Pore Structure Evolution of Coal After Supercritical CO2–Water–Rock Treatment: A Multifractal Analysis
Abstract
1. Introduction
2. Sampling and Experiments
2.1. Coal Samples
2.2. Low-Temperature N2/CO2 Gas Sorption Experiment
2.3. Supercritical CO2–Water–Rock Reaction Experiment
2.4. Multifractal Theory
2.5. Experimental Procedures
3. Results and Discussions
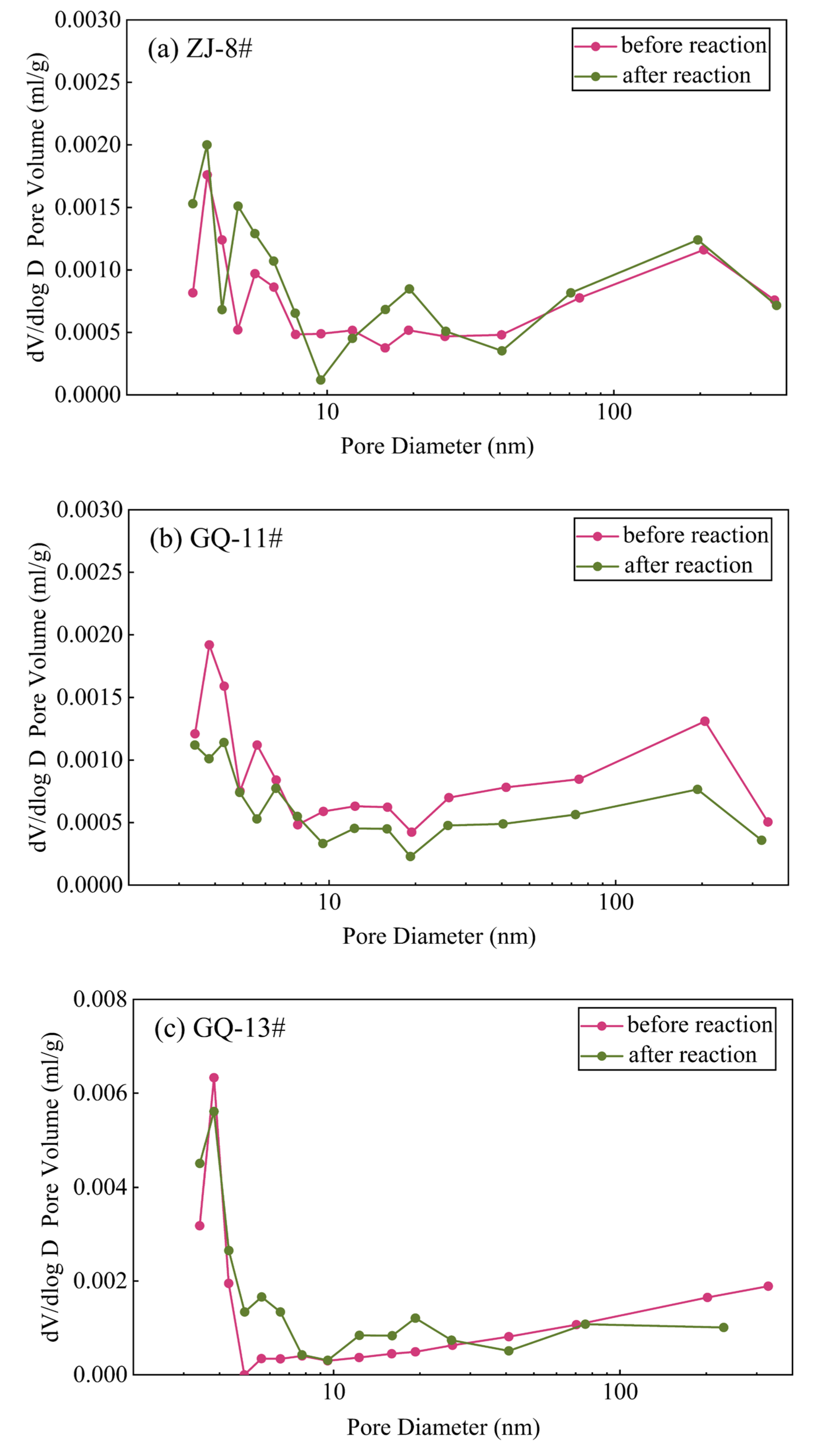
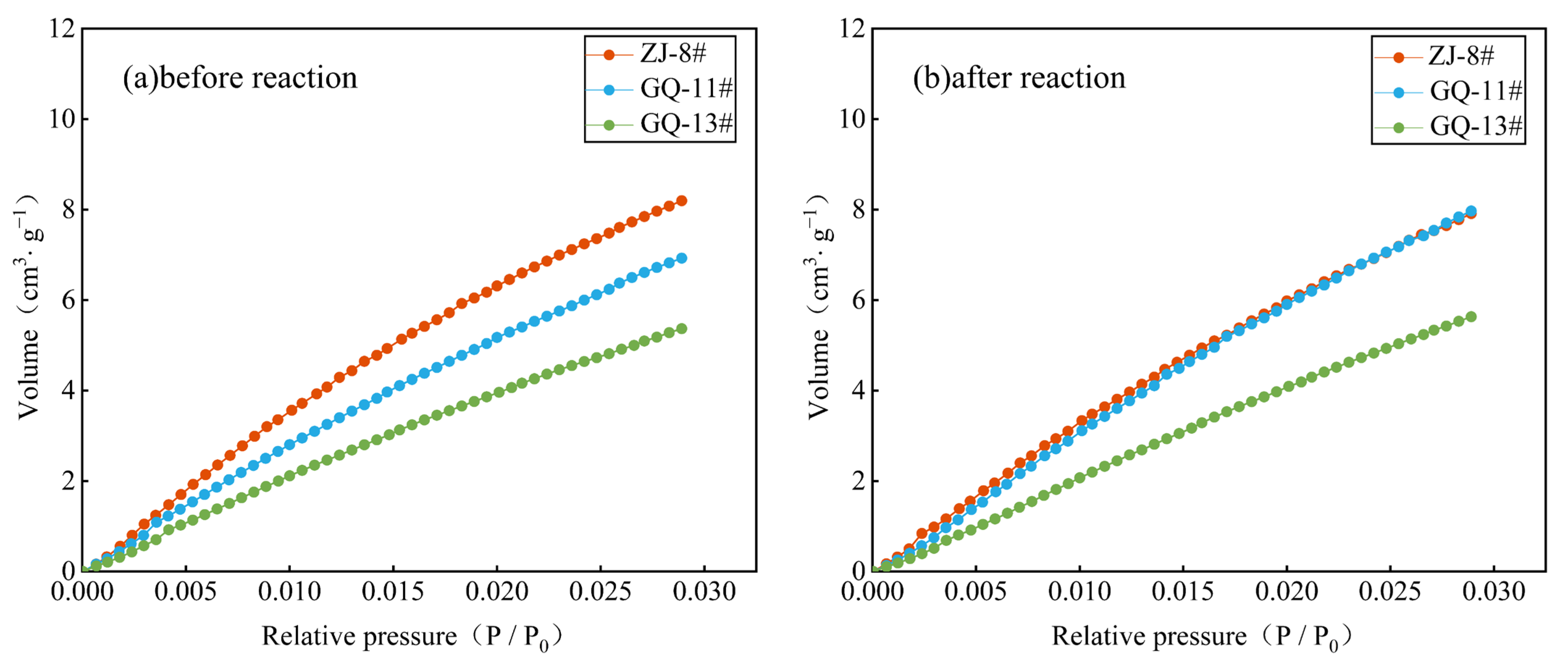
3.1. Low-Temperature N2 Adsorption Testing Results Before and After the ScCO2–Water–Rock Reaction
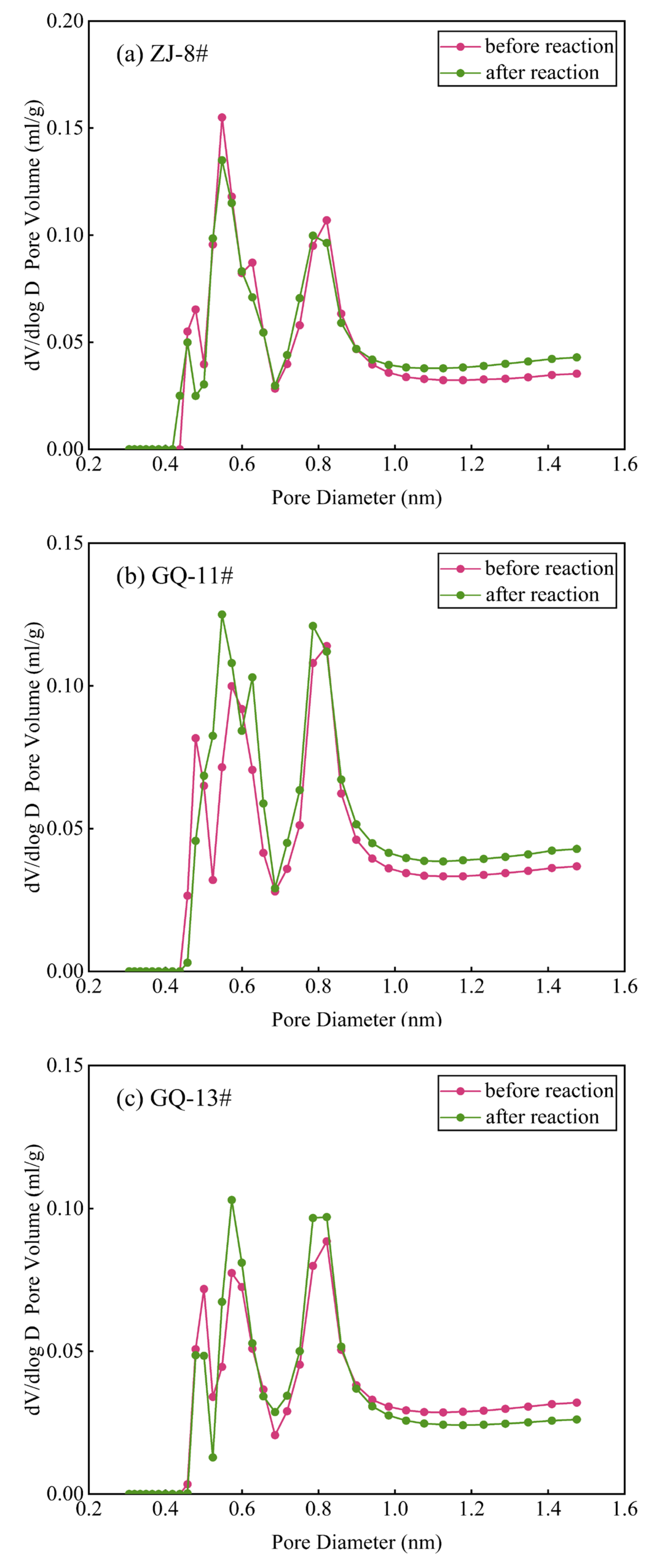
3.2. Data of Low-Temperature CO2 Testing Before and After the Reaction
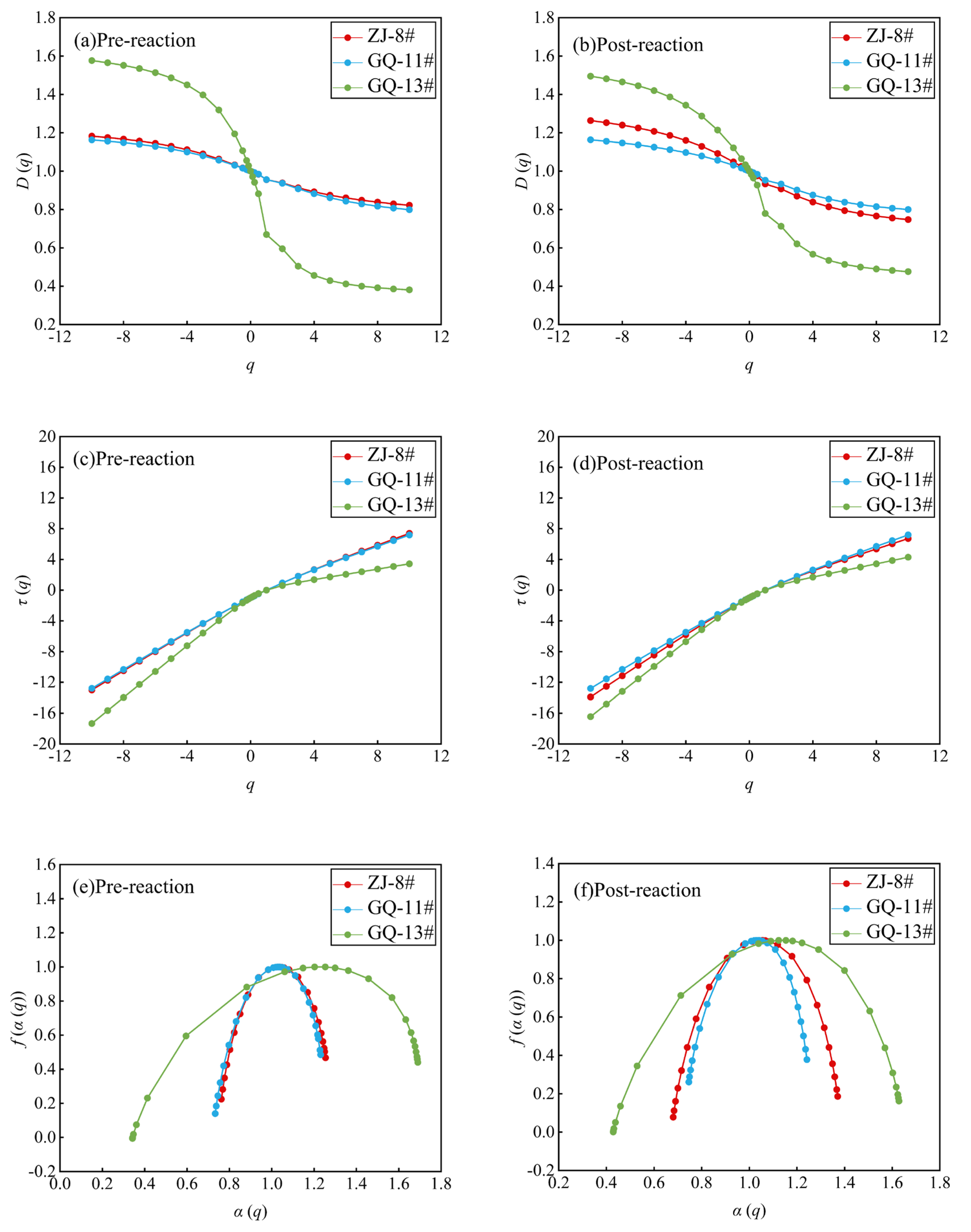
3.3. Multifractal Characteristics
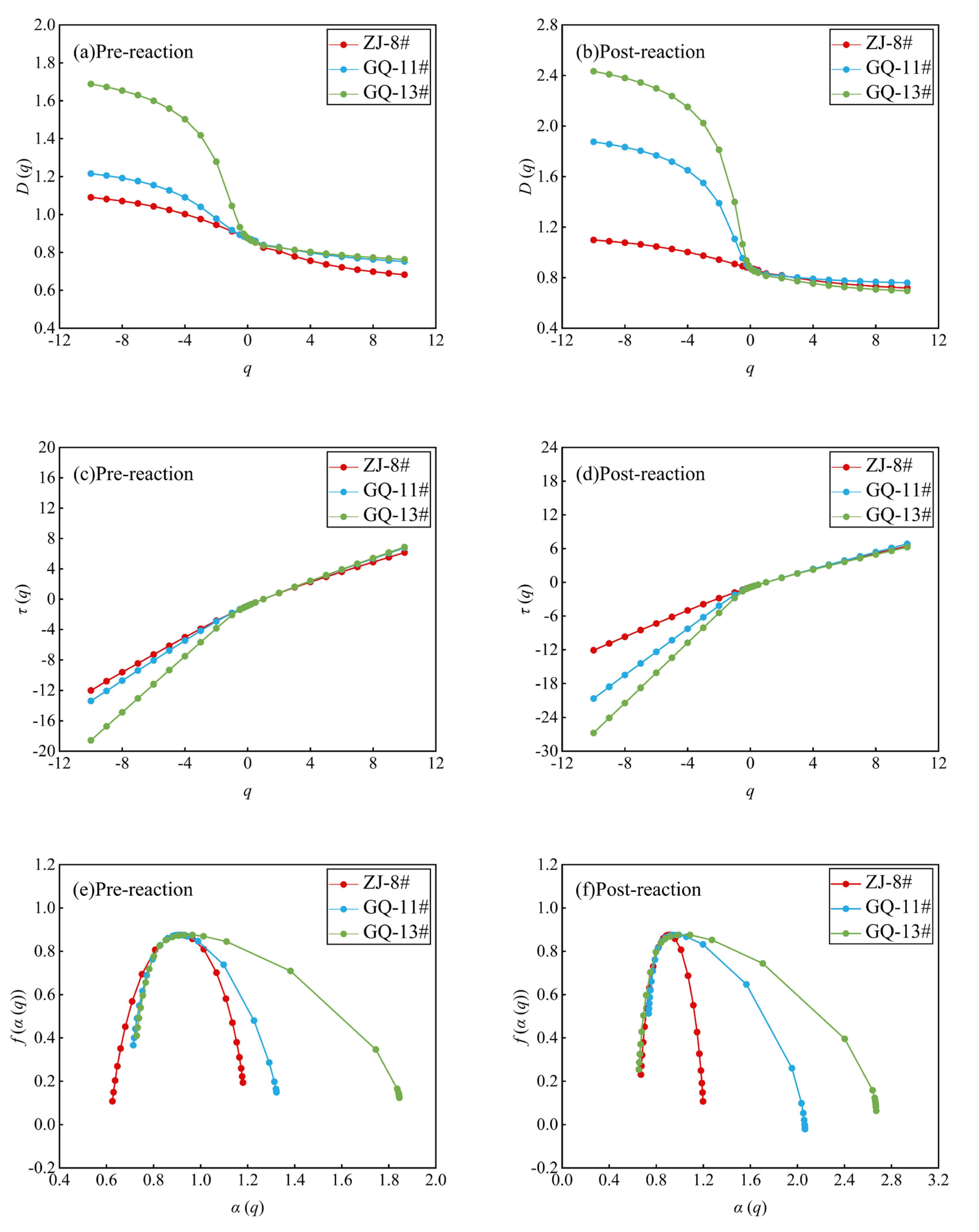
3.3.1. The Low-Temperature N2 Multifractal Characteristics
3.3.2. The Low-Temperature CO2 Multifractal Characteristics
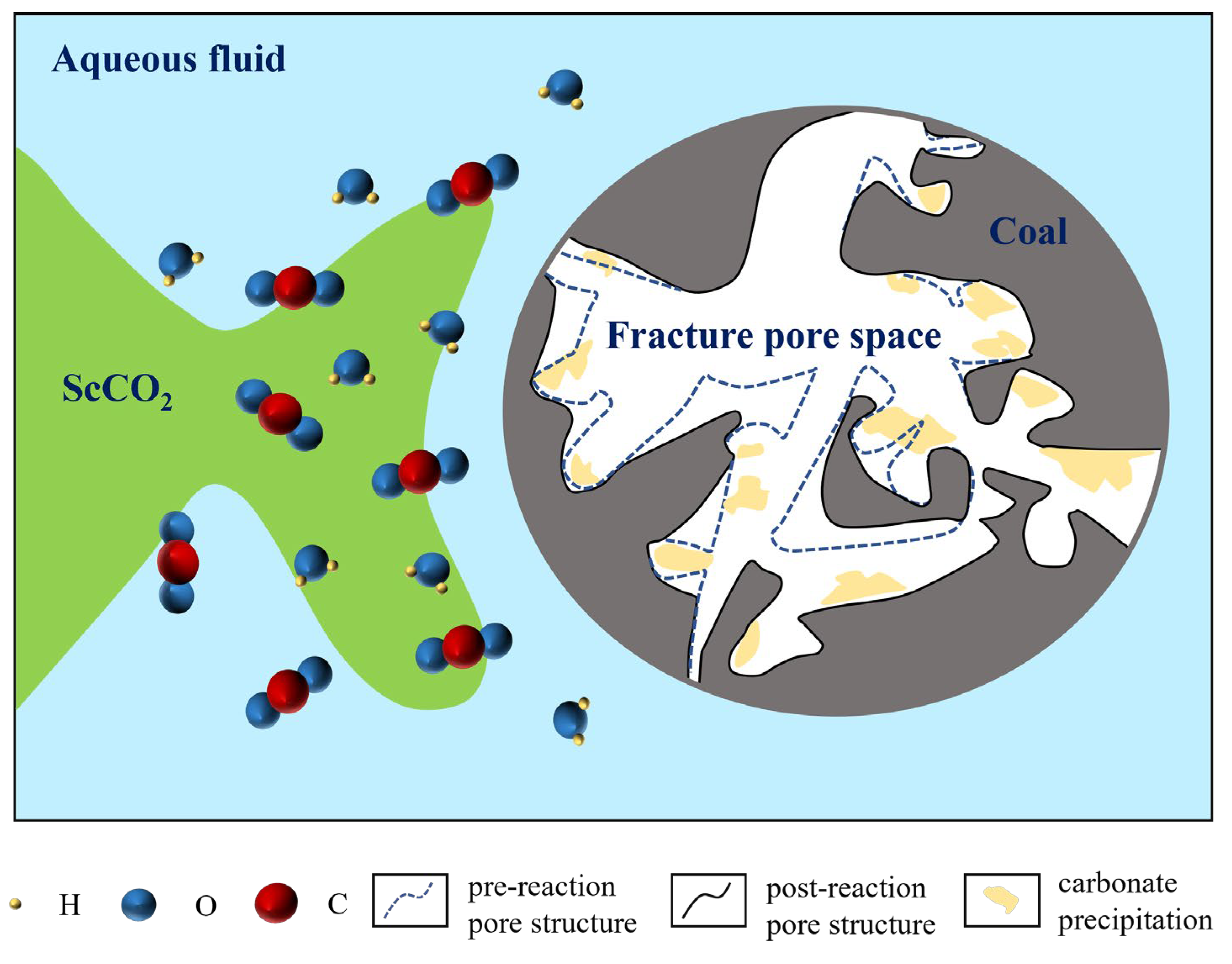
3.4. Mechanisms of Pore Structure Modification in Coal Reservoirs by Supercritical CO2–Water–Rock Interactions
4. Conclusions
- (1)
- The low-temperature N2 and CO2 testing offers a comprehensive characterization of coal’s small-scale pore structure features. The ScCO2–water–rock reaction minimally alters coal pore morphology, primarily influencing pore abundance. Specifically, the ZJ-8# sample’s micropore volume increases and its micropore–mesopore distribution becomes more complex under N2 testing conditions. In contrast, the GQ-11# sample experiences a reduction in micropore volume and count, whereas the GQ-13# sample shows no significant change in micropore volume. Within the CO2 sorption testing range, the ZJ-8# sample’s PSD remains largely unchanged before and after the reaction. The GQ-11# and GQ-13# samples display increased micropore abundance, with the GQ-11# sample exhibiting more pronounced micropore development after the reaction.
- (2)
- The pore size distributions from low-temperature N2 and CO2 sorption experiments reveal multifractal behavior. After the ScCO2–water–rock reaction, N2 testing of multifractal parameters showed a decrease in heterogeneity for the GQ-13# sample, an increase for the ZJ-8# sample, and no significant change for the GQ-11# sample. Moreover, the aggregation degree of mesopores across the