The Influence of Environmental Temperature on the Passive Oxidation Process in the C/SiC Composite
Abstract
1. Introduction
2. Oxidation Behavior of the C/SiC Composite
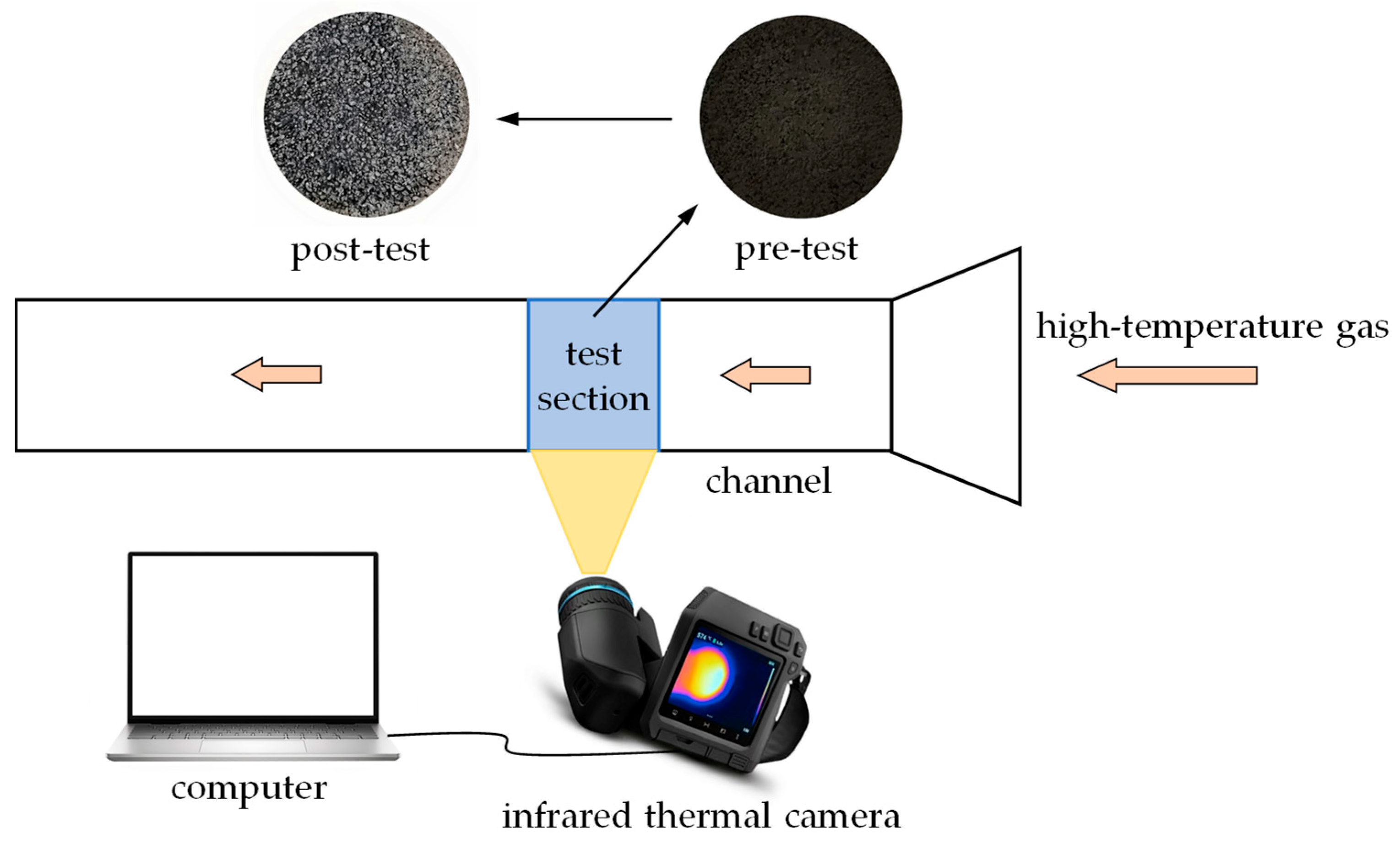
2.1. Heating Oxidation Experiment
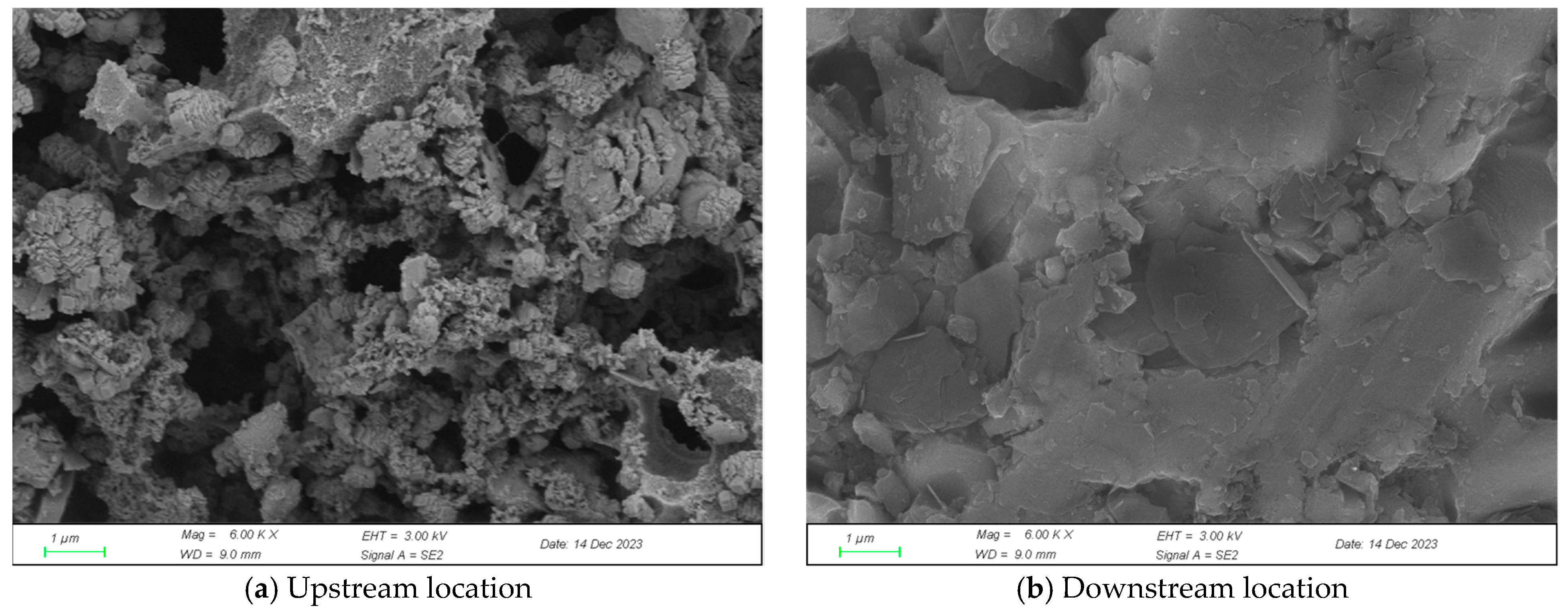
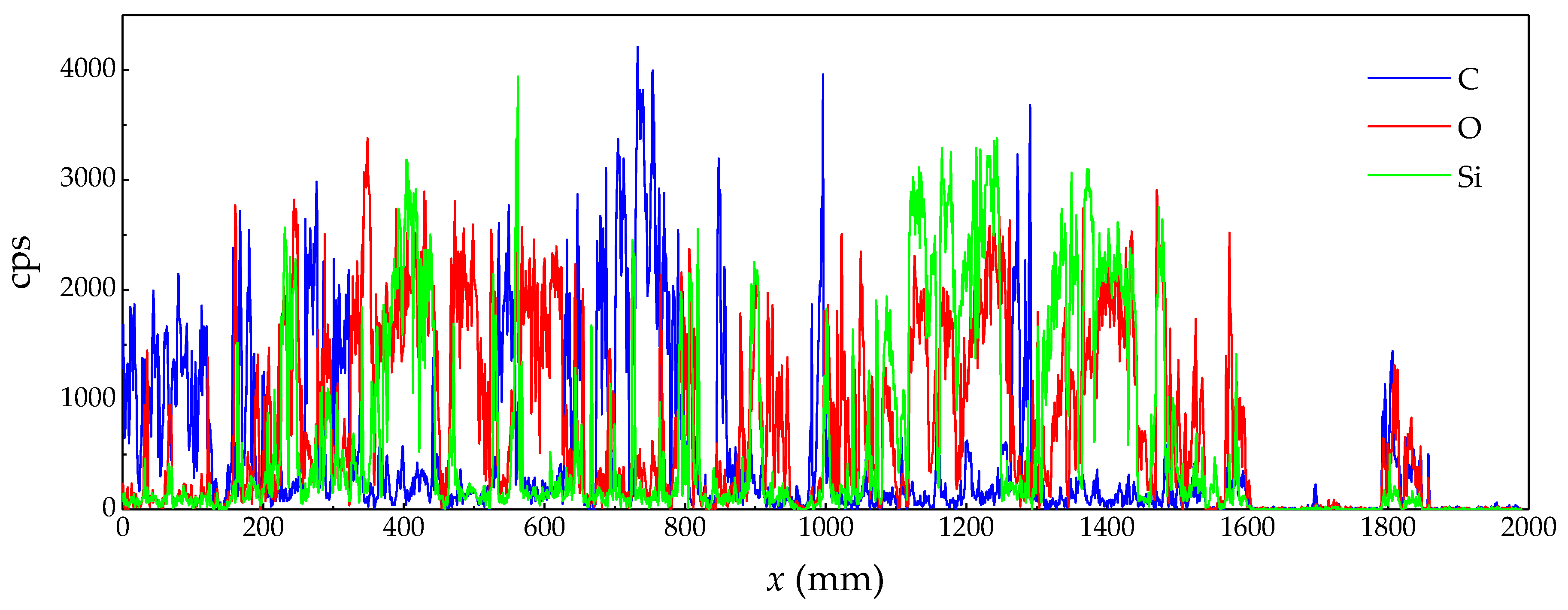
2.2. Analysis of Experimental Product
3. Physical Model
3.1. Fractal Theory
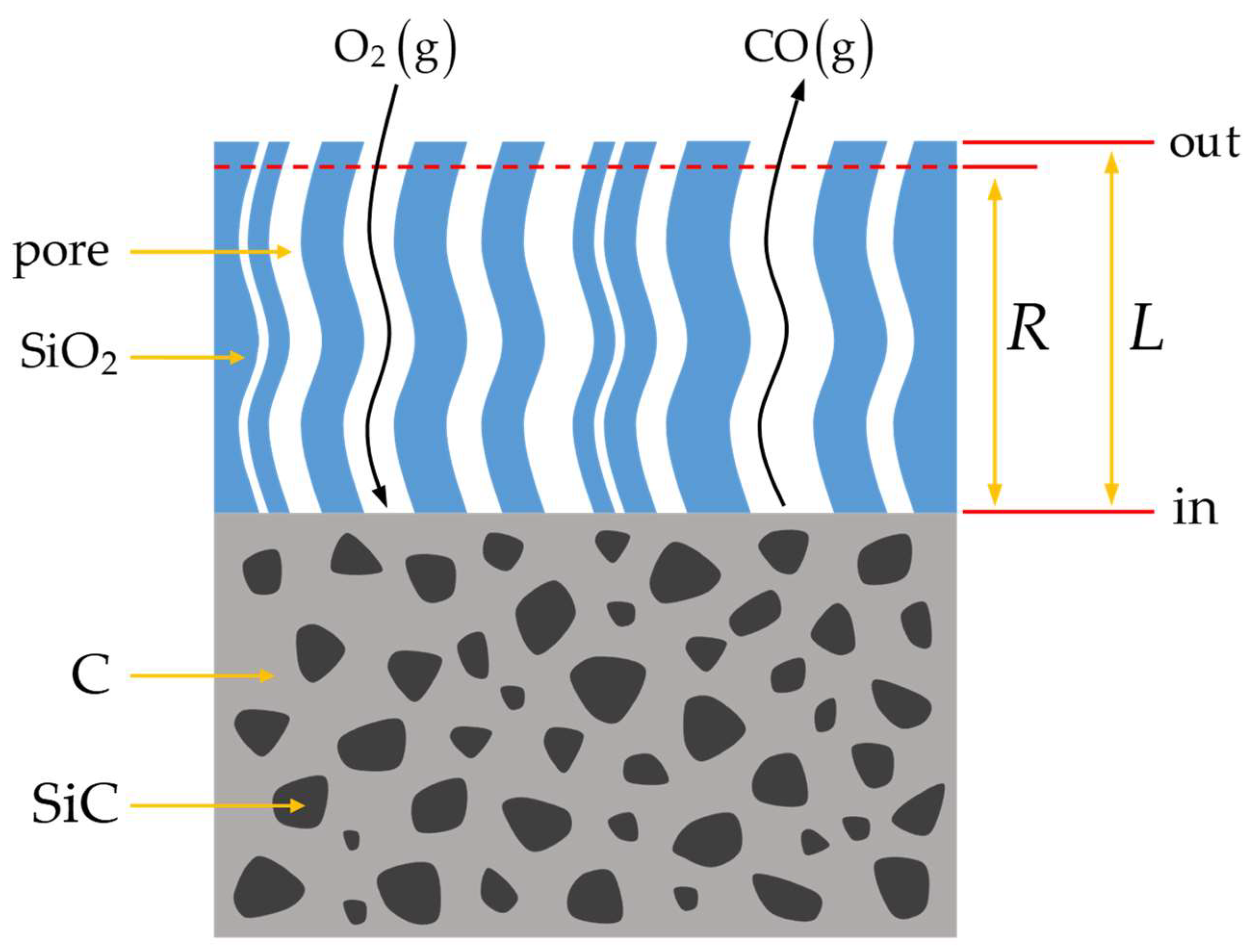
3.2. Oxidation Model
- The oxidation reaction was at a steady state, where the rate of oxygen consumption equaled the rate of oxygen diffusion flow;
- The oxidation recession rate of both C and SiC within the C/SiC composite was assumed to be identical;
- Capillaries with distinct pores were disconnected and the pore size within each individual capillary was considered uniform;
- The inlets and outlets of all orifices were positioned on the same plane.
3.3. Simplification of the Oxidation Model
4. Validation and Application of the Model
4.1. Validation of the Oxidation Model
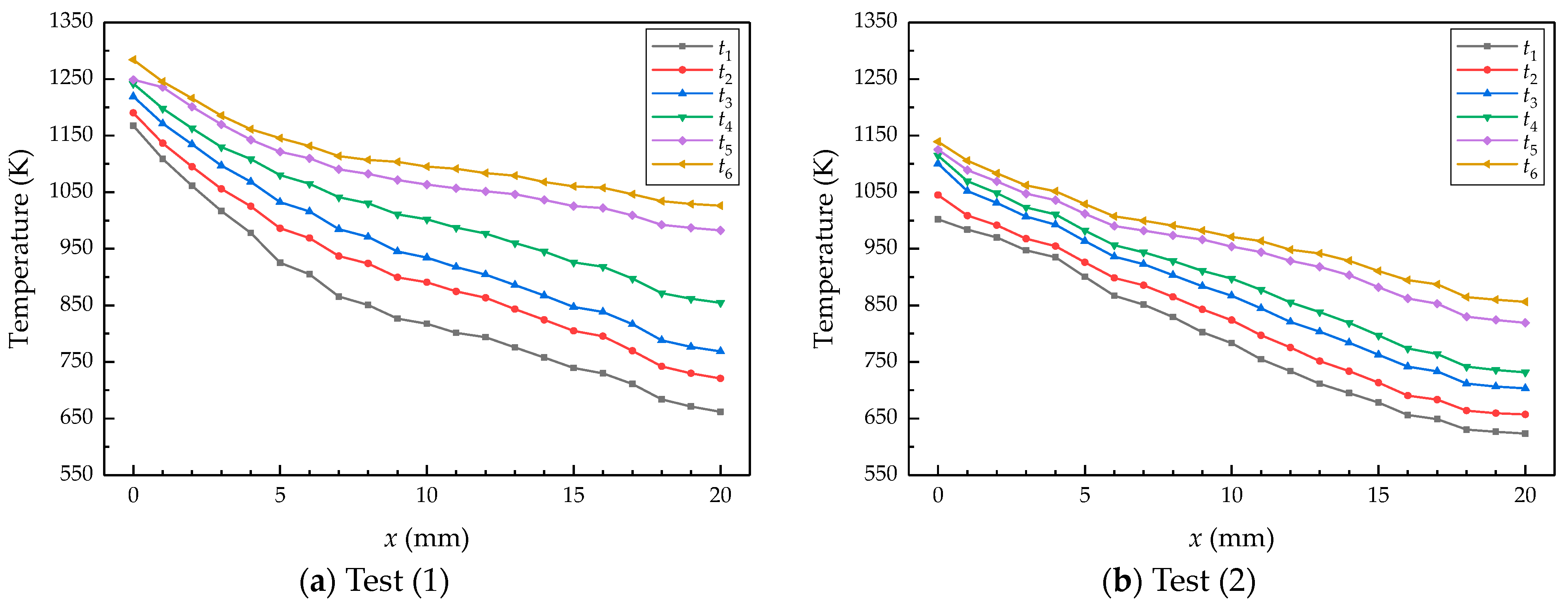
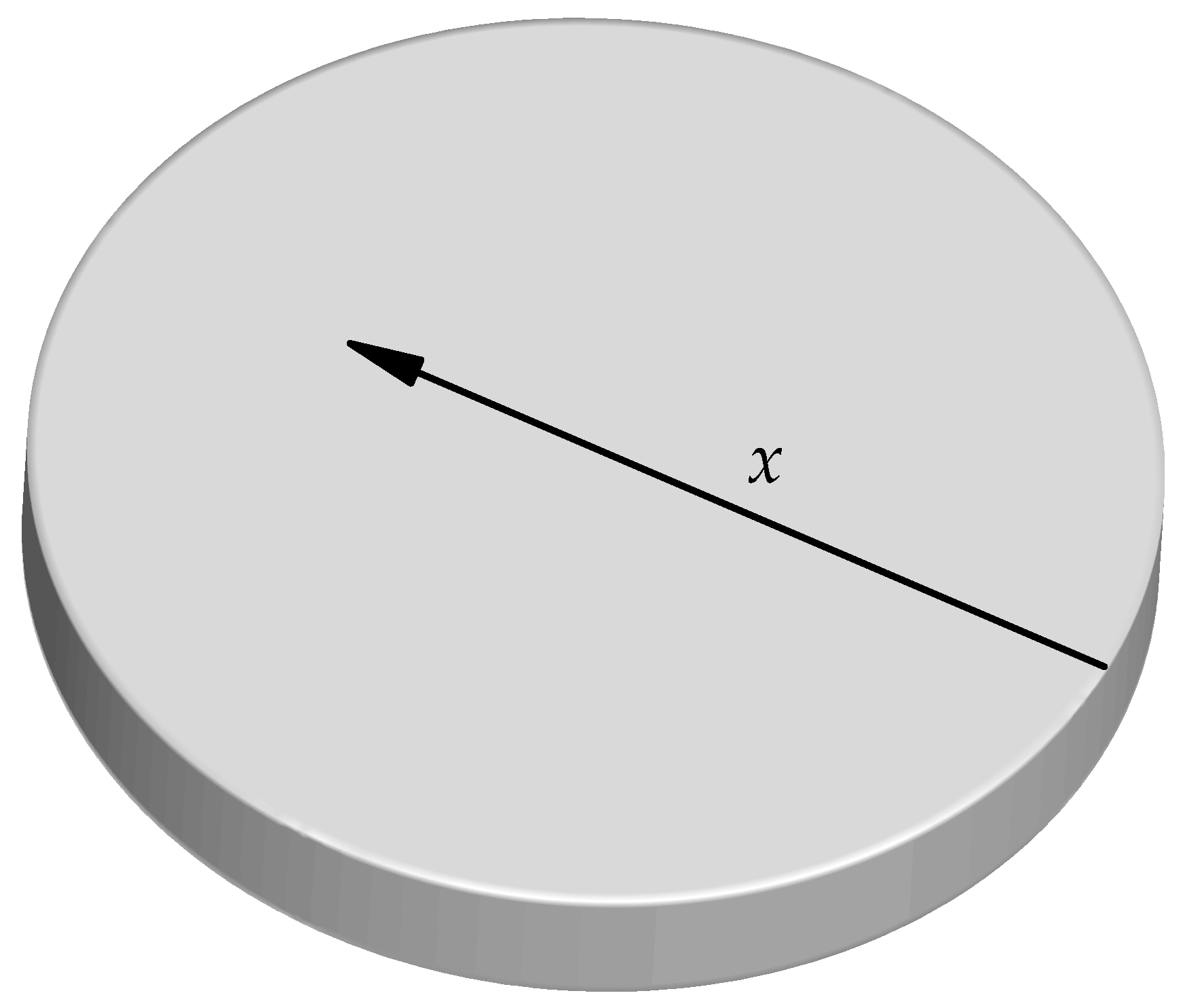
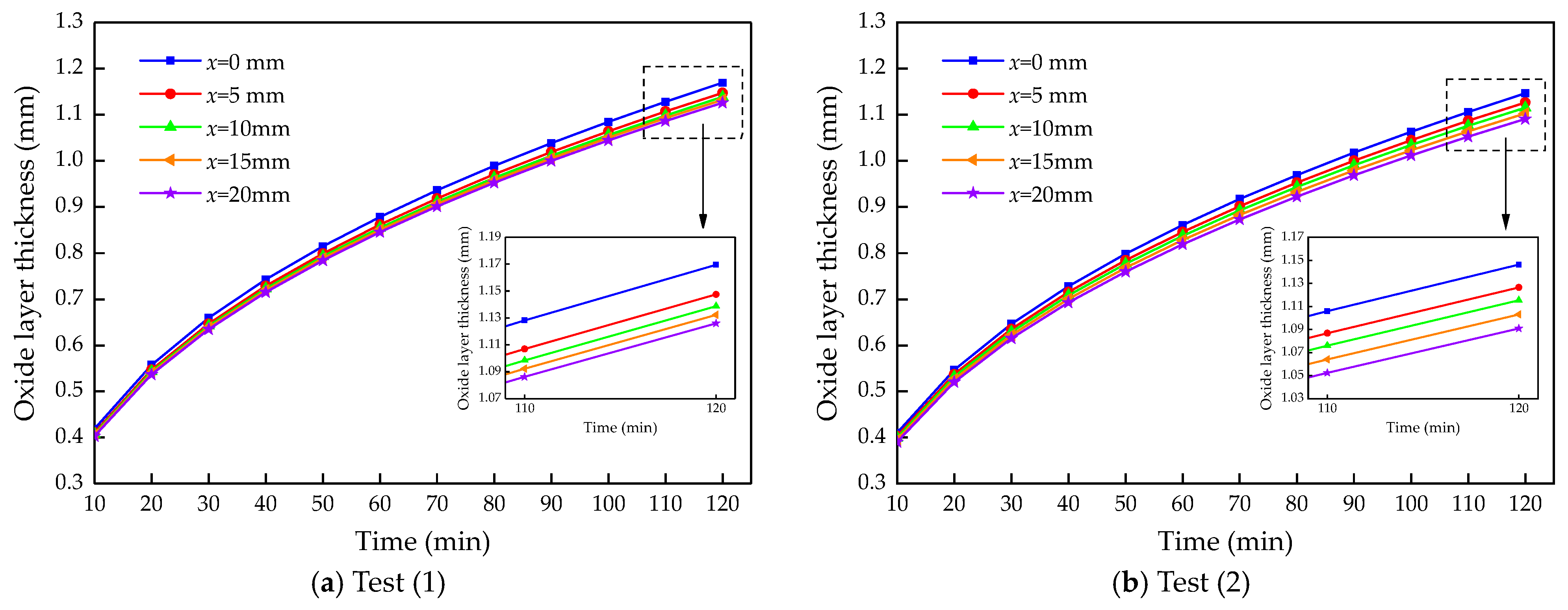
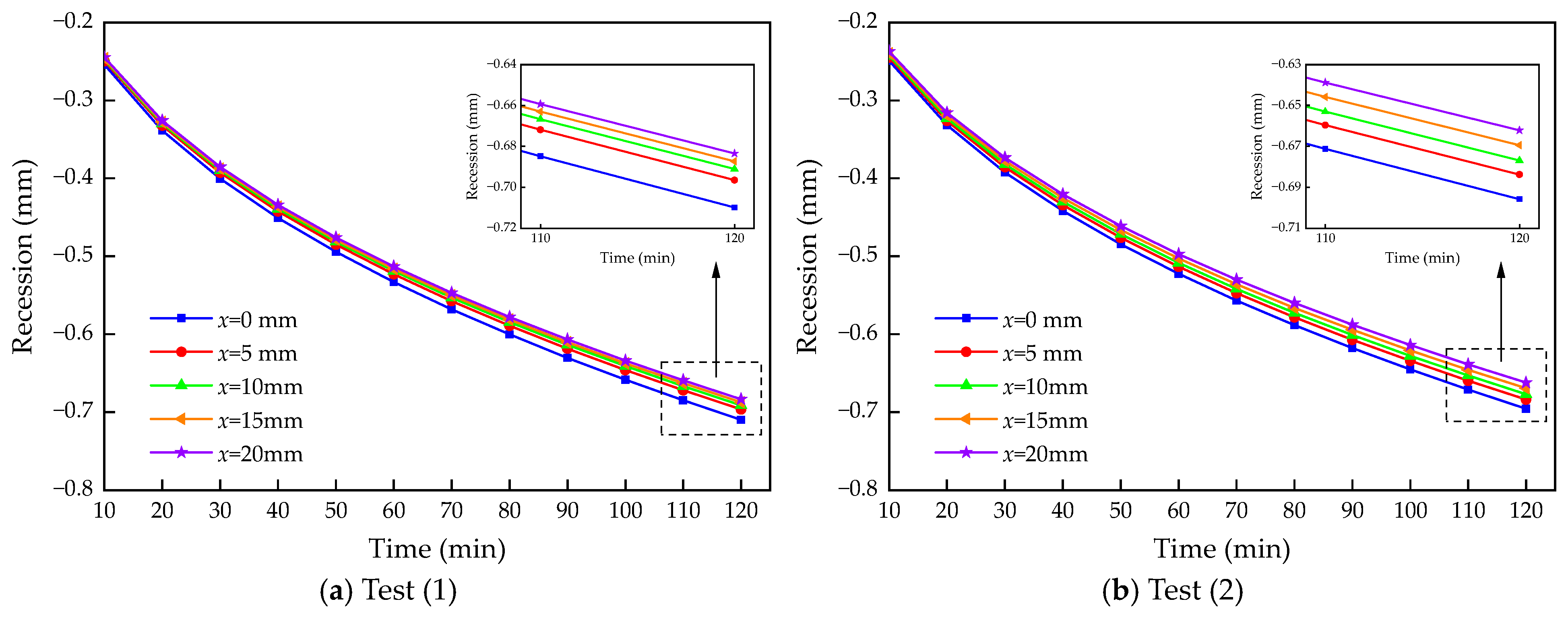
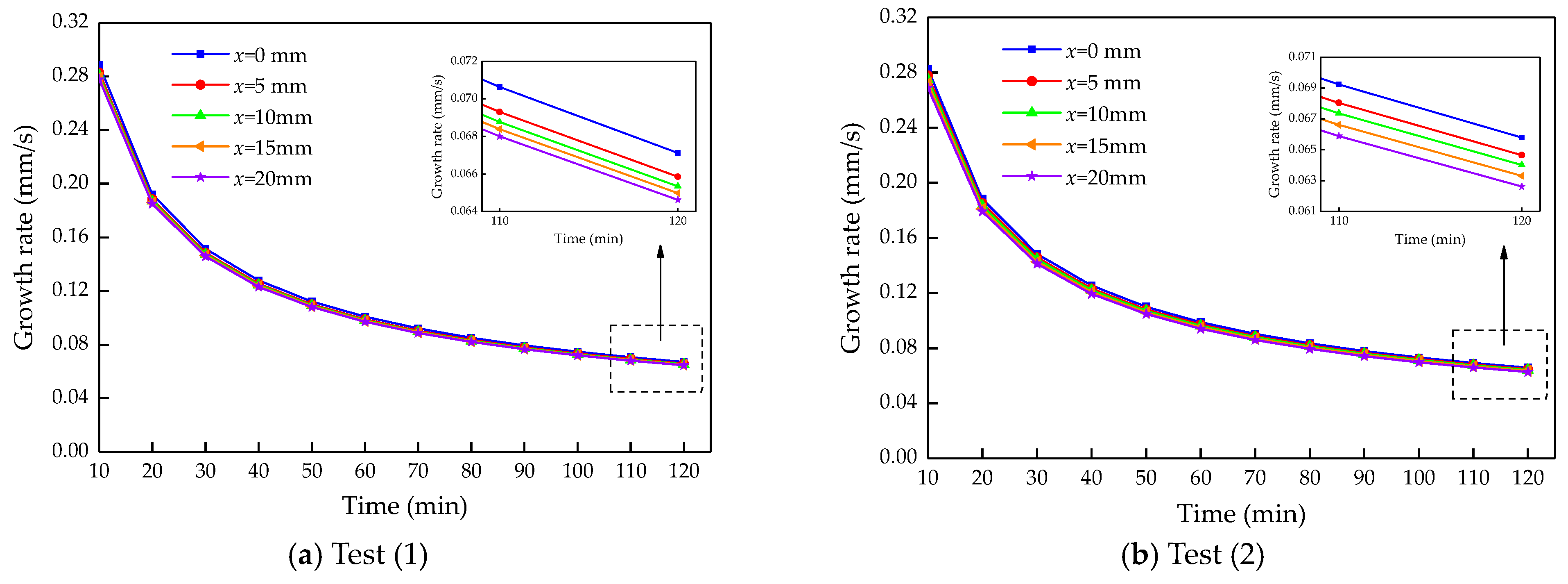
