Abstract
Plants that enter symbiotic relationships with nitrogen (N)-fixing microbes contribute some of their N to the community through leaf litter decomposition and mineralization processes. The speed of these processes varies greatly among tree species. Mesocosm methods were used to determine the speed of N and carbon (C) release from Cycas micronesica, Intsia bijuga, and Serianthes nelsonii leaf litter. Microcosm methods were used to determine soil respiration traits in soils containing the leaf litter. The speed of leaf litter N and C release during decomposition occurred in the order C. micronesica < I. bijuga < S. nelsonii. Soil carbon dioxide efflux was increased by adding leaf litter to incubation soils, and the increase was greatest for S. nelsonii and least for C. micronesica litter. Ammonium, nitrate, total N, organic C, and total C were increased by adding litter to incubation soils, and the differences among the species converged with incubation duration. The rate of increases in available N and decreases in organic C were greatest for S. nelsonii and least for C. micronesica litter. These findings indicate that S. nelsonii litter released N and C rapidly, C. micronesica litter released N and C slowly, and the leaf economic spectrum accurately predicted the differences.
1. Introduction
Biological nitrogen (N) fixation is a crucial component of the nitrogen cycle [1,2]. The group of plants known as N-fixers is not capable of N fixation directly [3], but they enter symbiotic relationships with N-fixing microbes in a manner that gives them access to the newly fixed N [4,5]. The largest group of plants with this consequential symbiosis is the Fabaceae where rhizobia bacteria reside in root nodules. Cyanobacteria have also evolved N-fixing capacity [6], and the ancient group of plants known as cycads develops ageotropic coralloid roots which are colonized by cyanobacteria [7,8].
Plants with N-fixing microbial symbionts contribute their N to other sympatric organisms through herbivory, organ abscission, or plant death. As a result, the ecosystem services rendered by their contributions of N are of interest in ecology research but also in holistic managed production systems such as permaculture or agroforestry. The decomposition of litterfall has been the primary focus for research on how trees transfer minerals and metals from the living tree to the bulk soil. Experimental incubation of litter and/or soils enclosed in screen litterbags or impervious bags or tubes has generated a robust literature on the subject [9,10]. Litter N does not act in isolation during decomposition processes but is linked to carbon (C) [11]. Moreover, litter decomposition is a critical component of the global C cycle [12]. Therefore, C and N dynamics are often studied in synchrony during the mineralization of elements from organic matter.
The Micronesian island of Guam is home to several native tree species that provide these ecosystem services. Intsia bijuga (Colebr.) Kuntze has a widespread indigenous range and is vulnerable to extinction due to over-exploitation for timber and loss of habitat [13]. The handsome legume tree is a member of the Caesalpinioideae subfamily and is designated as the official territorial tree of Guam where it is known as ifit [14]. Excessive harvesting of I. bijuga trees on Guam was reported more than a century ago [15]. Cycas micronesica K.D. Hill is an arborescent cycad species with an endemic range that includes Palau, Yap, and the southern Mariana Islands [16]. The local name is fadang. Invasive insect herbivore species have devastated the populations on Guam [17,18], and the mortality has led to the designation of endangered status [16]. Serianthes nelsonii Merr. has a restricted endemic range that includes the adjacent islands of Guam and Rota [19]. The critically endangered legume tree is a member of the Mimosoideae subfamily and exhibited a limited population size when it was described [20]. The list of threats to this large tree, known as Håyun lågu, is lengthy [19,21]. Seed production and seedling regeneration are considerable but recruitment is unsuccessful, and the single greatest conservation need is adaptive management research to determine the reasons for recruitment failures [22,23].
The conservation agenda for threatened plant species is often limited by lack of knowledge, and one approach for advancing conservation success is to expand species-specific research [24]. Studies that reveal the ecosystem contributions of the threatened tree species may aid conservationists by providing information about the benefits of saving the species. Indeed, conservation efforts are more likely to succeed if the science that underpins biodiversity conservation adds a focus on ecosystem services that are also threatened along with the threats to biodiversity [25,26]. The decomposition traits or nutrient release characteristics of litter from Guam’s C. micronesica, I. bijuga, and S. nelsonii trees have not been reported. The objectives were to use microcosm and mesocosm methods to incubate litter and soil samples to compare the speed of N and C release from leaf litter samples from these three Guam species. Based on personal in situ observations, the lifespan of an S. nelsonii leaf is less than one year, of an I. bijuga leaf is two to three years, and of a C. micronesica leaf is more than seven years (unpublished data). Employing the tenets of the leaf economic spectrum [27,28], I hypothesized that N and C release would be rapid for S. nelsonii leaf litter, slow for C. micronesica leaf litter, and intermediate for I. bijuga leaf litter.
2. Materials and Methods
Leaf litter was collected in northern Guam from trees that were growing in coastal karst substrates that were formed in slope alluvium, loess, and residuum overlying limestone (Clayey-skeletal, gibbsitic, nonacid, isohyperthermic Lithic Ustorthents) [29]. Litter was collected from October 2014 to September 2015 with frequent visits to in situ localities. The litter samples from October–November, December–January, February–March, April–May, June–July, and August–September were stored separately in paper bags to provide six replications to determine initial litter chemistry traits.