N2 testing range decreased uniformly across all samples. CO2 sorption testing multifractal parameters indicated no significant heterogeneity change for the ZJ-8# sample after the reaction, yet a notable increase for the GQ-11# and GQ-13# samples. Pore heterogeneity was more pronounced in the 0.45 to 0.70 nm range compared to the 0.70 to 1.08 nm range for all samples.
- (3)
- The ScCO2–water–rock reaction exerts complex and varied effects on coal samples. Findings from low-temperature N2/CO2 sorption tests and multifractal analysis have unveiled the dual effects of precipitation and dissolution in ScCO2–water–rock interactions with coal reservoir micropores. These interactions not only lead to the enlargement of existing pores or the formation of new fractures but also result in the precipitation of carbonates that may block the original pores. Furthermore, the analysis of multifractal-related parameters before and after the reaction confirms that the reaction occurs heterogeneously within the pores. Grasping the mechanisms underlying the ScCO2–water–rock interaction is essential for comprehending the intricate changes within the microstructure of coal during CO2 sequestration.
- (4)
- Our work introduces a novel approach to characterizing coal pore structures by integrating low-temperature N2/CO2 adsorption with multifractal theory, revealing significant alterations in pore size distribution and multifractal characteristics. However, the method cannot dynamically describe the changes in coal pores throughout the entire process of ScCO2–water–rock reactions. Future research should prioritize the application of advanced techniques, such as low-field NMR testing, to elucidate the dynamic changes in coal pore structures throughout the reaction process. Additionally, integrating experimental data with numerical simulations will offer a more holistic and comprehensive understanding of the underlying mechanisms.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cusworth, D.H.; Duren, R.M.; Thorpe, A.K.; Eastwood, M.L.; Green, R.O.; Dennison, P.E.; Frankenberg, C.; Heckler, J.W.; Asner, G.P.; Miller, C.E. Quantifying global power plant carbon dioxide emissions with imaging spectroscopy. AGU Adv. 2021, 2, e2020AV000350. [Google Scholar] [CrossRef]
