Relating the Morphology of Bipolar Neurons to Fractal Dimension
Abstract
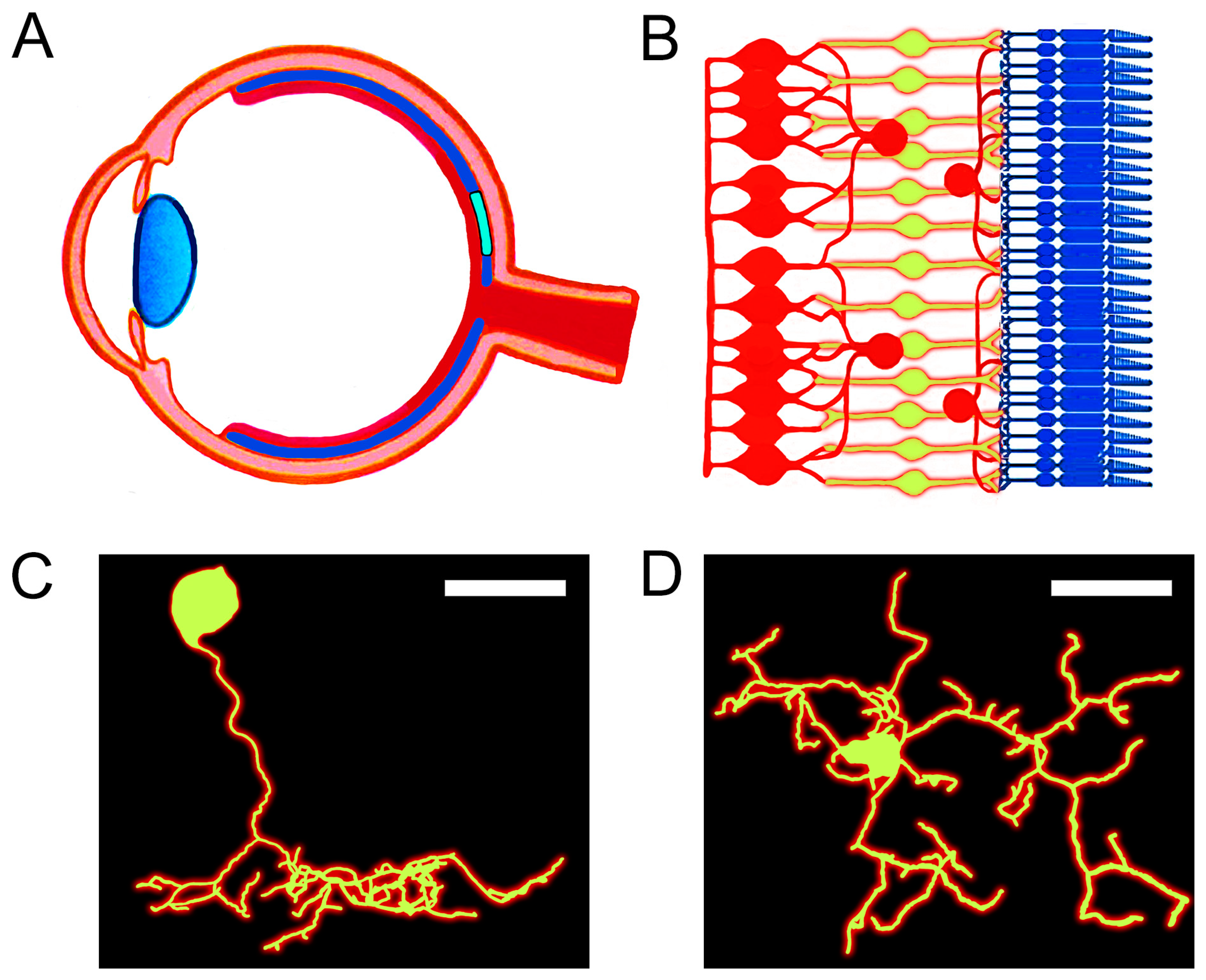
1. Introduction
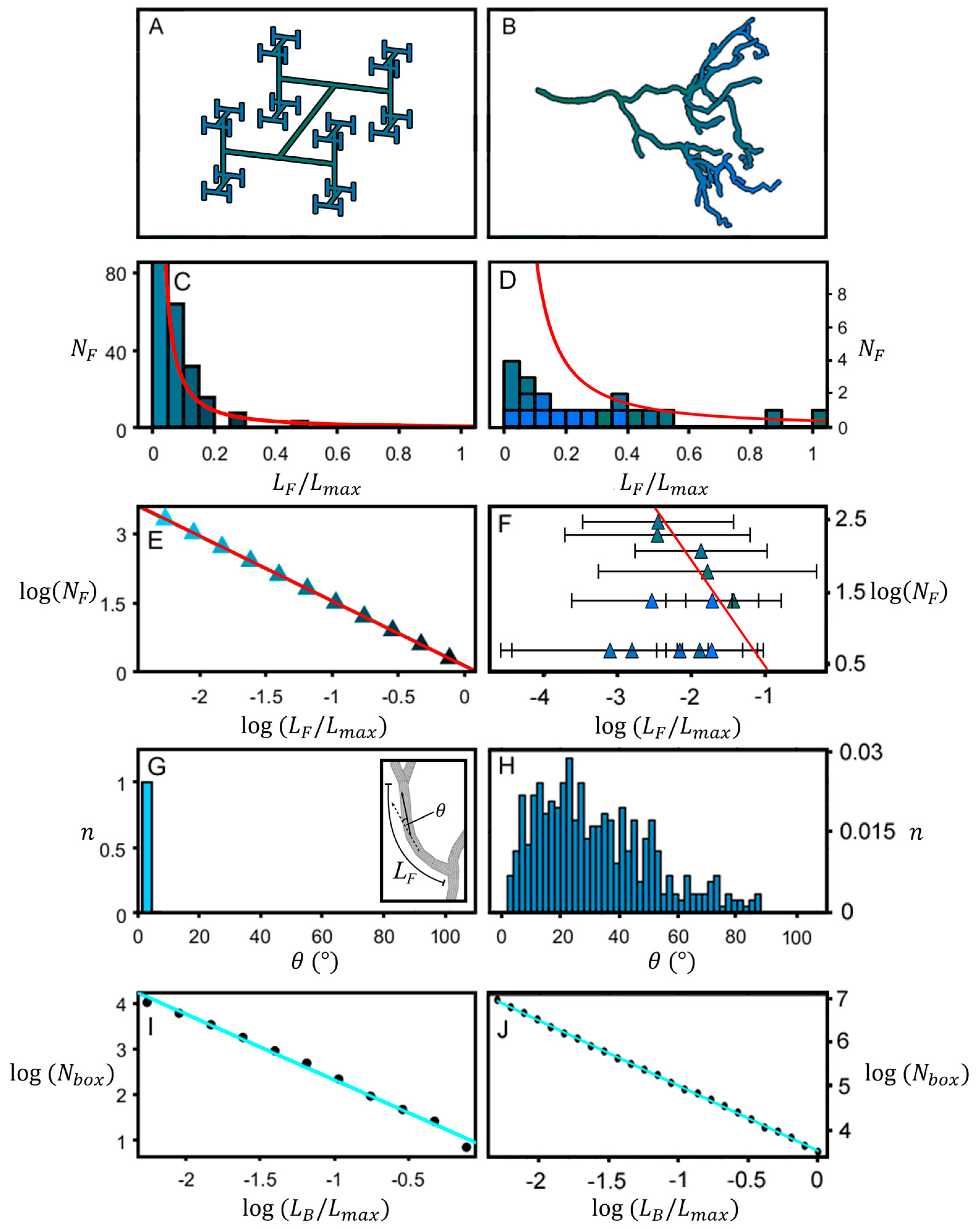
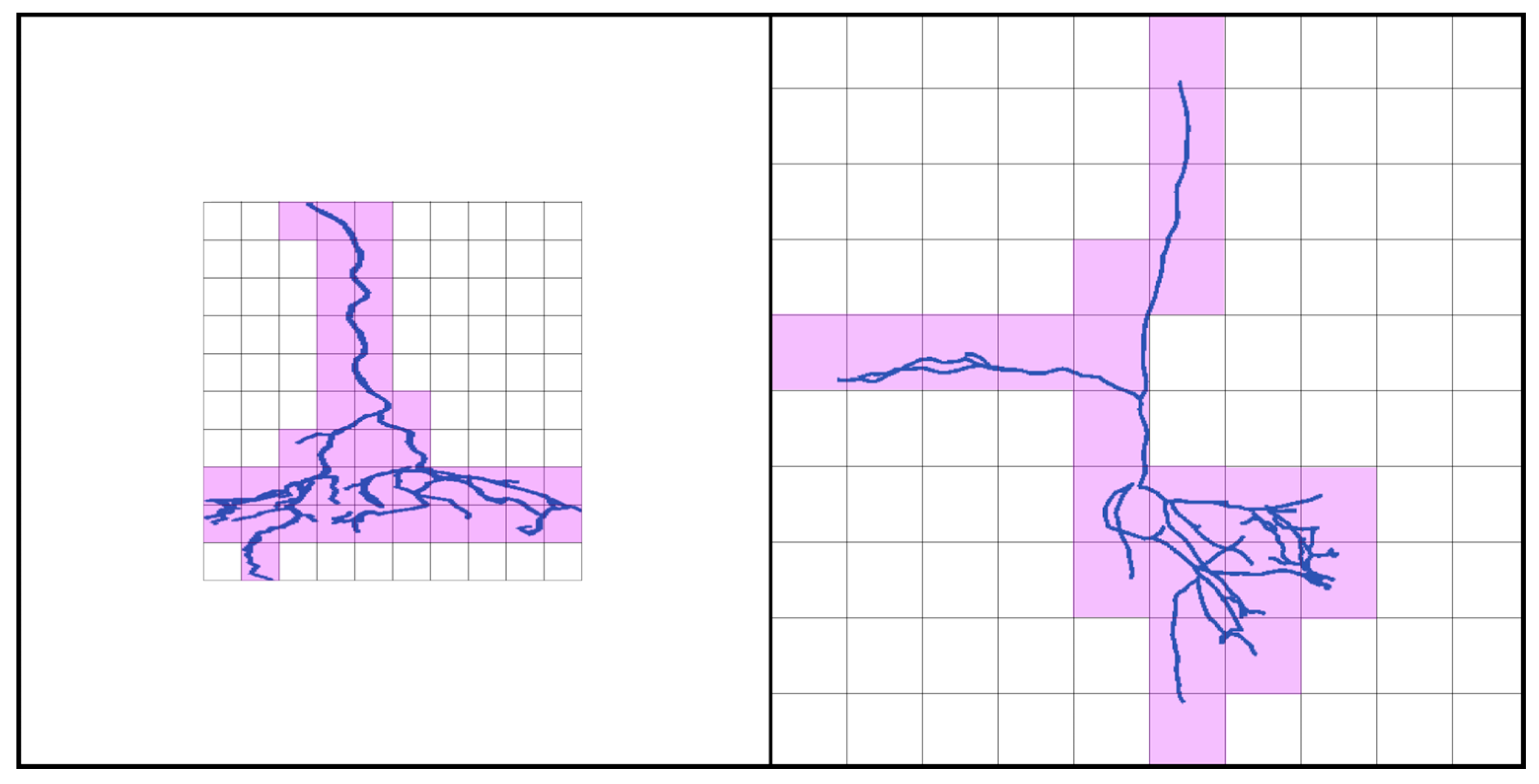
2. Materials and Methods
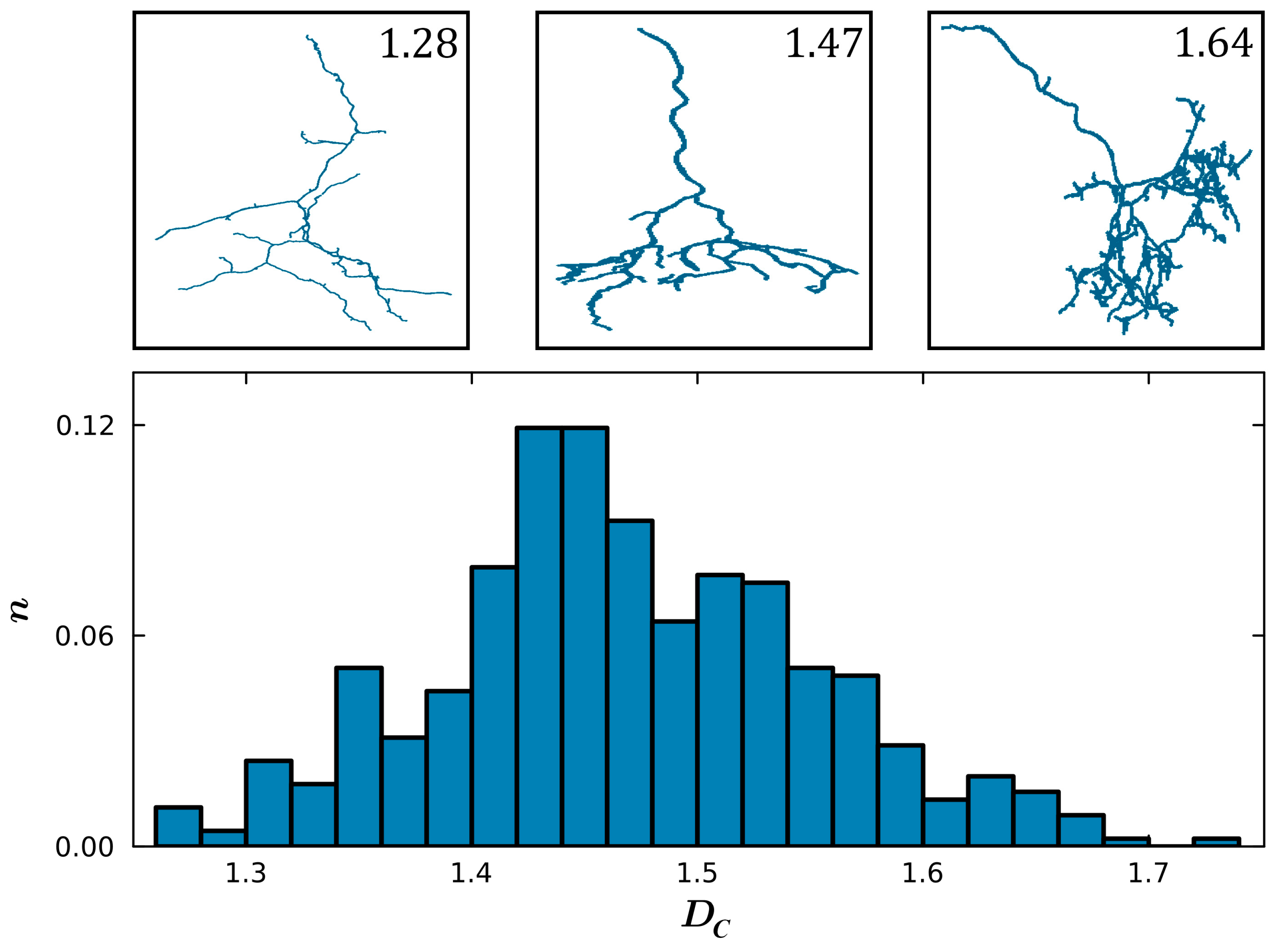
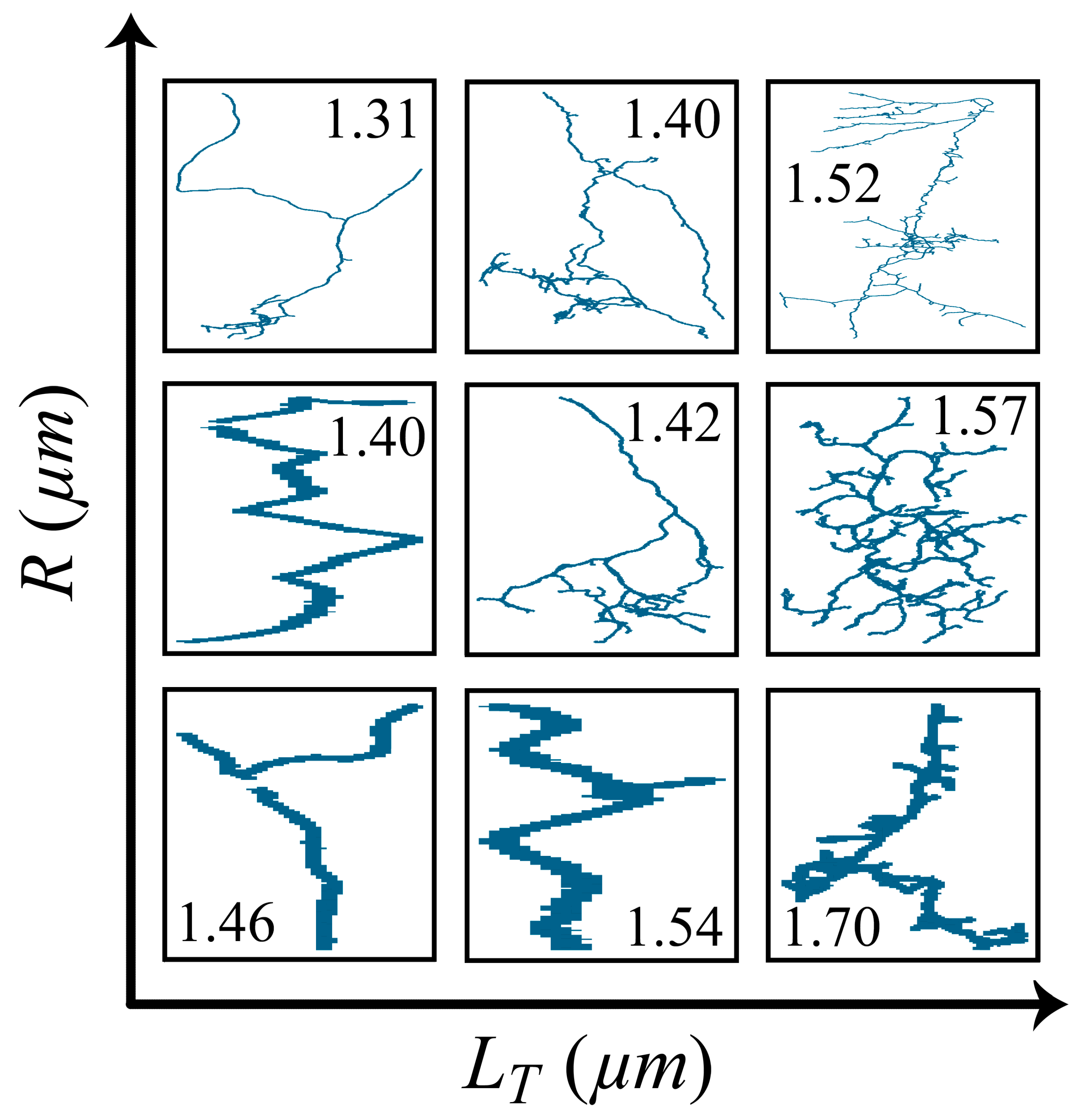
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chklovskii, D.B. Synaptic Connectivity and Neuronal Morphology: Two Sides of the Same Coin. Neuron 2004, 43, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.L.; Wang, Y.; Riachi, I.; Schürmann, F.; Markram, H. Statistical Connectivity Provides a Sufficient Foundation for Specific Functional Connectivity in Neocortical Neural Microcircuits. Proc. Natl. Acad. Sci. USA 2012, 109, E2885–E2894. [Google Scholar] [CrossRef]
- van Ooyen, A.; Carnell, A.; de Ridder, S.; Tarigan, B.; Mansvelder, H.D.; Bijma, F.; de Gunst, M.; van Pelt, J. Independently Outgrowing Neurons and Geometry-Based Synapse Formation Produce Networks with Realistic Synaptic Connectivity. PLoS ONE 2014, 9, e85858. [Google Scholar] [CrossRef]
- Laughlin, S.B.; de Ruyter van Steveninck, R.R.; Anderson, J.C. The Metabolic Cost of Neural Information. Nat. Neurosci. 1998, 1, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Dukkipati, S.S.; Garrett, T.L.; Elbasiouny, S.M. The Vulnerability of Spinal Motoneurons and Soma Size Plasticity in a Mouse Model of Amyotrophic Lateral Sclerosis. J. Physiol. 2018, 596, 1723–1745. [Google Scholar] [CrossRef] [PubMed]
- Kemper, T.L.; Bauman, M. Neuropathology of Infantile Autism. J. Neuropathol. Exp. Neurol. 1998, 57, 645–652. [Google Scholar] [CrossRef]