Cycas micronesica, I. bijuga, and S. nelsonii produce pinnately compound leaves, but the size of the leaflets and abscission traits following leaf senescence differ among the species. Therefore, litter collection methods were distinct for each species to ensure collection of recently senesced leaflets. Serianthes nelsonii produces bipinnately compound leaves with small leaflets of about 0.2 cm2 each. Entire leaves typically abscise with senescing leaflets attached, and freshly fallen leaves that contained yellow turgid leaflets were retrieved from the forest floor during weekly visits. There was no take from the living tree, but access to the site was enabled by the United States Endangered Species Act Recovery Permit TE-84876A-0. Pinnately compound I. bijuga leaves are generally comprised of two leaflet pairs, and the large leaflets that are up to 15 cm in length typically abscise individually. Leaflets were collected from the forest floor beneath I. bijuga trees by selecting freshly fallen yellow leaflets. Pinnately compound C. micronesica leaves are up to 180–200 cm in length and contain up to 150 lanceolate leaflets that are 24–28 cm in length. The leaves are usually retained on the plant after full senescence. Leaflets from C. micronesica were collected from attached leaves containing some leaflets that were turgid and yellow and some leaflets that were desiccated and brown. This approach ensured the collection of recently senesced leaflet litter. At this time Guam’s C. micronesica population had been attacked by the non-native armored scale Aulacaspis yasumatsui Takagi for many years [30]. The litter was collected from a protected plot that had received imidacloprid applications since 2007 to ensure the source leaves were not infested with non-native insect herbivores and exhibited natural longevity.
The C. micronesica and I. bijuga leaflets were cut into 2-cm sections, then all samples were stored in air-conditioned laboratory conditions at the University of Guam in order to air-dry. The start of the experiments occurred after litter dry weight became stable. A 3-g sample was removed from each of the six replications of litter per species. This sample was used to determine initial litter chemistry. The rest of the litter samples were combined into one homogenized sample for each of the species and used for three separate studies at the University of Guam as described below.
The initial litter samples were dried at 75 °C for 24 h then milled to pass through a 20-mesh screen. Carbon and nitrogen were determined by dry combustion (FLASH EA1112 CHN analyzer; Thermo Fisher, Waltham, MA, USA) [31]. Lignin was quantified with the acetyl-bromide method [32]. Cellulose was determined according to AOAC International [33]. Stoichiometric traits were calculated as C/N, lignin/N, and cellulose/N, all based on concentrations. Differences among the three species were determined by analysis of variance (PROC GLM, SAS Institute, Cary, NC, USA). Means separations for significant factors were conducted by Tukey’s honest significant difference test.
2.1. Litter Decomposition
The litterbag method was employed to determine C and N release during decomposition. Each litterbag was constructed with ≈3 g of enclosed litter from one of the three species, and there were 16 litterbags per species. The litterbags were constructed from a nylon screen with 1.5-mm mesh and were square with 15-cm sides.
Field soil was excavated from the surface 5 cm within the habitats where the experimental litter had been collected on January 9, 2016. Fresh litter was removed from the soil surface, but humus was included and homogenized with the soil. The collection sites did not include trees from any of the three species to ensure the home field advantage factors [34,35] would not complicate the species comparisons. The most common trees in the collection sites were Aglaia mariannensis Merr., Cordia subcordata Lam., Pandanus tectorius Parkinson, Ochrosia oppositifolia (Lam.) K. Schum., and Premna serratifolia L. A representative sample from this homogenized incubation soil was dried at 105 °C. Total carbon and nitrogen contents were determined by dry combustion (FLASH EA1112 CHN analyzer). Available P was determined by the Olsen method [36]. Extractable potassium, calcium, and magnesium were quantified following digestion with diethylenetriaminepentaacetic acid [37]. Elements were quantified by inductively coupled plasma optical emission spectrometry (Spectro Genesis; SPECTRO Analytical Instruments, Kleve, Germany).
A mesocosm was constructed at the University of Guam in an open-air room with a roof that excluded rainfall and direct sun exposure. The mesocosm was 0.5 m × 2.5 m and the homogenized incubation soil was added to 20-cm depth. The litterbags were placed on top of the soil within four blocks on January 10, 2016, with four litterbags per species per block. The 12 litterbags for each block were positioned randomly within each block. Poultry mesh was placed on top of the mesocosm, and a polyethylene sheet was placed on the mesh to ensure it was not in contact with the litterbags. The polyethylene sheet was removed once per week and the litterbags and soil were irrigated to apply about 2 cm of rainfall equivalent. The temperature of the soil at a depth of 5 cm was periodically observed with a thermometer and compared with the ambient temperature in the open-air room. The maximum and minimum temperature of the soil was not different from that of the ambient conditions, but diel hysteresis caused less rapid diurnal increases and nocturnal decreases in temperature in the soil.
One block was selected randomly after three months, and the 12 litterbags (3 species × 4 replications) within the block were harvested. The litter was extracted from the litterbags, adhering soil was rinsed, then litter was air-dried and stored in paper bags until chemical analyses. This procedure was repeated at six months and nine months. The remaining block was harvested at 12 months.
The C and N pools were calculated for every litter sample to determine loss with time. For the initial C and N pool, samples from each species were weighed as air-dry weight, then were dried at 75 °C for 24 h to obtain oven-dry weight. The estimated oven-dry weight of the initial ≈ 3-g samples was calculated from the oven-dry weight/air-dry weight quotients. Then initial C and N pools were calculated from the initial concentrations and multiplied by the estimated oven-dry weight. For samples of 3, 6, 9, and 12 months, the oven-dry weight was measured prior to analysis for C and N concentration using the methods described earlier. The total pool within the litter for each species and incubation time was calculated by multiplying the concentrations by the oven-dry weights.
There were three response variables: total C, total N, and the quotient C/N based on the total pool of each element (not concentration). The influence of 3 species × 5 incubation periods was assessed using repeated-measures analysis with four replications. The time of incubation was designated as the repeated measure, and the analysis used an autoregressive covariance structure (PROC MIXED, SAS). Means separation for significant factors was conducted by Tukey’s honest significant difference test.
2.2. Soil Respiration
A microcosm approach was employed to determine the influence of litter additions to soil on soil respiration. The microcosms were constructed from the base of 2-L plastic soda bottles. The bottles were cut at 6-cm height to create cylindrical microcosms that were 9.5 cm in diameter. Insulation foam with a width of 0.5 cm was affixed to the perimeter of the top of each microcosm to create a total diameter of 10.5 cm. Twelve 3-mm holes were drilled in the base of each microcosm to ensure adequate drainage and aeration.