- Qi, S.; Zheng, B.; Wang, Z.; Zhao, H.; Cui, Z.; Huang, T.; Guo, S.; Fu, L.; Dong, P. Geological evaluation for the carbon dioxide geological utilization and storage (CGUS) site: A review. Sci. China Earth Sci. 2023, 66, 1917–1936. [Google Scholar] [CrossRef]
- Yekeen, N.; Khan, J.A.; Ali, M.; Elraies, K.A.; Okunade, O.A.; Ridha, S.; Al-Yaseri, A. Impact of nanoparticles–surfactant solutions on carbon dioxide and methane wettabilities of organic-rich shale and CO2/brine interfacial tension: Implication for carbon geosequestration. ER 2022, 8, 15669–15685. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Rasouli, V.; Rabiei, M. Significant aspects of carbon capture and storage—A review. Petroleum 2019, 7, 335–340. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, F.; Liu, D.; Sun, X.; Wang, H. Quantitative characterization of the evolution of in-situ adsorption/free gas in deep coal seams: Insights from NMR fluid detection and geological time simulations. Int. J. Coal Geol. 2024, 285, 104474. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Z.; Cai, Y.; Sun, F. Characterizing coal gas reservoirs: A multiparametric evaluation based on geological and geophysical methods. Gondwana Res. 2024, 133, 91–107. [Google Scholar] [CrossRef]
- Liu, D.; Yao, Y.; Chang, Y. Measurement of adsorption phase densities with respect to different pressure: Potential application for determination of free and adsorbed methane in coalbed methane reservoir. Chem. Eng. J. 2022, 446, 137103. [Google Scholar] [CrossRef]
- Omotilewa, O.J.; Panja, P.; Vega-Ortiz, C.; McLennan, J. Evaluation of en-hanced coalbed methane recovery and carbon dioxide sequestration potential in high volatile bituminous coal. J. Nat. Gas. Sci. Eng. 2021, 91, 103979. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, R.; Wang, J.; Huang, J.; Li, X.; Wu, J. Desorption hysteresis of CO2 and CH4 in different coals with cyclic desorption experiments. J. CO2 Util. 2020, 40, 101200. [Google Scholar] [CrossRef]
- Vasilenko, T.A.; Kirillov, A.K.; Molchanov, A.N.; Pronskii, E.A. An NMR study of the ratio between free and sorbed methane in the pores of fossil coals. Solid Fuel Chem. 2018, 52, 361–369. [Google Scholar] [CrossRef]