- Goikolea-Vives, A.; Stolp, H.B. Connecting the Neurobiology of Developmental Brain Injury: Neuronal Arborisation as a Regulator of Dysfunction and Potential Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 8220. [Google Scholar] [CrossRef] [PubMed]
- Arikkath, J. Molecular Mechanisms of Dendrite Morphogenesis. Front. Cell. Neurosci. 2012, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Kalisman, N.; Silberberg, G.; Markram, H. Deriving Physical Connectivity from Neuronal Morphology. Biol. Cybern. 2003, 88, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Stepanyants, A.; Chklovskii, D.B. Neurogeometry and Potential Synaptic Connectivity. Trends Neurosci. 2005, 28, 387–394. [Google Scholar] [CrossRef]
- McAssey, M.P.; Bijma, F.; Tarigan, B.; van Pelt, J.; van Ooyen, A.; de Gunst, M. A Morpho-Density Approach to Estimating Neural Connectivity. PLoS ONE 2014, 9, e86526. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Stepanyants, A.; Elston, G.N.; Grosberg, A.Y.; Chklovskii, D.B. Maximization of the Connectivity Repertoire as a Statistical Principle Governing the Shapes of Dendritic Arbors. Proc. Natl. Acad. Sci. USA 2009, 106, 12536–12541. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, S.; Rowland, C.; Smith, J.H.; Watterson, W.J.; Miller, D.; Niell, C.M.; Alemán, B.J.; Perez, M.-T.; Taylor, R.P. Controlled Assembly of Retinal Cells on Fractal and Euclidean Electrodes. PLoS ONE 2022, 17, e0265685. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, S.; Rowland, C.; Smith, J.H.; Griffiths, W.; Watterson, W.J.; Niell, C.M.; Alemán, B.J.; Perez, M.-T.; Taylor, R.P. Comparison of Fractal and Grid Electrodes for Studying the Effects of Spatial Confinement on Dissociated Retinal Neuronal and Glial Behavior. Sci. Rep. 2022, 12, 17513. [Google Scholar] [CrossRef]
