Weed Sci. 1986, 34, 384–390. [Google Scholar] [CrossRef]
- Shilling, D.G.; Liebl, R.A.; Worsham, A.D. Rye (Secale cereale L.) and Wheat (Triticum aestivum L.) Mulch: The Suppression of Certain Broadleaved Weeds and the Isolation and Identification of Phytotoxins; ACS Publications: Washington, DC, USA, 1985. [Google Scholar]
- Basche, A.D.; Kaspar, T.C.; Archontoulis, S.V.; Jaynes, D.B.; Sauer, T.J.; Parkin, T.B.; Miguez, F.E. Soil water improvements with the long-term use of a winter rye cover crop. Agric. Water Manag. 2016, 172, 40–50. [Google Scholar] [CrossRef]
- Haruna, S.I.; Nkongolo, N.V. Cover crop management effects on soil physical and biological properties. Procedia Environ. Sci. 2015, 29, 13–14. [Google Scholar] [CrossRef]
- Weerasekara, C.S.; Udawatta, R.P.; Gantzer, C.J.; Kremer, R.J.; Jose, S.; Veum, K.S. Effects of cover crops on soil quality: Selected chemical and biological parameters. Commun. Soil Sci. Plant Anal. 2017, 48, 2074–2082. [Google Scholar] [CrossRef]
- Domagała-Świątkiewicz, I.; Siwek, P.; Bucki, P.; Rabiasz, K. Effect of hairy vetch (Vicia villosa Roth.) and vetch-rye (Secale cereale L.) biculture cover crops and plastic mulching in high tunnel vegetable production under organic management. Biol. Agric. Hortic. 2019, 35, 248–262. [Google Scholar] [CrossRef]
- Rosecrance, R.; McCarty, G.; Shelton, D.; Teasdale, J. Denitrification and N mineralization from hairy vetch (Vicia villosa Roth) and rye (Secale cereale L.) cover crop monocultures and bicultures. Plant Soil 2000, 227, 283–290. [Google Scholar] [CrossRef]
- Tabaglio, V.; Marocco, A.; Schulz, M. Allelopathic cover crop of rye for integrated weed control in sustainable agroecosystems. Ital. J. Agron. 2013, 8, 35–40. [Google Scholar] [CrossRef]
- Wells, M.S.; Brinton, C.M.; Reberg-Horton, S.C. Weed suppression and soybean yield in a no-till cover-crop mulched system as influenced by six rye cultivars. Renew. Agric. Food Syst. 2016, 31, 429–440. [Google Scholar] [CrossRef]
- Jabran, K. Rye allelopathy for weed control. In Manipulation of Allelopathic Crops for Weed Control; Springer: Cham, Switzerland, 2017; pp. 49–56. [Google Scholar]
- Canadian Agricultural Services Coordinating Committee. Soil Classification Working Group, National Research Council Canada. Agriculture, & Agri-Food Canada. Research Branch. In The Canadian System of Soil Classification; (No. 1646); NRC Research Press: Ottawa, ON, Canada, 1998. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; pp. 2–66. [Google Scholar]
- Jones, D.; Willett, V. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- CEAEQ. Détermination des Métaux et du Phosphore Assimilables: Méthode par Spectrométrie de Masse à Source Ionisante au Plasma D’argon; MA 200–Mét-P ass 10; Centre d’Expertise en Analyse Environnementale du Québec: Quebec City, QC, Canada, 2014; p. 15. [Google Scholar]
- Blair, G.J.; Lefroy, R.D.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Wells, M.; Reberg-Horton, S.; Smith, A.; Grossman, J. The reduction of plant-available nitrogen by cover crop mulches and subsequent effects on soybean performance and weed interference. Agron. J. 2013, 105, 539–545. [Google Scholar] [CrossRef]
- Williams, A.; Wells, M.S.; Dickey, D.A.; Hu, S.; Maul, J.; Raskin, D.T.; Reberg-Horton, S.C.; Mirsky, S.B. Establishing the relationship of soil nitrogen immobilization to cereal rye residues in a mulched system. Plant Soil 2018, 426, 95–107. [Google Scholar] [CrossRef]