- Akhondzadeh, H.; Keshavarz, A.; Awan, F.; Awan, F.U.R.; Zamani, A.; Iglauer, S.; Lebedev, M. Coal cleat network evolution through liquid nitrogen freeze-thaw cycling. Fuel 2022, 314, 123069. [Google Scholar] [CrossRef]
- Mejia-Ballesteros, J.E.; Savastano, H., Jr.; Fiorelli, J.; Rojas, M.F. Effect of mineral additions on the microstructure and properties of blended cement matrices for fiber-cement applications. Cem. Concr. Compos. 2019, 98, 49–60. [Google Scholar] [CrossRef]
- Youjun, J.; Vafai, K. Analysis of pore scale fluid migration in a porous medium-application to coal rock seam. Int. J. Numer. Methods Heat Fluid Flow 2017, 27, 1706–1719. [Google Scholar] [CrossRef]
- Qi, L.; Zhou, X.; Peng, X.; Chen, X.; Wang, Z.; An, F. Study on the difference of pore structure of anthracite under different particle sizes using low-temperature nitrogen adsorption method. Environ. Sci. Pollut. Res. 2023, 30, 5216–5230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tian, H.; Tang, J.; Zhang, X. Quantitative Characterization of Coal Shale Pores and Fractures Based on Combined High-Pressure Mercury Pressure and Low-Temperature N2/CO2 Adsorption Methods. ACS Omega 2024, 9, 20927–20936. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, Y.; Haghighi, M. Investigation of pore size distributions of coals with different structures by nuclear magnetic resonance (NMR) and mercury intrusion porosimetry (MIP). Measurement 2018, 116, 122–128. [Google Scholar] [CrossRef]
- Vranjes-Wessely, S.; Misch, D.; Issa, I.; Kiener, D.; Fink, R.; Seemann, T.; Liu, B.; Rantitsch, G.; Sachsenhofer, R.F. Nanoscale pore structure of Carboniferous coals from the Ukrainian Donets Basin: A combined HRTEM and gas sorption study. Int. J. Coal Geol. 2020, 224, 103484. [Google Scholar] [CrossRef]
- Shen, M.; Chen, X. Influence rules and mechanisms of mechanical vibration at different frequencies on dynamic process of gas diffusion from coal particles. Energy Explor. Exploit. 2021, 39, 1939–1957. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Zhao, W.; Chen, P. Effect of water intrusion on the characteristics of surface morphology and pore fracture spaces in argillaceous meagre coal. J. Nat. Gas. Sci. Eng. 2020, 81, 103404. [Google Scholar] [CrossRef]