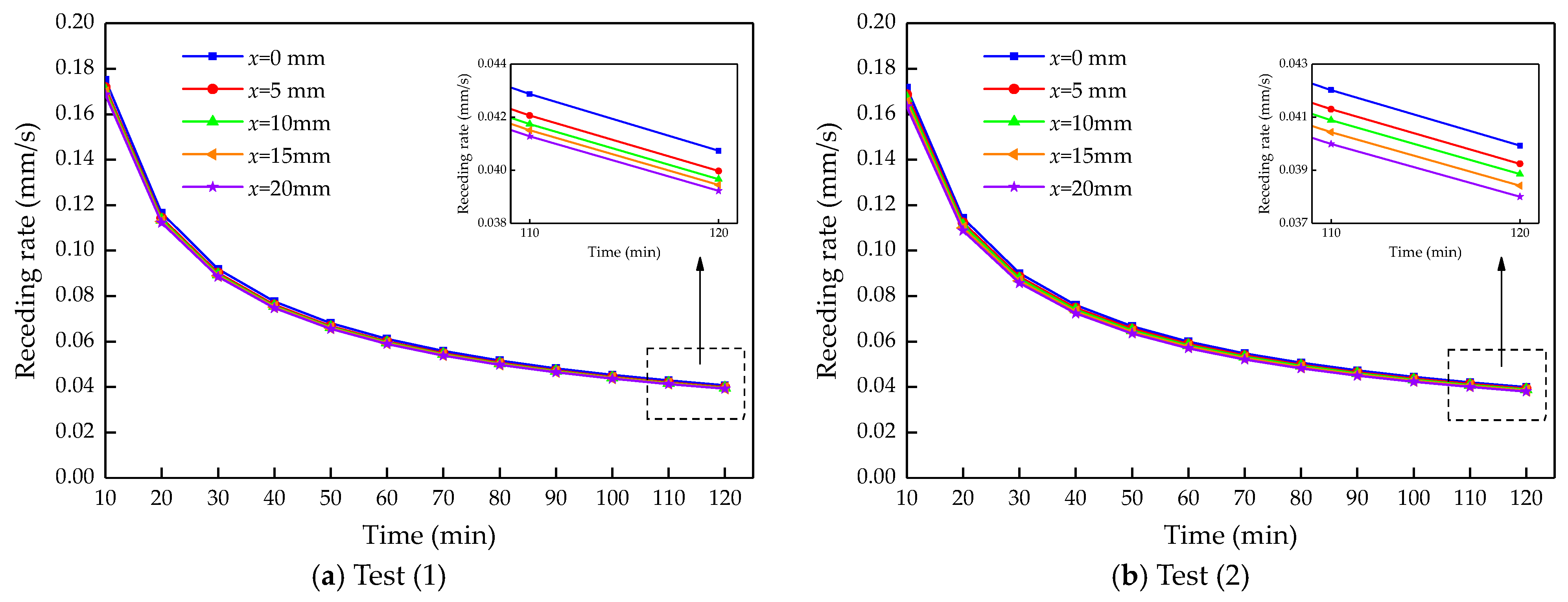
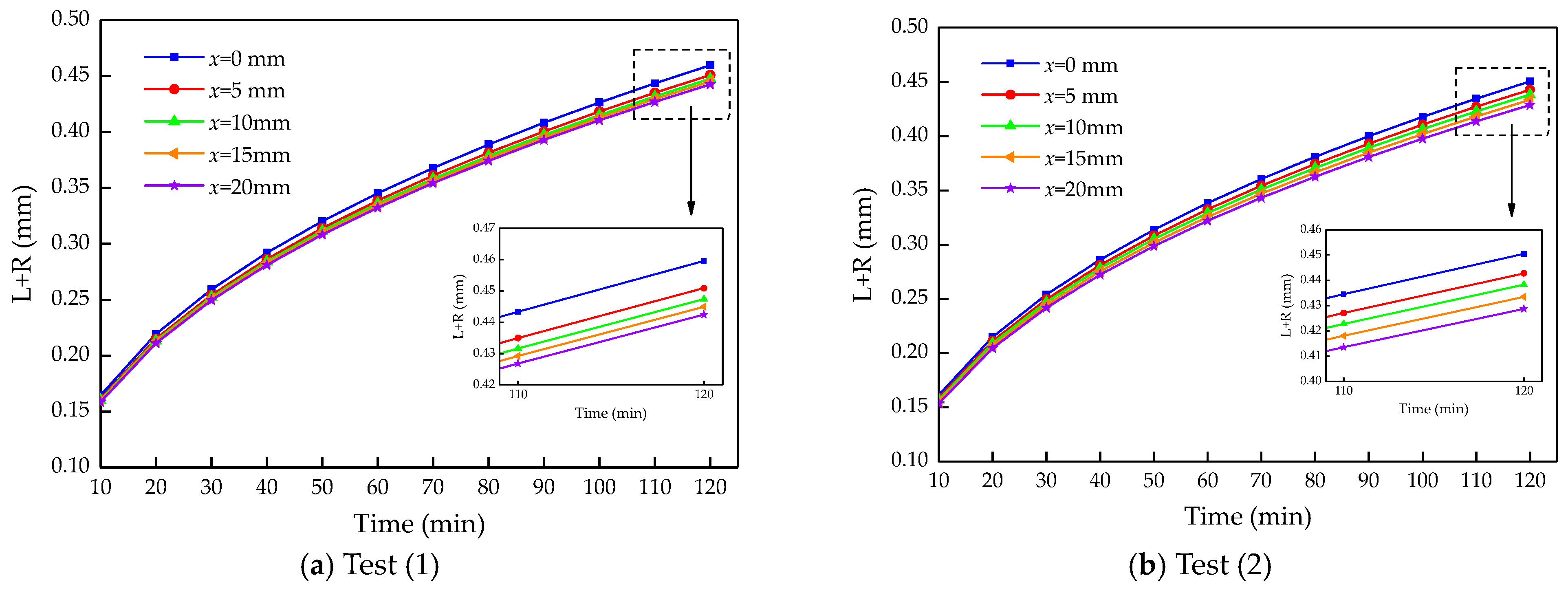
4.2. Calculation Results and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.Z.; Yang, J.L.; Fang, D.N. Research progress on thermal protection materials and structures of hypersonic vehicles. Appl. Math. Mech. Engl. Ed. 2008, 29, 51–60. [Google Scholar] [CrossRef]
- Uyanna, O.; Najafi, H. Thermal protection systems for space vehicles: A review on technology development, current challenges and future prospects. Acta Astronaut. 2020, 176, 341–356. [Google Scholar] [CrossRef]
- Rizzo, S.; Grasso, S.; Salvo, M.; Casalegno, V.; Reece, M.J.; Ferraris, M. Joining of C/SiC composites by spark plasma sintering technique. J. Eur. Ceram. Soc. 2014, 34, 903–913. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Yu, S.J. Ablation behavior and mechanism analysis of C/SiC composites. J. Mater. Res. Technol. 2016, 5, 170–182. [Google Scholar] [CrossRef]
- Wagner, C. Passivity during the Oxidation of Silicon at Elevated Temperatures. J. Appl. Phys. 1958, 29, 1295–1297. [Google Scholar] [CrossRef]
- Deal, B.E.; Grove, A.S. General relationship for the thermal oxidation of silicon. J. Appl. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef]