- Schröter, M.; Paulsen, O.; Bullmore, E.T. Micro-Connectomics: Probing the Organization of Neuronal Networks at the Cellular Scale. Nat. Rev. Neurosci. 2017, 18, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Erickson, P.A.; Fisher, S.K.; Anderson, D.H.; Stern, W.H.; Borgula, G.A. Retinal Detachment in the Cat: The Outer Nuclear and Outer Plexiform Layers. Investig. Ophthalmol. Vis. Sci. 1983, 24, 927–942. [Google Scholar]
- Wu, S.M. Feedback Connections and Operation of the Outer Plexiform Layer of the Retina. Curr. Opin. Neurobiol. 1992, 2, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Karamali, F.; Behtaj, S.; Babaei-Abraki, S.; Hadady, H.; Atefi, A.; Savoj, S.; Soroushzadeh, S.; Najafian, S.; Nasr Esfahani, M.H.; Klassen, H. Potential Therapeutic Strategies for Photoreceptor Degeneration: The Path to Restore Vision. J. Transl. Med. 2022, 20, 572. [Google Scholar] [CrossRef] [PubMed]
- Phelan, J.K.; Bok, D. A Brief Review of Retinitis Pigmentosa and the Identified Retinitis Pigmentosa Genes. Mol. Vis. 2000, 6, 116–124. [Google Scholar]
- Kralik, J.; Kleinlogel, S. Functional Availability of ON-Bipolar Cells in the Degenerated Retina: Timing and Longevity of an Optogenetic Gene Therapy. Int. J. Mol. Sci. 2021, 22, 11515. [Google Scholar] [CrossRef]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal Remodeling in Human Retinitis Pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef]
- Chichagova, V.; Hallam, D.; Collin, J.; Zerti, D.; Dorgau, B.; Felemban, M.; Lako, M.; Steel, D.H. Cellular Regeneration Strategies for Macular Degeneration: Past, Present and Future. Eye 2018, 32, 946–971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-J.; Ma, Y.; Jin, Z.-B. The Road to Restore Vision with Photoreceptor Regeneration. Exp. Eye Res. 2021, 202, 108283. [Google Scholar] [CrossRef] [PubMed]