- Li, F.; Jiang, B.; Song, Y.; Cheng, G.; Lu, G. Multifractal behavior of the micro- and mesopore structures of brittle tectonically deformed coals and its influence on methane adsorption capacity. Energy Fuel 2021, 35, 3042–3064. [Google Scholar] [CrossRef]
- Gould, D.; Vadakkan, T.; Poché, R.; Dickinson, M. Multifractal and Lacunarity analysis of microvascular morphology and remodeling. Microcirculation 2011, 18, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bai, Q.; Zhu, C. A methodology for determining the size distribution of broken rock masses in longwall mining goaf. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 113. [Google Scholar] [CrossRef]
- Bu, H.; Ju, Y.; Tan, J.; Wang, G.; Li, X. Fractal characteristics of pores in nonmarine shales from the Huainan coalfield, eastern China. J. Nat. Gas Sci. Eng. 2015, 24, 166–177. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, Y.; Liu, D.; Cai, Y.; Liu, Y. Characterizations of full-scale pore size distribution, porosity and permeability of coals: A novel methodology by nuclear magnetic resonance and fractal analysis theory. Int. J. Coal Geol. 2018, 196, 148–158. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Li, G.; Fu, X.; Duan, C.; Liu, H.; Xu, H. Fluid distribution and pore structure multifractal characteristics analysis of coal measure mudstone. J. Nat. Gas. Sci. Eng. 2021, 88, 103810. [Google Scholar]
- Chen, X.; Yao, G.; Cai, J.; Huang, Y.; Yuan, X. Fractal and multifractal analysis of different hydraulic flow units based on micro-CT images. J. Nat. Gas Sci. Eng. 2017, 48, 145–156. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Jin, Z.; Wu, C. Multifractal analysis of pore structure in middle-and high-rank coal by mercury intrusion porosimetry and low-pressure N2 adsorption. Nat. Resour. Res. 2021, 30, 4565–4584. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, J.; Yang, Y.; Cheng, J.; Zhou, J.; Cen, K. Fractal characteristic of pore structures in 13 coal specimens Relationship among fractal dimension, pore structure parameter, and slurry ability of coal. Fuel Process. Technol. 2016, 149, 256–267. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, Y.; Liu, D.; Cai, Y.; Liu, Y. Nuclear magnetic resonance surface relaxivity of coals. Int. J. Coal Geol. 2019, 205, 1–13. [Google Scholar] [CrossRef]