- Narushima, T.; Goto, T.; Hirai, T. High-temperature passive oxidation of chemically vapor deposited silicon carbide. J. Am. Ceram. Soc. 1989, 72, 1386–1390. [Google Scholar] [CrossRef]
- Sakraker, I.; Asma, C.O. Experimental investigation of passive/active oxidation behavior of SiC based ceramic thermal protection materials exposed to high enthalpy plasma. J. Eur. Ceram. Soc. 2013, 33, 351–359. [Google Scholar] [CrossRef]
- Chen, S.Y.; Body, I.D. Chemical equilibrium analysis of silicon carbide oxidation in oxygen and air. J. Am. Ceram. Soc. 2019, 102, 4272–4284. [Google Scholar] [CrossRef]
- Zhou, S.G.; Zeng, L.; Guo, Y.J.; Liu, X. Simulation of C/SiC Composite Oxidation Behavior in Static Environment. Phy. Gases. 2021, 6, 29–36. (In Chinese) [Google Scholar]
- Wang, L.; Pan, Y.; Deng, D.Y.; Xu, X.L.; Yu, J.J. Theoretical model and calculation method of oxidation ablation behavior of C/SiC materials. Missiles Space Veh. 2022, 4, 92–97. (In Chinese) [Google Scholar]
- Xi, Y.N.; Wang, L.J.; Gao, Y.; Lei, D. Numerical investigation on effective elastic modulus of multifractal porous materials. Fractal Fract. 2023, 7, 3. [Google Scholar] [CrossRef]