The fresh soil that was described in Section 2.1 was used for the incubations. In order to calculate the water weight of the fresh soil, six of the microcosms were initially filled to 5-cm height on January 10, 2016, then the soil was weighed to determine the fresh weight of 334 ± 11 g. The samples were dried for 24 h at 105 °C then re-weighed to obtain a dry weight of 252 ± 9 g. These data were used to estimate the dry weight of each soil fresh weight sample. The undisturbed bulk density of this soil series is 0.6–0.9 g·cm−3 [29].
A sample of 15 g was obtained from the litter for each of the three tree species on 11 January 2016, was milled to pass through a 20-mesh screen, then four 2.5-g samples were extracted and added to one of four soil samples of 334-g fresh weight. This created four soil incubation replications per species with milled litter added at an estimated 1% dry weight basis. In addition to the 12 replications with litter additions, there were four replications with no litter added that served as the control. The 16 microcosms were placed in an ambient laboratory room and irrigated on the first day until the first signs of drainage, then were irrigated again every other day.
The first respiration measurements were conducted on 13 January 2016, then were repeated every 2 d for the first 8 d, and every 5 to 7 d thereafter until the study was terminated on day 59. A CIRAS EGM-4 analyzer fitted with an SRC-1 close system chamber (PP Systems, Amesbury, MA, USA) was used to determine carbon dioxide efflux from the soil surface within each microcosm. The inside diameter of the chamber was 10 cm, enabling the insulation foam to create a seal when the top of a microcosm was forced into the chamber. The integrity of the seal was confirmed periodically by breathing onto the chamber-microcosm interface to reveal no change in the carbon dioxide concentration patterns. The EGM-4 recorded air temperature and carbon dioxide concentration change over a 2-min period. The measurements were made from 09:00 until 11:00 h on each day of measurement, and the ambient carbon dioxide was 405–415 µmol·mol−1. The carbon dioxide efflux was calculated from the carbon dioxide change with time based on the 71 cm2 of soil surface within the microcosms.
The data for each of the four soil treatments were plotted in a scatter plot with carbon dioxide flux on the vertical axis and date of measurement on the horizontal axis to reveal a non-linear decline with time. The curves for each of the four soil treatments were fitted with the highly significant model y = a/(x + b) + c. Thereafter, the data for each of the four replications per treatment were fitted to determine the regression model for each replication. Each of the three coefficients from the model was subjected to analysis of variance to determine differences among the four soil treatments.
2.3. Mineralization
The speed of C and N mineralization was determined using the buried bag method [38]. Fresh soil was collected from the field sites using the methods previously described during the first week of January 2017. The soil was thoroughly homogenized, then a sample was extracted to determine macronutrient concentrations as described in Section 2.1. About 20 L of the remaining soil was placed in a container with a bottom constructed with 1.5-mm screen fabric then irrigated until the first signs of drainage from the bottom of the container. The container was covered with plastic to stop surface evaporation then allowed to drain for two days. The percentage of water that remained in the soil was calculated as 39% from a comparison of fresh weight minus dry weight after one day in a forced-draft oven set at 105 °C. This was considered water holding capacity of the homogenized soil.
The soil was separated into 16 1-L samples for incubations. A 120-g sample was removed from the stored litter from each of the tree species. The litter for each species was milled to pass through a 20-mesh screen then was separated into four 30-g samples. Each of the 30-g samples was added to one of four 1-L samples of soil and homogenized to create four replications per tree species. This approach utilized 12 of the soil samples, and the remaining four samples served as a control with no added litter. Thus, there were four replications with four soil treatments.
Water was added to each 1-L replication to estimate 50% of water holding capacity to ensure adequate aeration. An initial soil sample of 100 mL was removed to quantify pre-incubation C and N relations, enclosed in a plastic bag, and frozen at −20 °C. The remaining soil for each replication was enclosed in a zip-lock plastic bag for incubations. The 16 bags were buried in the mesocosm described above in a randomized complete block design on January 8, 2017.
Each bag was opened and the soil was thoroughly mixed every 15 days to ensure the soil organic matter was redistributed and carbon dioxide did not accumulate in the bags. Fresh weights were used to determine if there was any water loss, and water was added to reach 50% of water holding capacity whenever needed. Additionally, 100-g samples were removed at 15, 30, 60, and 90 days and frozen at −20 °C. Therefore, there were five sampling periods including the initial sampling period.
The soils were thawed to ambient temperature, then N and C components of the soil samples were determined as previously described [39]. Total N and total C were determined by dry combustion, and organic C was determined by dichromate consumption with the modified Walkley-Black protocol [40]. Nitrate and ammonium were determined colorimetrically following 2M KCl extraction [41]. Net nitrification was calculated by subtracting concentration in one sampling period from nitrate concentration in the subsequent sampling period then dividing by the incubation duration. Net ammonification was calculated for each sampling time using the same methods as for nitrate. Available N was calculated as the sum of nitrate and ammonium concentrations.
The influence of four soil treatments over 120 d of incubation on the C and N variables were assessed using repeated-measures analysis of variance with four replications treated as blocks. The incubation time was designated as the repeated measure, and the analysis used a compound symmetry covariance structure (PROC MIXED, SAS). Means separation for significant factors was conducted by Tukey’s honest significant difference.
3. Results
The two bulk soil samples were collected one year apart but were similar in chemistry (Table 1). The chemistry of fresh leaf litter differed among the species for every measured trait except C concentration (Table 2). Nitrogen concentration was lowest for I. bijuga litter and was similar for C. micronesica and S. nelsonii litter. Lignin concentration decreased in the order C. micronesica > I. bijuga > S. nelsonii. Cellulose concentration was greatest for C. micronesica and was similar for the other two species. Collectively, these traits predicted litter quality and speed of decomposition would occur in the order C. micronesica < I. bijuga < S. nelsonii. The stoichiometric traits did not follow the same trends, in that I. bijuga litter exhibited the greatest values and S. nelsonii exhibited the least values. Collectively, the stoichiometry predicted litter quality and speed of decomposition would occur in the order I. bijuga < C. micronesica < S. nelsonii.