- Zheng, S.; Sang, S.; Yao, Y.; Liu, D.; Liu, S.; Wang, M.; Feng, G. A multifractal-based method for determination NMR dual T2 cutoffs in coals. J. Petrol. Sci. Eng. 2022, 214, 110488. [Google Scholar] [CrossRef]
- Fang, J.; Li, Q.; Yin, T.; Cai, Y. Investigation on the Nanopore Heterogeneity in Coals and its Influence on Methane Adsorption: A Multifractal Theory Study. ACS Omega 2024, 9, 19504–19516. [Google Scholar] [CrossRef] [PubMed]
- Vasilenko, T.; Kirillov, A.; Islamov, A.; Doroshkevich, A.; Łudzik, K.; Chudoba, D.M.; Mita, C. Permeability of a coal seam with respect to fractal features of pore space of fossil coals. Fuel 2022, 329, 125113. [Google Scholar] [CrossRef]
- GB/T 19222-2003; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, National Standardization Administration. Sampling of Coal Petrology. Standards Press of China: Beijing, China, 2003.
- GB/T 16773-2008; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, National Standardization Administration. Method of Preparing Coal Samples for the Coal Petrographic Analysis. Standards Press of China: Beijing, China, 2009.
- GB/T 21650.3-2011; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, National Standardization Administration. Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption—Part 3: Analysis of Micropores by Gas Adsorption. Standards Press of China: Beijing, China, 2012.
- Li, W.; Liu, H.; Song, X. Multifractal analysis of Hg pore size distributions of tectonically deformed coals. Int. J. Coal Geol. 2015, 144, 138–152. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, G.; Dong, Y.; Danesh, N.; Chen, Z.; Zhang, T. Comparison of low-field NMR and microfocus X-ray computed tomography in fractal characterization of pores in artificial cores. Fuel 2017, 210, 217–226. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, Y.; Liu, D.; Cai, Y.; Liu, Y.; Li, X. Nuclear magnetic resonance T2 cutoffs of coals: A novel method by multifractal analysis theory. Fuel 2019, 241, 715–724. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, C.; Li, G.; Fu, X.; Zhong, Q.; Liu, H.; Dong, Z. Determinations of the multifractal characteristics of the pore structures of low-, middle-, and high-rank coal using high-pressure mercury injection. J. Pet. Sci. Eng. 2021, 203, 108656. [Google Scholar] [CrossRef]