- Kaspar, T.; Bakker, M. Biomass production of 12 winter cereal cover crop cultivars and their effect on subsequent no-till corn yield. J. Soil Water Conserv. 2015, 70, 353–364. [Google Scholar] [CrossRef]
- Malpassi, R.; Kaspar, T.; Parkin, T.; Cambardella, C.; Nubel, N. Oat and rye root decomposition effects on nitrogen mineralization. Soil Sci. Soc. Am. J. 2000, 64, 208–215. [Google Scholar] [CrossRef]
- Sievers, T.; Cook, R.L. Aboveground and root decomposition of cereal rye and hairy vetch cover crops. Soil Sci. Soc. Am. J. 2018, 82, 147–155. [Google Scholar] [CrossRef]
- Duguet, F.; Parent, L.E.; Ndayegamiye, A. Compositional indices of net nitrification in cultivated organic soils. Soil Sci. 2006, 171, 886–901. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. How plant root exudates shape the nitrogen cycle. Trends Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef]
- Dion, P.-P.; Jämtgård, S.; Bertrand, A.; Pepin, S.; Dorais, M. Organic nitrogen uptake and assimilation in Cucumis sativus using position-specific labeling and compound-specific isotope analysis. Front. Plant Sci. 2018, 9, 1596. [Google Scholar] [CrossRef]
- Enggrob, K.L.; Jakobsen, C.M.; Pedersen, I.F.; Rasmussen, J. Newly depolymerized large organic N contributes directly to amino acid uptake in young maize plants. New Phytol. 2019, 224, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Healey, J.R.; Willett, V.B.; Farrar, J.F.; Hodge, A. Dissolved organic nitrogen uptake by plants—An important N uptake pathway? Soil Biol. Biochem. 2005, 37, 413–423. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Harasim, E.; Gawęda, D.; Wesołowski, M.; Kwiatkowski, C.; Gocół, M. Cover cropping influences physico-chemical soil properties under direct drilling soybean. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 85–94. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, S.; Wei, W.; Liang, B.; Li, J.; Ding, X. Straw and optimized nitrogen fertilizer decreases phosphorus leaching risks in a long-term greenhouse soil. J. Soils Sed. 2020, 20, 1199–1207. [Google Scholar] [CrossRef]
- Austin, E.E.; Wickings, K.; McDaniel, M.D.; Robertson, G.P.; Grandy, A.S. Cover crop root contributions to soil carbon in a no-till corn bioenergy cropping system. Gcb. Bioenergy 2017, 9, 1252–1263. [Google Scholar] [CrossRef]




| Site 1 | Site 2 | |
|---|---|---|
| Total surface area (control + rye treatment) | 1.8 ha | 1.0 ha |
| Rye variety | Guttino | Gauthier |
| Rye seeding rate (kg/ha) | 225 | 165 |
| Rye seeding date | 16 October 2018 | 25 September 2018 |
| Rye scrolling date | 15 May 2019 | 01 June 2019 |
| Rye yield (Mg ha−1) | 6 | 2.5 |
| Fertilization | 100 kg N/ha, 20 kg P2O5/ha, and 160 kg K2O/ha | 60 kg N/ha, 30 kg P2O5/ha, and 185kg K2O/ha |
| Lettuce variety | Global | Bergams Green |
| Lettuce planting method | PlantTape automated transplanting system | Planting in cubic blocks |
| Lettuce planting date | 30 June (seedlings were about 5 days old) | 10 July (seedlings were about 15 days old) |
| Lettuce harvest date | 08 August | 08 August |
| Lettuce yield: control (g per lettuce plant) | 556 | 648 |
| Lettuce yield: rye mulch cover (g per lettuce plant) | 101 | 605 |
| Nitrogen | Phosphorus | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | Site 1 | Site 2 | Site 1 | Site 2 | ||||
| F Values | Pr > F † | F Values | Pr > F | F Values | Pr > F | F Values | Pr > F | |
| Sampling zone | 20.4 | <0.001 | 0.837 | 0.036 | 0.189 | 0.664 | 2.075 | 0.156 |