Table 1.
Concentration of macronutrients in soils used for decomposition, mineralization, and respiration studies.

Table 2.
Chemical traits of leaf litter of three tree species from Guam. Mean ± SE, n = 6.
3.1. Litter Decomposition
The total pool of C within the litter samples was influenced by the main factor of time (p < 0.001), the main factor of species (p < 0.001), and the interaction of time × species (p < 0.001). The initial total C pool in the 3-g litter samples was similar among the three species in the fresh litter samples (Figure 1a). From 3 to 12 months of decomposition, the amount of remaining C in the litter increased in the order S. nelsonii < I. bijuga < C. micronesica. The total pool of N within the litter samples was influenced by the main factor of time (p < 0.001), the main factor of species (p < 0.001), and the interaction of time × species (p < 0.001). The initial total N pool in the 3-g litter samples was similar for C. micronesica and S. nelsonii but was greatly reduced for I. bijuga (Figure 1b). The 3-month loss of N from S. nelsonii litter greatly exceeded that of C. micronesica and I. bijuga litter. From 3 to 12 months of decomposition, the amount of remaining N in the litter increased in the order S. nelsonii < I. bijuga < C. micronesica. The quotient C/N within the litter samples was influenced by the main factor of time (p < 0.001), the main factor of species (p < 0.001), and the interaction of time × species (p = 0.017). The quotient C/N was greatest in I. bijuga litter for every sampling period throughout the study (Figure 1c). Alternatively, the C/N of C. micronesica litter was not different from that of S. nelsonii litter for any of the sampling periods.
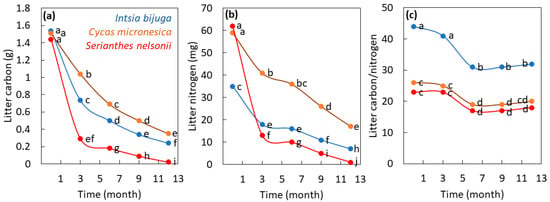
Figure 1.
The influence of decomposition time on leaf litter traits for three Guam tree species. (a) Total pool of carbon in litter that was 3 g initially; (b) Total pool of nitrogen in litter that was 3 g initially; (c) The carbon/nitrogen quotient within litter that was 3 g initially. Markers are mean of 4 replications, and markers with the same letters are not different according to Tukey’s honest significant difference.
The greatest amount of C and N release occurred in the first 3-month incubation period for I. bijuga and S. nelsonii. The release of these elements from C. micronesica litter was more gradual over time of incubation. The release of C and N from these litter samples was almost 100% for S. nelsonii litter during the study. These decomposition data indicated the absolute chemical concentrations of lignin and cellulose in the initial litter were more important for predicting actual elemental release than were initial stoichiometry traits (Table 2).
The daily maximum temperature for the incubation period was 30 °C, and the daily minimum temperature was 24.8 °C.
3.2. Soil Respiration
The carbon dioxide efflux of soils incubated in microcosms was greatest 2 days after leaf litter additions, which was the first day of gas exchange measurements (Figure 2). A non-linear decrease occurred with time of incubation, and the trends were described by the model y = a/(x + b) + c for all four treatments. Coefficients a, b, and c all differed among the treatments (p < 0.001). The non-linear changes in carbon dioxide efflux caused the differences among the treatments to converge with time such that efflux was similar for all treatments by day 49. In conformity with the litter decomposition, the initial concentrations of lignin and cellulose accurately predicted maximum carbon dioxide efflux from the microcosms and more accurately than did initial stoichiometry traits (Table 2).
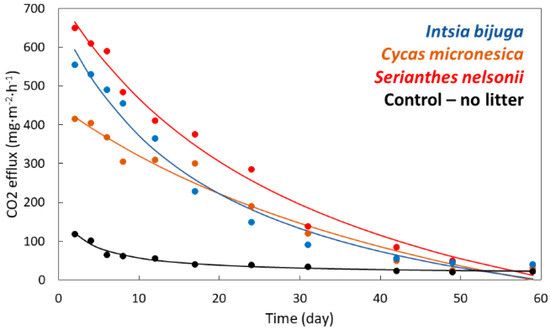
Figure 2.
The influence of incubation time on carbon dioxide (CO2) efflux from soil following addition of 1% leaf litter from three Guam tree species. Markers are mean of 4 replications. Control: y = 584.172/(x + 3.44) + 13.194; Cycas micronesica: y = 54,333.726/(x + 59.809) − 458.228; Intsia bijuga: y = 17,333.339/(x + 19.363) − 217.959; Serianthes nelsonii: y = 35,929.52/(x + 32.299) − 382.979.
The mean temperature for the incubation period was at 28.9 °C, with a mean minimum of 27.6 °C and a mean maximum of 30.2 °C. The mean chamber temperature during gas exchange measurements was 28.5 °C.
3.3. Mineralization
Ammonium concentration during buried bag incubation was influenced by the main factor of time (p < 0.001), the main factor of soil treatment (p < 0.001), and the interaction of time × species (p < 0.001). The patterns of ammonium concentration during the 120-day incubation period were highly erratic among the four treatments (Figure 3a). Ammonium concentration of the S. nelsonii litter treatment was greatest on day 15 then declined over time. Ammonium concentration for the I. bijuga litter remained elevated from day 15–90 then declined by day 120. Ammonium concentration of the C. micronesica litter increased gradually until day 90 then declined by day 120. Ammonium concentration was similar for the four treatments on day 120. These highly inconsistent trends in ammonium concentration were mediated by the generation of ammonium from organic matter concomitant with the conversion of ammonium to nitrate.
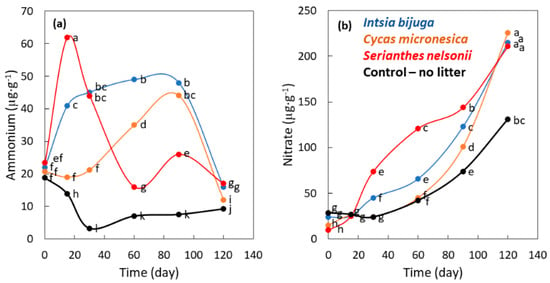
Figure 3.