- Li, Q.; Liu, D.; Cai, Y.; Zhou, Y.; Yin, T. Investigation on the methane adsorption capacity in coals: Considerations from nanopores by multifractal analysis. Energy Fuels 2021, 35, 6633–6643. [Google Scholar] [CrossRef]


| Sample No. | Proximate Analysis (%) | Porosity (%) | Ro (%) | Maceral Composition (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vd | FCd | V | I | E | M | |||
| ZJ-8# | 1.24 | 16.59 | 32.79 | 50.63 | 2.36 | 0.748 | 94.2 | 4.1 | 1.7 | 0.0 |
| GQ-11# | 1.05 | 14.47 | 33.8 | 51.72 | 2.55 | 0.675 | 88.9 | 8.5 | 2.5 | 0.1 |
| GQ-13# | 1.28 | 20.99 | 27.52 | 51.49 | 1.14 | 0.621 | 94.2 | 4.2 | 1.6 | 0.0 |
| Sample ID | Burial Depth (m) | Temperature (K) | Pressure (MPa) | Time (d) |
|---|---|---|---|---|
| ZJ-8# | 600 | 305 | 7 | 7 |
| GQ-11# | 700 | 308 | 8 | 7 |
| GQ-13# | 800 | 308 | 8 | 7 |
| Sample No. | BET Specific Surface Area (m2/g) | BJH Pore Volume (mL/g) | Pore Diameter Dv (d) (nm) | |
|---|---|---|---|---|
| Before reaction | ZJ-8# | 0.196 | 0.002 | 3.812 |
| GQ-11# | 0.37 | 0.002 | 3.819 | |
| GQ-13# | 0.713 | 0.006 | 3.823 | |
| After reaction | ZJ-8# | 0.385 | 0.002 | 3.808 |
| GQ-11# | 0.201 | 0.002 | 3.402 | |
| GQ-13# | 1.107 | 0.003 | 3.822 |
| Sample No. | Surface Area (m2/g) | Pore Volume (mL/g) | Average Pore Width (nm) | |
|---|---|---|---|---|
| Before reaction | ZJ-8# | 85.125 | 0.03 | 0.573 |
| GQ-11# | 73.793 | 0.027 | 0.501 | |
| GQ-13# | 57.728 | 0.022 | 0.501 | |
| After reaction | ZJ-8# | 83.01 | 0.03 | 0.548 |
| GQ-11# | 83.755 | 0.031 | 0.573 | |
| GQ-13# | 58.506 | 0.022 | 0.501 |
| Sample No. | Dmin | D-2 | D-1 | D0 | D1 | D2 | Dmax | D-10– D10 | D-10 –D0 | D0– D10 | Δα | α0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZJ-8# | 1.182 | 1.063 | 1.032 | 1.000 | 0.955 | 0.938 | 0.822 | 0.36 | 0.182 | 0.178 | 0.492 | 1.0368 |
| GQ-11# | 1.163 | 1.057 | 1.030 | 1.000 | 0.957 | 0.937 | 0.799 | 0.364 | 0.163 | 0.201 | 0.498 | 1.0352 |
| GQ-13# | 1.576 | 1.319 | 1.194 | 1.000 | 0.670 | 0.595 | 0.381 | 1.195 | 0.576 | 0.619 | 1.348 | 1.2528 |
| Sample No. | Dmin | D-2 | D-1 | D0 | D1 | D2 | Dmax | D-10– D10 | D-10 –D0 | D0– D10 | Δα | α0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZJ-8# | 1.263 | 1.092 | 1.048 | 1.000 | 0.933 | 0.907 | 0.747 | 0.516 | 0.263 | 0.253 | 0.691 | 0.9756 |