- Palanker, D.; Goetz, G. Restoring Sight with Retinal Prostheses. Phys. Today 2018, 71, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, H.; Song, Y.M.; Park, J.-U. Implantation of Electronic Visual Prosthesis for Blindness Restoration. Opt. Mater. Express OME 2019, 9, 3878–3894. [Google Scholar] [CrossRef]
- Chenais, N.A.L.; Airaghi Leccardi, M.J.I.; Ghezzi, D. Photovoltaic Retinal Prosthesis Restores High-Resolution Responses to Single-Pixel Stimulation in Blind Retinas. Commun. Mater. 2021, 2, 28. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Chen, Z.C.; Bhuckory, M.; Huang, T.; Shin, A.; Zuckerman, V.; Ho, E.; Rosenfeld, E.; Galambos, L.; Kamins, T.; et al. Electronic Photoreceptors Enable Prosthetic Visual Acuity Matching the Natural Resolution in Rats. Nat. Commun. 2022, 13, 6627. [Google Scholar] [CrossRef]
- Keremane, S.; Rowland, C.; Brouse, B.; Uehara, H.; Ambati, B.K.; Taylor, R. Quantification of Neuronal Dendrite Structure in Mouse Retinal Bipolar Cells Using Fractal Dimension, D. Investig. Ophthalmol. Vis. Sci. 2024, 65, 6685. [Google Scholar]
- Golestanirad, L.; Elahi, B.; Molina Arribere, A.; Mosig, J.R.; Pollo, C.; Graham, S.J. Analysis of Fractal Electrodes for Efficient Neural Stimulation. Front. Neuroeng. 2013, 6, 3. [Google Scholar] [CrossRef]
- Watterson, W.J.; Montgomery, R.D.; Taylor, R.P. Modeling the Improved Visual Acuity Using Photodiode Based Retinal Implants Featuring Fractal Electrodes. Front. Neurosci. 2018, 12, 277. [Google Scholar] [CrossRef]
- De Berg, M.; Cheong, O.; Van Kreveld, M.; Overmars, M. Computational Geometry: Algorithms and Applications; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-77973-5. [Google Scholar]