- Xu, T.; Moore, I.D.; Gallant, J.C. Fractals, fractal dimensions and landscapes—A review. Geomorphology 1993, 8, 245–262. [Google Scholar] [CrossRef]
- Jevrić, M.; Knežević, M.; Kalezić, J.; Kopitović-Vuković, N.; Ćipranić, I. Application of fractal geometry in urban pattern design. Teh. Vjesn. 2014, 21, 873–879. [Google Scholar]
- Perrier, E.; Bird, N.; Rieu, M. Generalizing the fractal model of soil structure: The pore–solid fractal approach. Geoderma 1999, 88, 137–164. [Google Scholar] [CrossRef]
- Sergeyev, Y. Evaluating the exact infinitesimal values of area of Sierpinski’s carpet and volume of Menger’s sponge. Chaos Solitons Fractals 2009, 42, 3042–3046. [Google Scholar] [CrossRef]
- Yu, B.M. Analysis of flow in fractal porous media. Appl. Mech. Rev. 2008, 61, 050801. [Google Scholar] [CrossRef]
- Yu, B.M.; Li, J.H. Some fractal characters of porous media. Fractals 2001, 9, 365–372. [Google Scholar] [CrossRef]
- Yu, B.M.; Cheng, P. A fractal permeability model for bi-dispersed porous media. Int. J. Heat Mass Transf. 2002, 45, 2983–2993. [Google Scholar] [CrossRef]
- Cai, J.C.; Yu, B.M. A discussion of the effect of tortuosity on the capillary imbibition in porous media. Transp. Porous Media 2011, 89, 251–263. [Google Scholar] [CrossRef]