| GQ-11# | 1.163 | 1.057 | 1.031 | 1.000 | 0.953 | 0.932 | 0.800 | 0.363 | 0.163 | 0.200 | 0.496 | 0.9834 |
| GQ-13# | 1.495 | 1.214 | 1.121 | 1.000 | 0.779 | 0.713 | 0.476 | 1.019 | 0.495 | 0.524 | 1.119 | 0.9272 |
| Sample No. | Dmin | D-2 | D-1 | D0 | D1 | D2 | Dmax | D-10– D10 | D-10 –D0 | D0– D10 | Δα | α0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZJ-8# | 1.090 | 0.945 | 0.911 | 0.876 | 0.826 | 0.807 | 0.683 | 0.407 | 0.214 | 0.193 | 0.555 | 0.857 |
| GQ-11# | 1.216 | 0.978 | 0.918 | 0.876 | 0.839 | 0.828 | 0.752 | 0.464 | 0.34 | 0.124 | 0.608 | 0.860 |
| GQ-13# | 1.689 | 1.279 | 1.046 | 0.876 | 0.836 | 0.825 | 0.764 | 0.925 | 0.813 | 0.112 | 1.117 | 0.852 |
| Sample No. | Dmin | D-2 | D-1 | D0 | D1 | D2 | Dmax | D-10– D10 | D-10 –D0 | D0– D10 | Δα | α0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZJ-8# | 1.099 | 0.943 | 0.909 | 0.876 | 0.833 | 0.819 | 0.718 | 0.381 | 0.223 | 0.158 | 0.528 | 0.859 |
| GQ-11# | 1.875 | 1.389 | 1.107 | 0.876 | 0.826 | 0.815 | 0.760 | 1.115 | 0.999 | 0.116 | 1.329 | 0.847 |
| GQ-13# | 2.433 | 1.813 | 1.399 | 0.876 | 0.814 | 0.797 | 0.696 | 1.737 | 1.557 | 0.180 | 2.018 | 0.839 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Liu, Y.; Huang, F.; Liu, S.; Sang, S.; Dai, X.; Wang, M. Pore Structure Evolution of Coal After Supercritical CO2–Water–Rock Treatment: A Multifractal Analysis. Fractal Fract. 2025, 9, 144. https://doi.org/10.3390/fractalfract9030144
Zheng S, Liu Y, Huang F, Liu S, Sang S, Dai X, Wang M. Pore Structure Evolution of Coal After Supercritical CO2–Water–Rock Treatment: A Multifractal Analysis. Fractal and Fractional. 2025; 9(3):144. https://doi.org/10.3390/fractalfract9030144
Chicago/Turabian StyleZheng, Sijian, Yanzhi Liu, Fansheng Huang, Shiqi Liu, Shuxun Sang, Xuguang Dai, and Meng Wang. 2025. "Pore Structure Evolution of Coal After Supercritical CO2–Water–Rock Treatment: A Multifractal Analysis" Fractal and Fractional 9, no. 3: 144. https://doi.org/10.3390/fractalfract9030144
APA StyleZheng, S., Liu, Y., Huang, F., Liu, S., Sang, S., Dai, X., & Wang, M. (2025). Pore Structure Evolution of Coal After Supercritical CO2–Water–Rock Treatment: A Multifractal Analysis. Fractal and Fractional, 9(3), 144. https://doi.org/10.3390/fractalfract9030144