- Morigiwa, K.; Tauchi, M.; Fukuda, Y. Fractal Analysis of Ganglion Cell Dendritic Branching Patterns of the Rat and Cat Retinae. Neurosci. Res. Suppl. 1989, 10, S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Ishikawa, A.; Ohtomo, K.; Kobayashi, Y.; Matsuoka, T. Fractal Dimension of Dendritic Tree of Cerebellar Purkinje Cell during Onto- and Phylogenetic Development. Neurosci. Res. 1992, 13, 19–31. [Google Scholar] [CrossRef]
- Bassingthwaighte, J.B.; Liebovitch, L.S.; West, B.J. Fractal Physiology; American Physiological Society: Rockville, MD, USA, 1994; ISBN 978-0-19-508013-1. [Google Scholar]
- Caserta, F.; Eldred, W.D.; Fernandez, E.; Hausman, R.E.; Stanford, L.R.; Bulderev, S.V.; Schwarzer, S.; Stanley, H.E. Determination of Fractal Dimension of Physiologically Characterized Neurons in Two and Three Dimensions. J. Neurosci. Methods 1995, 56, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, P.M.; Khokha, M. Fractal Geometry in Biological Systems: An Analytical Approach; CRC Press: Boca Raton, FL, USA, 1996; ISBN 978-0-8493-7636-8. [Google Scholar]
- Smith, T.G.; Lange, G.D.; Marks, W.B. Fractal Methods and Results in Cellular Morphology--Dimensions, Lacunarity and Multifractals. J. Neurosci. Methods 1996, 69, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, V.V.; Pushchina, E.V.; Karetin, Y.A. The Quasi-Fractal Structure of Fish Brain Neurons. Russ. J. Mar. Biol. 2004, 30, 127–134. [Google Scholar] [CrossRef]
- Wearne, S.L.; Rodriguez, A.; Ehlenberger, D.B.; Rocher, A.B.; Henderson, S.C.; Hof, P.R. New Techniques for Imaging, Digitization and Analysis of Three-Dimensional Neural Morphology on Multiple Scales. Neuroscience 2005, 136, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Milosević, N.T.; Ristanović, D. Fractality