- Zheng, Q.; Yu, B.M.; Wang, S.F.; Luo, L. A diffusivity model for gas diffusion through fractal porous media. Chem. Eng. Sci. 2012, 68, 650–655. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Yang, W.B.; Yu, H.Z. Study on the permeability of red sandstone via image enhancement. Fractals 2017, 25, 1750055. [Google Scholar] [CrossRef]
- Wang, S.X.; Chen, Z.L.; Qi, F.; Xu, C.H.; Wang, C.J.; Chen, T.; Guo, H. Fractal geometry and convolutional neural networks for the characterization of thermal shock resistances of ultra-high temperature ceramics. Fractal Fract. 2022, 6, 605. [Google Scholar] [CrossRef]
- Kou, L.; Li, W.X.; Wu, J.J. Permeability prediction of saturated geomaterials with revised pore–solid fractal model and critical path analysis. Fractal Fract. 2022, 6, 351. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, J.; Yang, B.; Yu, B.M. Research on the effective gas diffusion coefficient in dry porous media embedded with a fractal-like tree network. Phys. A 2013, 392, 1557–1566. [Google Scholar] [CrossRef]
- Kou, J.L.; Chen, Y.Y.; Zhou, X.Y.; Lu, H.J.; Wu, F.M.; Fan, J.T. Optimal structure of tree-like branching networks for fluid flow. Phys. A 2014, 393, 527–534. [Google Scholar] [CrossRef]