The influence of incubation time on nitrogen traits as influenced by the addition of leaf litter of three Guam tree species to incubation soil. (a) Ammonium; (b) Nitrate. Markers are mean of 4 replications, and markers with the same letters are not different according to Tukey’s honest significant difference.
Nitrate concentration during buried bag incubation was influenced by the main factor of time (p < 0.001), the main factor of soil treatment (p < 0.001), and the interaction of time × species (p < 0.001). The litter additions to soils increased nitrate concentrations above the control soils beginning on day 30 (Figure 3b). The increase for S. nelsonii litter exceeded that of the other treatments from 30–90 days. The increase for C. micronesica and I. bijuga litter exceeded that of the other treatments from 90–120 days. Nitrate concentration for the three litter treatments was about 1.7-fold greater than the control soil treatment on day 120. In general, as ammonium concentration declined, nitrate concentration increased and the N cycling dynamics shifted from ammonium-centered status at the beginning of incubation to nitrate-centered by the end of incubation.
Available N concentration during buried bag incubation was influenced by the main factor of time (p < 0.001), the main factor of soil treatment (p < 0.001), and the interaction of time × species (p < 0.001). The I. bijuga and S. nelsonii litter additions to soils increased available N concentrations in a consistent pattern from the first day until day 120 (Figure 4a). The available N concentration for the C. micronesica litter treatment was similar to the control treatment from day 0 to 60. Thereafter, the available N concentration for the C. micronesica litter treatment rapidly increased such that the content was similar to the litter of the other two species by day 120. Available N concentration for the three litter treatments was about 1.7-fold greater than the control soil treatment on day 120. The similarities between nitrate N and available N revealed that nitrate controlled available N to a greater degree than ammonium.
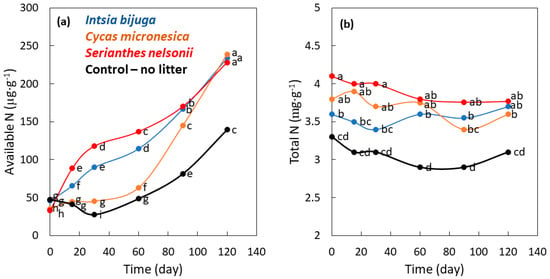
Figure 4.
The influence of incubation time on nitrogen (N) traits as influenced by the addition of leaf litter of three Guam tree species to incubation soil. (a) Available N; (b) Total N. Markers are mean of 4 replications, and markers with the same letters are not different according to Tukey’s honest significant difference.
Total N concentration during buried bag incubation was influenced by the main factor of time (p < 0.001), the main factor of soil treatment (p < 0.001), and the interaction of time × species (p < 0.001). There was a considerable overlap of concentrations among the species and incubation durations. The initial concentrations of total N increased in the order control < I. bijuga ≤ C. micronesica ≤ S. nelsonii (Figure 4b). The total N concentration for each of the four treatments did not significantly change during the 120 d incubation period. The total N concentration of the control soil treatment was 85% of that for the three litter treatments on day 120.
The changes in N forms enabled a calculation of mineralization components within each of the incubation periods between sampling dates or throughout the 120 day incubation period. Net ammonification (calculated from Figure 3a data) was negative for all four treatments over the 120 day study and was the most negative for the control soil treatment (−0.08 µg·g−1·day−1). Net ammonification ranged from −0.05 to −0.07 µg·g−1·day−1 for the three litter treatments. Net nitrification (calculated from Figure 3b data) of the three litter treatments ranged from 1.59 to 1.76 µg·g−1·day−1 and exceeded that of the control treatment (0.85 µg·g−1·day−1). The net mineralization (calculated from Figure 4a) was similar to net nitrification. Net mineralization of the three litter treatments over the 120-day study ranged from 1.54 to 1.69 µg·g−1·day−1 and exceeded that of the control treatment (0.77 µg·g−1·day−1). The timing of maximum net mineralization differed among the tree species. For example, S. nelsonii litter additions to the soils generated the greatest net mineralization of 2.83 µg·g−1·day−1 during the initial 30-day incubation period. During this same period, the net mineralization of C. micronesica litter soils was minimal. Alternatively, C. micronesica litter additions to the soils generated the greatest net mineralization of 3.13 µg·g−1·day−1 during the final 30-day incubation period.
Soil organic C concentration during buried bag incubation was influenced by the main factor of time (p < 0.001), the main factor of soil treatment (p < 0.001), and the interaction of time × species (p < 0.001). The initial concentrations of organic C were similar for the three litter addition treatments, which were 1.6-fold greater than organic C of the control soil treatment (Figure 5a). Organic C of the control soil treatment was stable throughout the incubation period, but organic C of the litter addition treatments declined with time of incubation. Organic C of the S. nelsonii litter treatment declined rapidly and was not different from the control soil treatment by 60 d of incubation. Organic C of the I. bijuga litter treatment declined more slowly and was not different from the control soil treatment by 90 d of incubation. Organic C of the C. micronesica litter treatment declined with time of incubation but remained greater than the other three treatments from day 60 to120. Organic C concentration of the control soil treatment was 83% of that for the C. micronesica litter treatment on day 120.
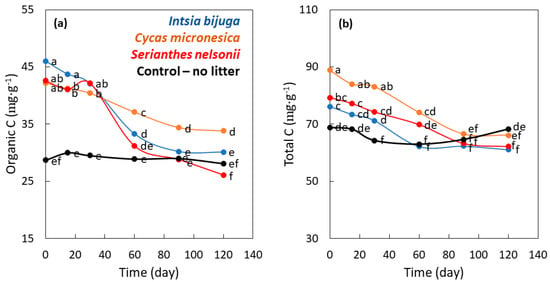
Figure 5.