| Soil cover | 128 | <0.001 | 25.9 | <0.001 | 194 | <0.001 | 13.9 | <0.001 |
| N pools | 5.65 | 0.018 | 2.13 | 0.147 | n.d. ‡ | n.d. | n.d. | n.d. |
| Sampling zone: soil cover | 0.37 | 0.545 | 1.94 | 0.017 | 3.56 | 0.062 | 6.18 | 0.016 |
| Sampling zone: N pools | 0.67 | 0.413 | 1.14 | 0.287 | n.d. | n.d. | n.d. | n.d. |
| Soil cover: N pools | 7.78 | 0.006 | 2.52 | 0.115 | n.d. | n.d. | n.d. | n.d. |
| Sampling zone: soil cover: N pools | 0.01 | 0.906 | 0.28 | 0.599 | n.d. | n.d. | n.d. | n.d. |
| Mehlich-III P (mg kg−1) | Mineral N (mg kg−1) | Soluble organic N (mg kg−1) | Total N (%) | Active C (mg kg−1) | Total C (%) | Soil C/N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | ||
| Soil cover | |||||||||||||||
| Rye | 83.2 | 82.7 | 46.9 | 48.0 a | 95.3 b | 111.4 a | 2.0 b | 2.09 b | 9247.9 b | 9209.7 | 48.3 | 45.1 a | 23.8 a | 21.0 a | |
| Control | 66.5 | 70.1 | 51.8 | 75.5 b | 80.2 a | 120.1 b | 1.8 a | 2.15 a | 7260.6 a | 9435.0 | 46.1 | 46.5 b | 26.1 b | 22.3 b | |
| Depth | |||||||||||||||
| 0–20 | 118.7 c | 112.3 c | 70.4 b | 86.8 b | 92.2 b | 129.8 b | 1.9 | 2.1 | 10,134.6 b | 10,537.8 | 46.1 | 44.3 a | 25.1 | 20.8 a | |
| 20–40 | 76.7 b | 92.0 b | 46.4 a | 53.8 a | 88.5 a | 124.5 b | 1.9 | 2.1 | 7913.3 a | 8655.6 | 46.9 | 44.5 a | 24.6 | 20.9 a | |
| 40–60 | 29.2 a | 24.9 a | 31.3 a | 44.7 a | 82.5 a | 93.1 a | 1.9 | 2.1 | 6714.9 a | 8773.6 | 48.6 | 48.6 b | 25.2 | 23.3 b | |
| Soil cover X depth interaction | |||||||||||||||
| Rye | 0–20 | 136.8 | 121.0 | 68.5 | 50.0 a | 96.9 c | 121.0 | 2.0 | 2.1 | 11,926.0 c | 10,573.0 | 46.5 | 43.2 | 22.9 a | 20.1 |
| 20–40 | 91.2 | 106.9 | 46.3 | 52.9 a | 100.1 c | 124.0 | 2.1 | 2.2 | 8760.3 b | 8309.5 | 48.2 | 43.5 | 22.6 a | 20.2 | |
| 40–60 | 21.8 | 20.3 | 25.9 | 41.0 a | 88.8 bc | 89.3 | 2.0 | 2.1 | 7057.5 ab | 8746.5 | 50.4 | 48.7 | 25.8 b | 22.8 | |
| Control | 0–20 | 100.7 | 103.6 | 72.3 | 123.5 b | 87.6 ab | 138.5 | 1.7 | 2.1 | 8343.3 ab | 10,502.5 | 45.7 | 45.5 | 27.2 b | 21.5 |
| 20–40 | 62.3 | 77.2 | 46.5 | 54.6 a | 76.9 a | 125.0 | 1.7 | 2.1 | 7066.3 ab | 9001.8 | 45.6 | 45.5 | 26.5 b | 21.7 | |
| 40–60 | 36.7 | 29.4 | 36.7 | 48.4 a | 76.2 a | 96.9 | 1.9 | 2.1 | 6372.3 a | 8800.8 | 46.8 | 48.5 | 24.6 a | 23.7 | |
| Statistical significance | |||||||||||||||
| Soil cover | NS | NS | NS | *** | *** | * | ** | ** | *** | NS | NS | ** | *** | *** | |
| Depth | *** | *** | ** | *** | ** | *** | NS | NS | *** | NS | NS | *** | NS | *** | |
| Soil cover X depth | NS | NS | NS | *** | * | NS | NS | NS | * | NS | NS | NS | *** | NS | |
| SE (18 df; n = 24) | 6.2 | 4.5 | 3.8 | 1.9 | 1.1 | 2.0 | 0.04 | 0.01 | 219.8 | 349.0 | 1.0 | 0.23 | 0.16 | 0.16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessureault-Rompré, J.; Gloutney, A.; Caron, J. Nutrient Availability under Lettuce Grown in Rye Mulch in Histosols. Nitrogen 2020, 1, 137-150. https://doi.org/10.3390/nitrogen1020012
Dessureault-Rompré J, Gloutney A, Caron J. Nutrient Availability under Lettuce Grown in Rye Mulch in Histosols. Nitrogen. 2020; 1(2):137-150. https://doi.org/10.3390/nitrogen1020012
Chicago/Turabian StyleDessureault-Rompré, Jacynthe, Alexis Gloutney, and Jean Caron. 2020. "Nutrient Availability under Lettuce Grown in Rye Mulch in Histosols" Nitrogen 1, no. 2: 137-150. https://doi.org/10.3390/nitrogen1020012
APA StyleDessureault-Rompré, J., Gloutney, A., & Caron, J. (2020). Nutrient Availability under Lettuce Grown in Rye Mulch in Histosols. Nitrogen, 1(2), 137-150. https://doi.org/10.3390/nitrogen1020012