- Miao, T.J.; Chen, A.M.; Zhang, L.W.; Yu, B.M. A novel fractal model for permeability of damaged tree-like branching networks. Int. J. Heat Mass Transf. 2018, 127, 278–285. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, H.L.; Jiang, J.; Xu, C. Fractal analysis of surface roughness effects on gas diffusion in porous nanofibers. Fractals 2020, 28, 2050125. [Google Scholar] [CrossRef]
- Altay, F.; Velipasalar, S. The use of thermal cameras for pedestrian detection. IEEE Sens. J. 2022, 22, 11489–11498. [Google Scholar] [CrossRef]
- Wilson, A.N.; Gupta, K.A.; Koduru, B.H.; Kumar, A.; Jha, A.; Cenkeramaddi, L.R. Recent advances in thermal imaging and its applications using machine learning: A review. IEEE Sens. J. 2023, 23, 3395–3407. [Google Scholar] [CrossRef]
- Olbert, M.; Nedela, V.; Jirak, J.; Hudec, J. Size and shape analysis of micro- to nano-particles of quartz powders using advanced electron microscopy and laser diffraction methods. Powder Technol. 2024, 433, 119250. [Google Scholar] [CrossRef]
- Abdennouri, A.; Zouaoui, E.; Ferkous, H.; Hamza, A.; Grimes, M.; Boukabou, A. An improved symmetric chaotic war strategy optimization algorithm for efficient scanning electron microscopy image segmentation: Calcium oxide catalyst case. Chemom. Intell. Lab. Syst. 2024, 244, 105043. [Google Scholar] [CrossRef]
- Schappo, H.; Gindri, I.M.; Cubillos, P.O.; Maru, M.M.; Salmoria, G.V.; Roesler, C.R. Scanning electron microscopy and energy-dispersive X-ray spectroscopy as a valuable tool to investigate the ultra-high-molecular-weight polyethylene wear mechanisms and debris in hip implants. J. Arthroplast. 2018, 33, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.J.; Mustafa, H.A.; Zaslansky, P.; Mantouvalou, I. Chemical mapping of teeth in 2D and 3D: X-ray fluorescence reveals hidden details in dentine surrounding fillings. Acta Biomater. 2020, 109, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Mtanis, T.; Biadsee, A.; Ormianer, Z. Assessing the cleanliness of dental implants using scanning electron microscopy and energy-dispersive X-ray spectroscopy analysis—A SEM and EDS in vitro study. J. Func. Biomater. 2023, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.Y.; Luo, X.G.; Chen, S.Y.; Yu, J.J.; Ai, B.C. The active-to-passive oxidation transition mechanism and engineering prediction method of C/SiC composites. Sci. China Technol. Sci. 2013, 56, 1403–1408. [Google Scholar] [CrossRef]