The influence of incubation time on carbon (C) traits as influenced by the addition of leaf litter of three Guam tree species to incubation soil. (a) Organic C; (b) Total C. Markers are mean of 4 replications, and markers with the same letters are not different according to Tukey’s honest significant difference.
Total C concentration during buried bag incubation was influenced by the main factor of time (p < 0.001), the main factor of soil treatment (p < 0.001), and the interaction of time × species (p < 0.001). The initial concentrations of total C were greatest for the C. micronesica litter treatment, intermediate for the I. bijuga and S. nelsonii litter treatments, and least for the control soil treatment (Figure 5b). The total C concentration for each of the three litter treatments declined with time of incubation and total C concentration was similar for the four treatments by 90 day.
The N and C transformations from the soil treatments differed among the species in patterns that were predicted by the initial lignin and cellulose concentrations of the leaf litter. The mean temperature for the incubation period was 26.8 °C, with nocturnal minima of 23.8 °C and diurnal maxima of 30.5 °C.
4. Discussion
The speed of N and C release from leaf litter contrasted sharply among three native tree species which associate with N-fixing microbial symbionts on the island of Guam. Quantification of litter release of N and C in litterbags, soil respiration following additions of litter to incubation soils in microcosms, and transformations in N and C forms with incubation time using the buried bag methods provided three contrasting approaches to confirm predications using the leaf economics spectrum [27,28] based on differences in observed leaf longevity. All three approaches indicated that components of S. nelsonii litter were the most labile, components of C. micronesica litter were the most recalcitrant, and components of I. bijuga litter were intermediate in the speed of turnover.
Observed leaf longevity was not the only trait that accurately predicted the rates of N and C release from the litter of these three species. First, meta-analyses and multiple biome litter transplant studies indicate that litter identity explains more variation in leaf litter decomposition than climatic variables or local soil characteristics [42,43,44]. This study aligned with this literature in that initial litter chemistry was also useful for predicting the outcomes. The differences in initial lignin and cellulose concentrations among the three species collectively predicted the observed disparity in the speed of N and C release from the litter samples. Second, the forests in the western Pacific islands are subjected to tropical cyclone damage more often than the forests in most locations [45,46]. The physiognomy of Guam’s forests at any point in time is controlled by the antecedent damaging tropical cyclone, and the island’s vegetation has been called “typhoon forests” [47]. During Guam’s tropical cyclones, S. nelsonii trees are easily defoliated, I. bijuga leaves exhibit intermediate damage, and C. micronesica leaves are relatively undamaged (personal observations), patterns that mirror the differences in leaf longevity.
The conservation of threatened organisms is a complicated agenda that is rife with myriad ecological nuances and human behaviors that limit successes. The determination and dissemination of information about ecosystem services provided by threatened organisms may improve conservation successes [25,26]. Trees that associate with N-fixing symbionts contribute N in a manner that influences habitat biogeochemistry through various factors such as tree density and spatial distribution [48]. Indeed, the influences of S. nelsonii on soil chemistry verified the accumulation of greater C and N concentrations in soils beneath the canopy than soils away [49]. Similar patterns were determined for C. micronesica trees [50,51]. The influence of I. bijuga on soil chemistry has not been reported to date. Clearly, studying the influence of threatened tree species on spatiotemporal dynamics of biogeochemistry may provide conservationists and resource managers with a reference for the development of adaptive management strategies in conservation plans or how to structure restoration sites. For Guam, the results from this study may be used by conservation managers to more fully understand how these three tree species influence biogeochemical processes through ecosystem services.
Carbon plays a commanding role in plant metabolism [52]. Plants remove carbon dioxide from the air through photosynthesis and convert it to organic products that lead to C sequestration in the biosphere, then plants release this C through direct respiration and indirect respiration during litter decomposition. Soil respiration measurements are critical for increasing our knowledge of terrestrial C cycling [53]. The results from this study indicate the C found in C. micronesica leaves will remain sequestered for greater durations during the leaf after-life stages, and C found in S. nelsonii leaves will be sequestered for shorter durations during after-life.
This initial look at the after-life trends of N and C turnover for three important Guam tree species illuminates several issues that require further study. First, microcosm and mesocosm studies are of critical importance for understanding various aspects of biology, especially when objectives are to compare various treatments that need to be observed in homogeneous settings. However, in situ studies are required to increase the relevance of findings from controlled studies. Decomposition trials under in situ temperature, light, and rainfall patterns remain to be conducted. Second, the evolution of S. nelsonii, C. micronesica, and I. bijuga was likely sympatric on Guam and Rota. The three species occur in the same locality on Guam today, S. nelsonii and I. bijuga occur in the same localities on Rota, and C. micronesica and I. bijuga occur in the same localities on Rota (personal observations). Single species litter that is decomposed in isolation often does not correlate with decomposition of multi-species litter mixtures [54,55,56]. Litter species mixture phenomena remain to be studied for these three Guam trees and the other sympatric tree species that share the forests. Third, the soil legacy effects of I. bijuga trees have not been determined, and this should be corrected with further study. Fourth, a home-field advantage often occurs during litter decomposition whereby litter from a tree species will decompose more rapidly in soils that are imprinted by the same tree species than in distant soils [34,35]. A full understanding of the N and C turnover during leaf after-life of these three tree species will require home versus away decomposition and mineralization methods. Fifth, tropical cyclones exert profound influences on biogeochemical cycling by defoliation of high-quality leaf material [57,58,59,60] or generating partial leaf mortality prior to the natural nutrient resorption processes that reduce leaf litter quality [61]. Global change phenomena predict minimal change in tropical cyclone frequency but increased intensity of future tropical cyclones [46]. Therefore, the disequilibrium in biogeochemical cycling that is caused by tropical cyclones may become more influential with global change [62]. The differences among the senesced litter from the three tree species in the present study may not align with that of green leaf litter, and these phenomena remain to be studied. Sixth, I. bijuga [14], C. micronesica [30], and S. nelsonii [19] are chronically damaged by native and non-native insect herbivores. Herbivory of leaves often increases litter quality [63,64], one of the factors that alter ecological processes. Further study of the direct and indirect effects of herbivory on ecosystem services provided by these three important tree species is needed.