of Dendritic Arborization of Spinal Cord Neurons. Neurosci. Lett. 2006, 396, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Werner, G. Fractals in the Nervous System: Conceptual Implications for Theoretical Neuroscience. Front. Physiol. 2010, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwon, N.; Chang, S.; Kim, K.-T.; Lee, D.; Kim, S.; Yun, S.J.; Hwang, D.; Kim, J.W.; Hwu, Y.; et al. Altered Branching Patterns of Purkinje Cells in Mouse Model for Cortical Development Disorder. Sci. Rep. 2011, 1, 122. [Google Scholar] [CrossRef] [PubMed]
- Di Ieva, A.; Grizzi, F.; Jelinek, H.; Pellionisz, A.J.; Losa, G.A. Fractals in the Neurosciences, Part I: General Principles and Basic Neurosciences. Neuroscientist 2014, 20, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, H.F.; Cornforth, D.J.; Roberts, A.J.; Landini, G.; Bourke, P.; Iorio, A. Image Processing of Finite Size Rat Retinal Ganglion Cells Using Multifractal and Local Connected Fractal Analysis. In AI 2004: Advances in Artificial Intelligence; Webb, G.I., Yu, X., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 3339, pp. 961–966. ISBN 978-3-540-24059-4. [Google Scholar]
- Murray, J.D. Use and Abuse of Fractal Theory in Neuroscience. J. Comp. Neurol. 1995, 361, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Cuntz, H.; Mathy, A.; Häusser, M. A Scaling Law Derived from Optimal Dendritic Wiring. Proc. Natl. Acad. Sci. USA 2012, 109, 11014–11018. [Google Scholar] [CrossRef]