- Zheng, Q.; Yu, B.M.; Duan, Y.G.; Fang, Q.T. A fractal model for gas slippage factor in porous media in the slip flow regime. Chem. Eng. Sci. 2013, 87, 209–215. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Jie, H.; Lu, S.; Li, Z. The Influence of Environmental Temperature on the Passive Oxidation Process in the C/SiC Composite. Fractal Fract. 2024, 8, 192. https://doi.org/10.3390/fractalfract8040192
Zhu Q, Jie H, Lu S, Li Z. The Influence of Environmental Temperature on the Passive Oxidation Process in the C/SiC Composite. Fractal and Fractional. 2024; 8(4):192. https://doi.org/10.3390/fractalfract8040192
Chicago/Turabian StyleZhu, Qingyong, Haixin Jie, Shun Lu, and Zhihui Li. 2024. "The Influence of Environmental Temperature on the Passive Oxidation Process in the C/SiC Composite" Fractal and Fractional 8, no. 4: 192. https://doi.org/10.3390/fractalfract8040192
APA StyleZhu, Q., Jie, H., Lu, S., & Li, Z. (2024). The Influence of Environmental Temperature on the Passive Oxidation Process in the C/SiC Composite. Fractal and Fractional, 8(4), 192. https://doi.org/10.3390/fractalfract8040192