Funding
This research was funded in part by the United States Forest Service Cooperative Agreement number 13-DG-11052021-210 and number 17-DG-11052021-217.
Acknowledgments
In this section you can acknowledge any support given which is not covered by the author contribution or funding sections.
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Postgate, J. Nitrogen Fixation, 3rd ed.; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Smil, V. Cycles of Life: Civilization and the Biosphere; W.H. Freeman: New York, NJ, USA, 2000. [Google Scholar]
- Marler, T.E.; Terral, O. It is what it is, but it shouldn’t be: The science of ambiguity. HortScience 2014, 49, 1234–1236. [Google Scholar]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2011, 16, 263–276. [Google Scholar]
- Latysheva, N.; Junker, V.L.; Palmer, W.J.; Codd, G.A.; Barker, D. The evolution of nitrogen fixation in cyanobacteria. Bioinformatics 2012, 28, 603–606. [Google Scholar]
- Norstog, K.J.; Nicholls, T.J. The Biology of the Cycads; Cornell University Press: Ithaca, NY, USA, 1997. [Google Scholar]
- Rai, A.N.; Söderbäck, E.; Bergman, B. Cyanobacterium-plant symbioses. N. Phytol. 2000, 147, 449–481. [Google Scholar]
- Khanna, P.K.; Raison, R.J. In situ core methods for estimating soil mineral-N fluxes: Re-evaluation based on 25 years of application and experience. Soil Biol. Biochem. 2013, 64, 203–210. [Google Scholar]
- Xie, Y. A meta-analysis of critique of litterbag method used in examining decomposition of leaf litters. J. Soils Sediments 2020, 20, 1881–1886. [Google Scholar]
- Pei, G.; Liu, J.; Peng, B.; Wang, C.; Jiang, P.; Bai, E. Nonlinear coupling of carbon and nitrogen release during litter decomposition and its responses to nitrogen addition. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005462. [Google Scholar] [CrossRef]
- Chapin, I.F.S.; Matson, P.A.; Vitousek, P. Principles of Terrestrial Ecosystem Ecology; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- World Conservation Monitoring Centre. Intsia bijuga. The IUCN Red List of Threatened Species. 1998. Available online: https://dx.doi.org/10.2305/IUCN.UK.1998.RLTS.T32310A9694485.en (accessed on 17 September 2020).
- Marler, T.E. Balancing growth and wood quality of Intsia bijuga under management: Complexity of silviculture conservation decisions. J. Trop. Forest Sci. 2015, 27, 429–434. [Google Scholar]
- Safford, W.E. The Useful Plants of the Island of Guam; Government Printing Office: Washington, DC, USA, 1905.
- Marler, T.; Haynes, J.; Lindstrom, A. Cycas Micronesica. The IUCN Red List of Threatened Species. 2010. Available online: https://dx.doi.org/10.2305/IUCN.UK.2010-3.RLTS.T61316A12462113.en (accessed on 17 September 2020).
- Marler, T.E.; Lawrence, J.H. Demography of Cycas micronesica on Guam following introduction of the armoured scale Aulacaspis yasumatsui. J. Tropical Ecol. 2012, 28, 233–242. [Google Scholar]
- Marler, T.E.; Krishnapillai, M.V. Longitude, forest fragmentation, and plant size influence Cycas micronesica mortality following island insect invasions. Diversity 2020, 12, 194. [Google Scholar] [CrossRef]
- Wiles, G.; Williams, E. Serianthes Nelsonii. The IUCN Red List of Threatened Species. 2017. Available online: https://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T30437A98715973.en (accessed on 17 September 2020).
- Merrill, E.D. Additions to the flora of Guam. Philipp. J. Sci. 1919, 15, 539–544. [Google Scholar]
- Marler, T.; Musser, C. Potential stressors leading to seedling mortality in the endemic Håyun lågu tree (Serianthes nelsonii Merr.) in the island of Guam. Trop. Conserv. Sci. 2015, 8, 738–744. [Google Scholar] [CrossRef]
- Marler, T.E.; Cascasan, A.N. Number of emerged seedlings and seedling longevity of the non-recruiting, critically endangered Håyun lågu tree Serianthes nelsonii Merr. (Fabales: Leguminosae) are influenced by month of emergence. J. Threatened Taxa 2015, 7, 8221–8225. [Google Scholar] [CrossRef]
- Marler, T.E.; Cruz, G.N. Extreme wind events influence seed rain and seedling dynamics of Guam’s Serianthes nelsonii Merr. Trop. Conserv. Sci. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. The value of research to selling the conservation of threatened species: The case of Cycas micronesica (Cycadopsida: Cycadales: Cycadaceae). J. Threat. Taxa 2014, 6, 6523–6528. [Google Scholar] [CrossRef]
- Berkes, F.; Folke, C. (Eds.) Linking Social and Ecological Systems: Management Practices and Social Mechanisms for Building Resilience; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Van Oudenhoven, A.P.E.; Martín-López, B.; Schröter, M.; de Groot, R. Advancing science on the multiple connections between biodiversity, ecosystems and people. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2018, 14, 127–131. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The world-wide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. N. Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef]
- Young, F.J. Soil Survey of Territory of Guam; U. S. Dept. of Agric. Soil Conservation Service: Washington, DC, USA, 1988.
- Marler, T.E. Cycad aulacaspis scale invades the Mariana Islands. Mem. N. Y. Bot. Garden 2012, 106, 20–35. [Google Scholar]
- Dumas, J.B.A. Procedes de L’analyse Organique. Ann. Chim. Phys. 1831, 47, 198–205. [Google Scholar]
- Iiyama, K.; Wallis, A.F.A. Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J. Sci. Food Agric. 1990, 51, 145–161. [Google Scholar] [CrossRef]
- AOAC. Official Method 973. 18 fibre (acid detergent) and lignin in animal feed. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1997; pp. 28–29. [Google Scholar]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar] [CrossRef]
- Lin, D.; Dou, P.; Yang, G.; Qian, S.; Wang, H.; Zhao, L.; Yang, Y.; Mi, X.; Ma, K.; Fanin, N. Home-field advantage of litter decomposition differs between leaves and fine roots. N. Phytol. 2020, 227, 995–1000. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U. S. Dept. of Agric.: Washington, DC, USA, 1954; p. 19.