- Rowland, C.; Moslehi, S.; Smith, J.H.; Harland, B.; Dalrymple-Alford, J.; Taylor, R.P. Fractal Resonance: Can Fractal Geometry Be Used to Optimize the Connectivity of Neurons to Artificial Implants? Adv. Neurobiol. 2024, 36, 877–906. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.H.; Rowland, C.; Harland, B.; Moslehi, S.; Montgomery, R.D.; Schobert, K.; Watterson, W.J.; Dalrymple-Alford, J.; Taylor, R.P. How Neurons Exploit Fractal Geometry to Optimize Their Network Connectivity. Sci. Rep. 2021, 11, 2332. [Google Scholar] [CrossRef] [PubMed]
- Ascoli, G.A.; Donohue, D.E.; Halavi, M. NeuroMorpho.Org: A Central Resource for Neuronal Morphologies. J. Neurosci. 2007, 27, 9247–9251. [Google Scholar] [CrossRef] [PubMed]
- Helmstaedter, M.; Briggman, K.L.; Turaga, S.C.; Jain, V.; Seung, H.S.; Denk, W. Connectomic Reconstruction of the Inner Plexiform Layer in the Mouse Retina. Nature 2013, 500, 168–174. [Google Scholar] [CrossRef]
- Denk, W.; Horstmann, H. Serial Block-Face Scanning Electron Microscopy to Reconstruct Three-Dimensional Tissue Nanostructure. PLoS Biol. 2004, 2, e329. [Google Scholar] [CrossRef]
- Berning, M.; Boergens, K.M.; Helmstaedter, M. SegEM: Efficient Image Analysis for High-Resolution Connectomics. Neuron 2015, 87, 1193–1206. [Google Scholar] [CrossRef]
- Inglis, A.; Cruz, L.; Roe, D.L.; Stanley, H.E.; Rosene, D.L.; Urbanc, B. Automated Identification of Neurons and Their Locations. J. Microsc. 2008, 230, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, I.; Taylor, R. Fractal Analysis of Time-Series Data Sets: Methods and Challenges. In Fractal Analysis; IntechOpen: London, UK, 2018; ISBN 978-1-78985-433-6. [Google Scholar]
- Rowland, C.; Harland, B.; Smith, J.H.; Moslehi, S.; Dalrymple-Alford, J.; Taylor, R.P. Investigating Fractal Analysis as a Diagnostic Tool That Probes the Connectivity of Hippocampal Neurons. Front. Physiol. 2022, 13, 932598. [Google Scholar] [CrossRef]