- Berghage, R.D.; Krauskopf, D.M.; Warncke, D.D.; Widders, I. Micronutrient Testing of Plant Growth Media Extractant, Identification and Evaluation. Commun. Soil Sci. Plant Anal. 1987, 18, 1089–1109. [Google Scholar] [CrossRef]
- Eno, C.F. Nitrate production in the field by incubating the soil in polyethylene bags. Proc. Soil Sci. Soc. Amer. 1960, 24, 277–279. [Google Scholar] [CrossRef]
- Marler, T.E.; Dongol, N.; Cruz, G.N. Leucaena leucocephala and adjacent native limestone forest habitats contrast in soil properties on Tinian Island. Commun. Integr. Biol. 2016, 9, e1212792. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series No. 5; SSSA and ASA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Cataldo, D.A.; Haroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; Handa, I.T.; Hattenschwiler, S.; van Ruijven, J.; van Bodegom, P.M.; Aerts, R. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 2012, 15, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E. Tropical cyclones and perennial species in the Mariana Islands. Hortscience 2001, 36, 264–268. [Google Scholar] [CrossRef]
- Marler, T.E. Pacific island tropical cyclones are more frequent and globally relevant, yet less studied. Front. Environ. Sci. 2014, 2, 42. [Google Scholar] [CrossRef]
- Stone, B.C. America’s Asiatic flora: The plants of Guam. Amer. Sci. 1971, 59, 308–319. [Google Scholar]
- Wilcots, M.E.; Taylor, B.N.; Kuprewicz, E.K.; Menge, D.N.L. Small traits with big consequences: How seed traits of nitrogen-fixing plants might influence ecosystem nutrient cycling. Oikos 2019, 128, 668–679. [Google Scholar] [CrossRef]
- Marler, T.E. Late successional tree species in Guam create biogeochemical niches. Commun. Integr. Biol. 2019, 12, 86–90. [Google Scholar] [CrossRef][Green Version]
- Marler, T.E.; Krishnapillai, M.V. Cycas micronesica trees alter local soil traits. Forests 2018, 9, 565. [Google Scholar] [CrossRef]
- Marler, T.E.; Calonje, M. Two cycad species affect the carbon, nitrogen, and phosphorus content of soils. Horticulturae 2020, 6, 24. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Phillips, C.L.; Bond-Lamberty, B.; Desai, A.R.; Lavoie, M.; Risk, D.; Tang, J.; Todd-Brown, K.; Vargas, R. The value of soil respiration measurements for interpreting and modeling terrestrial carbon cycling. Plant Soil 2017, 413, 1–25. [Google Scholar] [CrossRef]
- Cassart, B.; Basia, A.A.; Jonard, M.; Ponette, Q. Average leaf litter quality drives the decomposition of single-species, mixed-species and transplanted leaf litters for two contrasting tropical forest types in the Congo Basin (DRC). Ann. For. Sci. 2020, 77, 33. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Song, Q.; Compson, Z.G.; LeRoy, C.J.; Luan, F.; Wang, H.; Hu, Y.; Yang, Q. Synergistic effects: A common theme in mixed-species litter decomposition. New Phytol. 2020, 227, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Butenschoen, O.; Barantal, S.; Handa, I.T.; Makkonen, M.; Vos, V.; Aerts, R.; Berg, M.P.; McKie, B.; Van Ruijven, J.; et al. Decomposition of leaf litter mixtures across biomes: The role of litter identity, diversity and soil fauna. J. Ecol. 2020. [Google Scholar] [CrossRef]
- Steinwandter, M.; Schlick-Steiner, B.C.; Steiner, F.M.; Seeber, J. One plus one is greater than two: Mixing litter types accelerates decomposition of low-quality alpine dwarf shrub litter. Plant Soil 2019, 438, 405–419. [Google Scholar] [CrossRef]
- Lodge, D.; Scatena, F.; Asbury, C.; Sanchez, M. Fine litterfall and related nutrient inputs resulting from Hurricane Hugo in subtropical wet and lower montane rain forests of Puerto Rico. Biotropica 1991, 23, 336–342. [Google Scholar] [CrossRef]
- Xu, X. Nutrient dynamics in decomposing needles of Pinus luchuensis after typhoon disturbance in a subtropical environment. Ann. For. Sci. 2006, 63, 707–713. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, X.; Zou, X.; Lodge, D.J.; Stankavich, S.; González, G.; Cantrell, S.A. Responses of soil labile organic carbon to a simulated hurricane disturbance in a tropical wet forest. Forests 2018, 9, 420. [Google Scholar] [CrossRef]
- Marler, T.E.; Ferreras, U.F. Disruption of leaf nutrient remobilization in coastal Cycas trees by tropical cyclone damage. J. Geogr. Nat. Disasters 2015, 5, 1421–1427. [Google Scholar]
- Luo, Y.; Weng, E. Dynamic disequilibrium of the terrestrial carbon cycle under global change. Trends Ecol. Evol. 2011, 26, 96–104. [Google Scholar] [CrossRef]
- Marler, T.E.; Dongol, N. Three invasive insects alter Cycas micronesica leaf chemistry and predict changes in biogeochemical cycling. Commun. Integr. Biol. 2016, 9, e1208324. [Google Scholar] [CrossRef]
- Gandhi, K.J.K.; Herms, D.A. Direct and indirect effects of alien insect herbivores on ecological processes and interactions on forests of eastern North America. Biol. Invas. 2010, 12, 389–405. [Google Scholar]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).