- Rowland, C.; Smith, J.H.; Moslehi, S.; Harland, B.; Dalrymple-Alford, J.; Taylor, R.P. Neuron Arbor Geometry Is Sensitive to the Limited-Range Fractal Properties of Their Dendrites. Front. Netw. Physiol. 2023, 3, 1072815. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.P.; Moslehi, S.; Brouse, B.; Keremane, S.; Philliber, S.; Griffiths, W.; Rowland, C.; Smith, J.H.; Taylor, R.P. Evolution of Retinal Neuron Fractality When Interfacing with Carbon Nanotube Electrodes. Bioengineering 2024, 11, 823. [Google Scholar] [CrossRef]
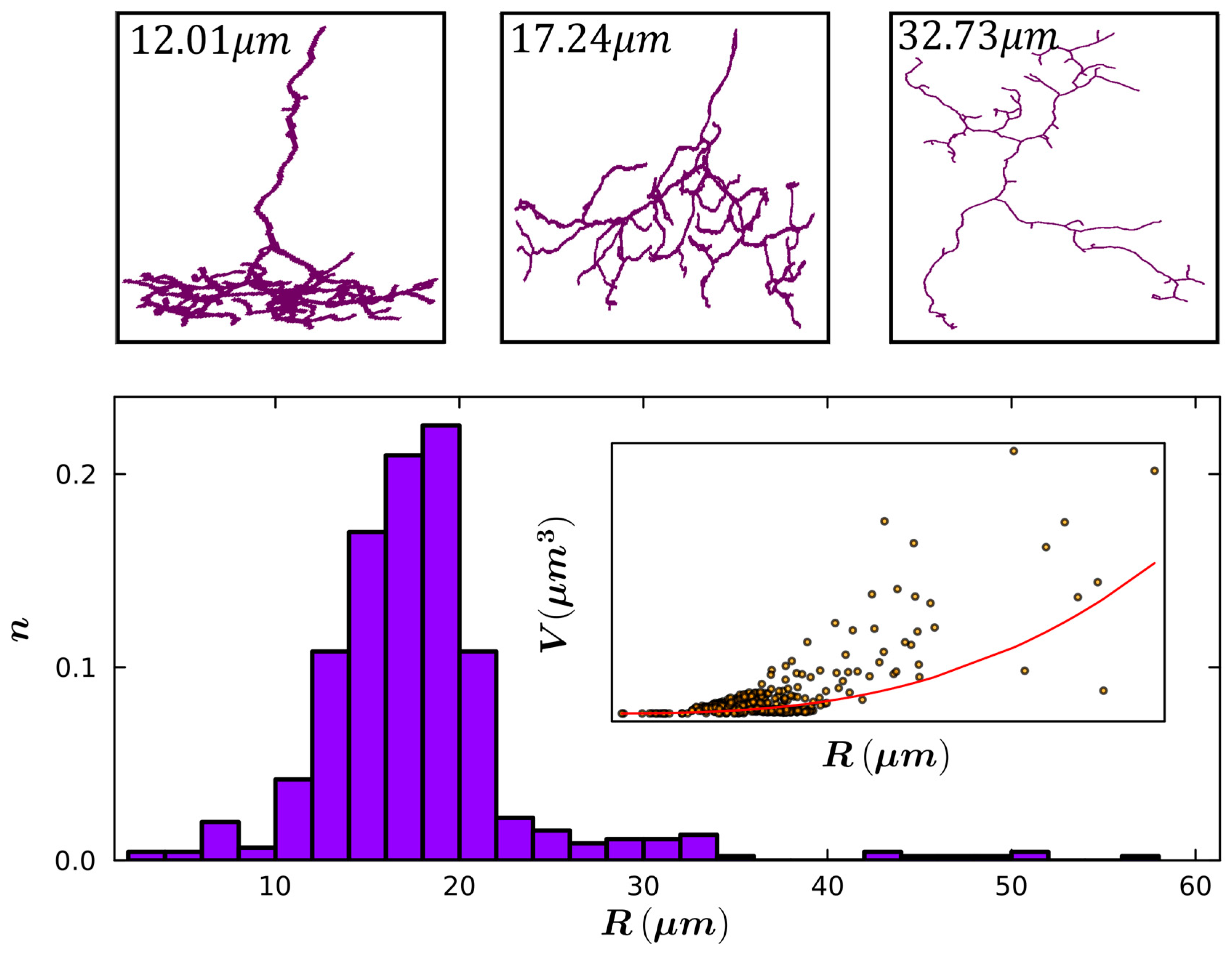
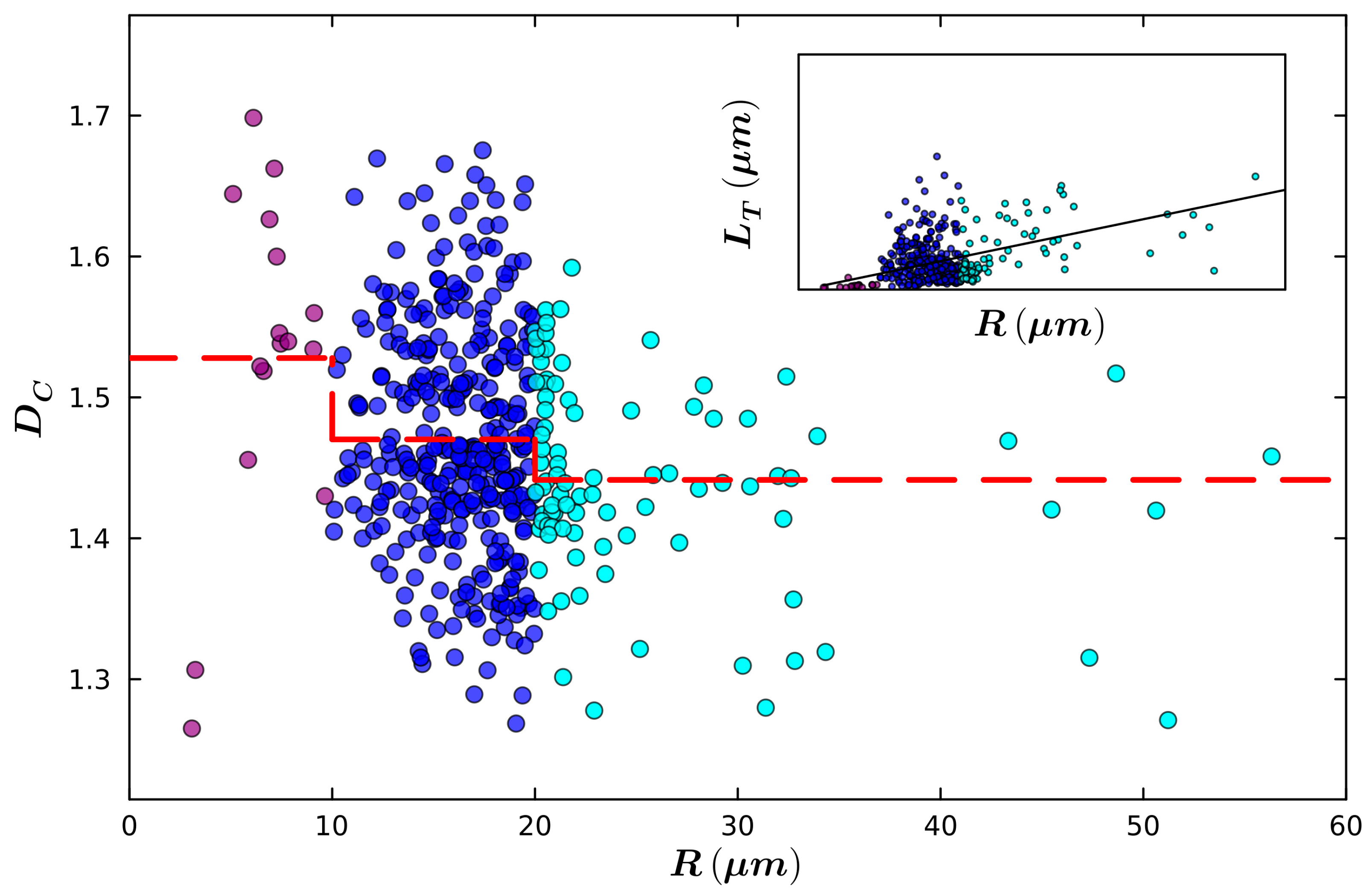
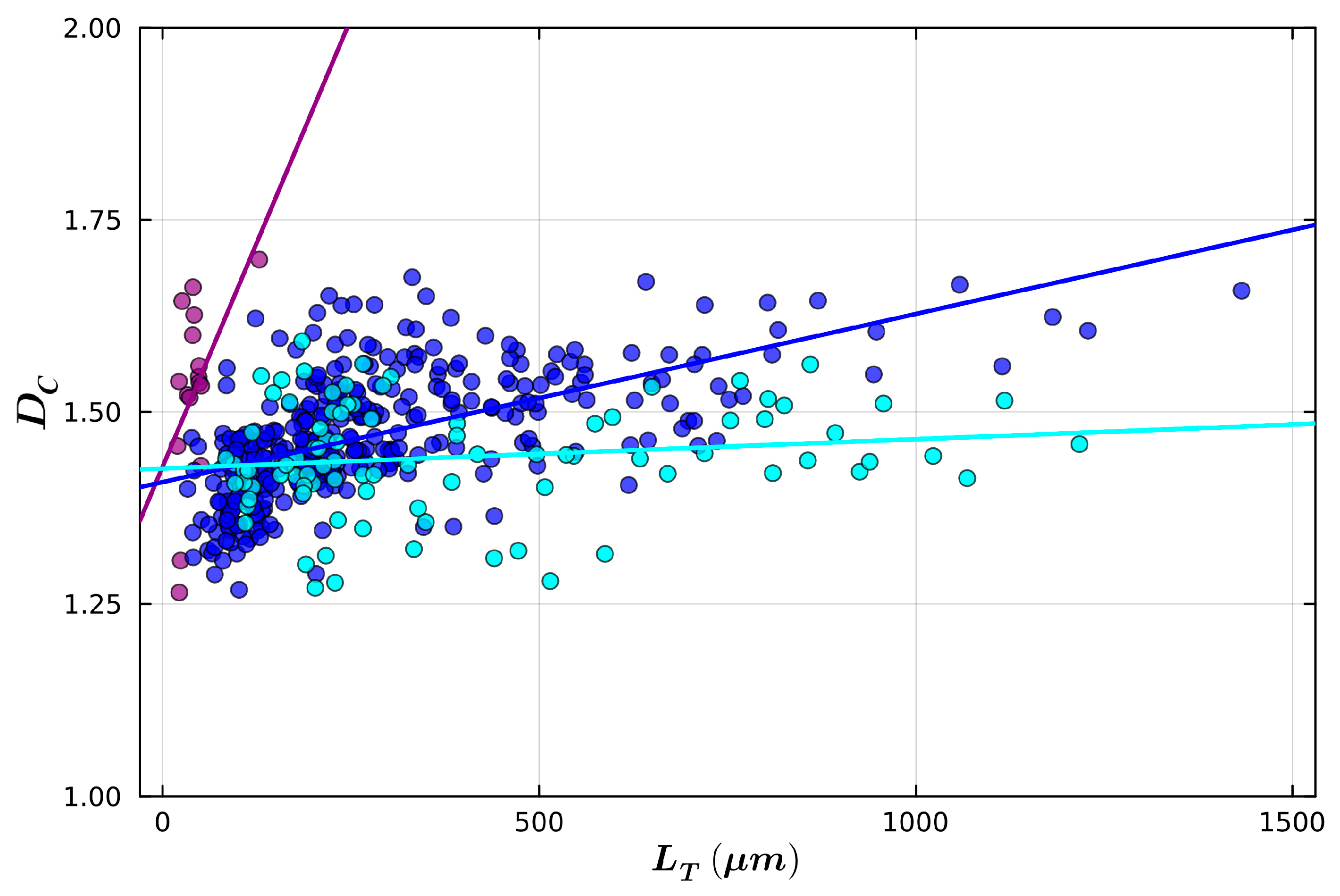
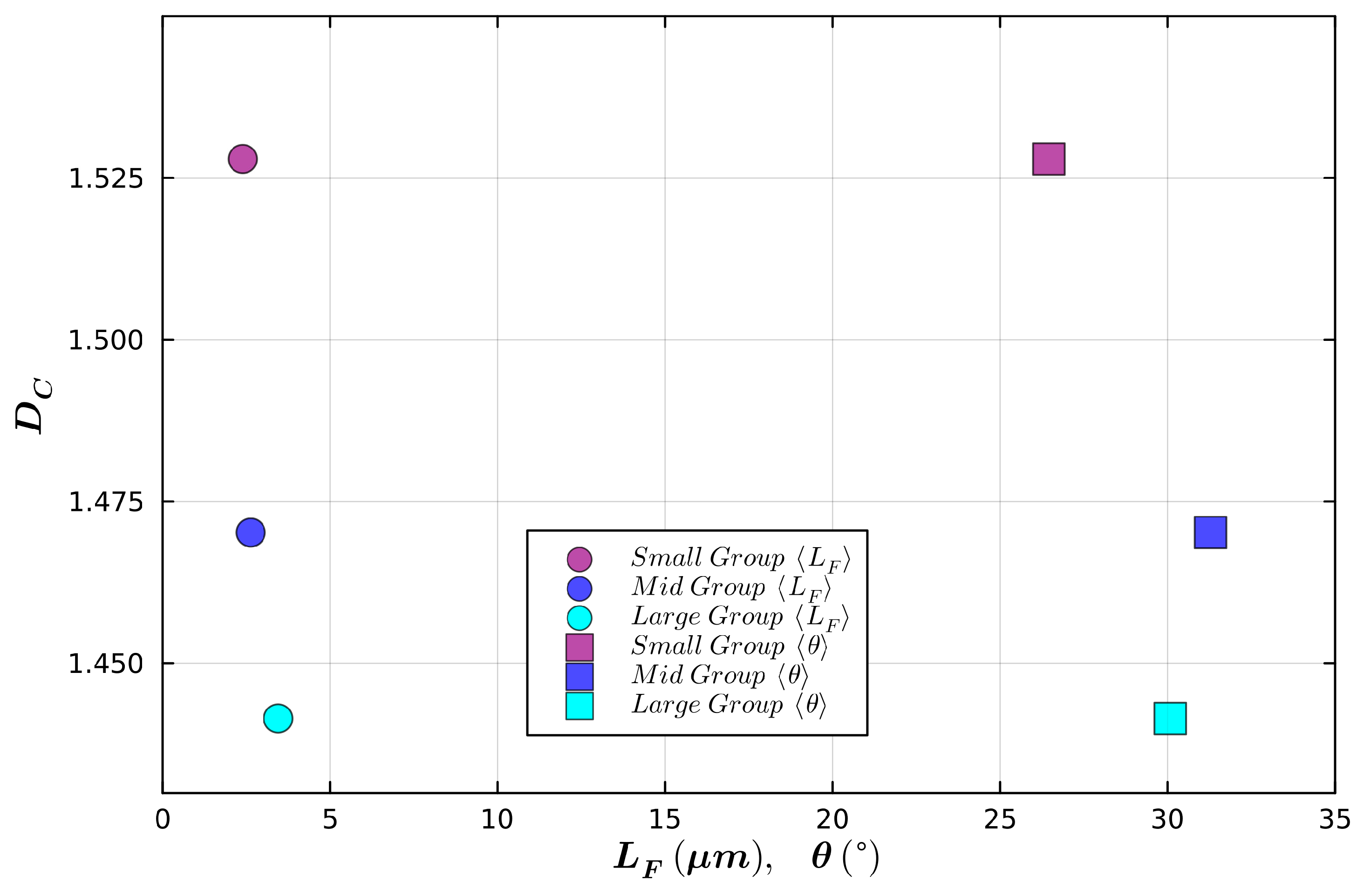
| Small Radius | Medium Radius | Large Radius | |
|---|---|---|---|
| Mean Radius (µm) | 6.8 | 16.2 | 25.6 |
| Mean Total Length (µm) | 42.6 | 281.0 | 394.0 |
| Mean Fork Length (µm) | 2.2 | 2.6 | 3.4 |
| Mean Weave Angle (°) | 26.5 | 31.3 | 30.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brouse, B., Jr.; Rowland, C.; Taylor, R.P. Relating the Morphology of Bipolar Neurons to Fractal Dimension. Fractal Fract. 2025, 9, 9. https://doi.org/10.3390/fractalfract9010009
Brouse B Jr., Rowland C, Taylor RP. Relating the Morphology of Bipolar Neurons to Fractal Dimension. Fractal and Fractional. 2025; 9(1):9. https://doi.org/10.3390/fractalfract9010009
Chicago/Turabian StyleBrouse, Bret, Jr., Conor Rowland, and Richard P. Taylor. 2025. "Relating the Morphology of Bipolar Neurons to Fractal Dimension" Fractal and Fractional 9, no. 1: 9. https://doi.org/10.3390/fractalfract9010009
APA StyleBrouse, B., Jr., Rowland, C., & Taylor, R. P. (2025). Relating the Morphology of Bipolar Neurons to Fractal Dimension. Fractal and Fractional, 9(1), 9. https://doi.org/10.3390/fractalfract9010009






