Fractional-Calculus Analysis of the Dynamics of a Vector-Borne Infection with Preventive Measures
Abstract
1. Introduction
2. Essential Concepts and Theory
3. Evaluation of the Fractional Dynamics
4. Existence Theory
- (P1)
- For q in , one can find and in a manner such that the following holds.
- (P2)
- One can find for all and in associated with the following condition:In set , define mapping as follows:
- (C1):
- First, we will validate the continuity of operator . Assuming is continuous for , this implies that is continuous. After that, considering , in a manner that , we have .Furthermore, let us takeThus, the continuity of is ensured from the continuity of , which implies that is continuous.
- (C2):
- Now, we will verify the bounded nature of . Let the following conditions be satisfied by operator for every :Subsequently, we demonstrate the boundedness of in subset T of , where T is bounded. Given , one may determine a non-negative value U in the following way due to the bounded nature of S:Accordingly, the outcome for any within the set T is derived from the above expression as follows:This implies that is a bounded operator.
- (C3):
- For equi-continuity, take and in , in which . Subsequently, we obtain the following:This uses the Arzelà–Ascoli theorem to guarantee the relative compactness of .
- (C4):
- For the final stage, take the below set:To determine the boundedness of , we assume that is a member of . All t in satisfy the below:Consequently, the set is bounded. The operator B has a fixed point that can be found using Schaefer’s theorem. Therefore, the malaria model (11) has at least one solution.
5. Stability Analysis
- (a)
- in which
- (b)
- (a)
- in which
- (b)
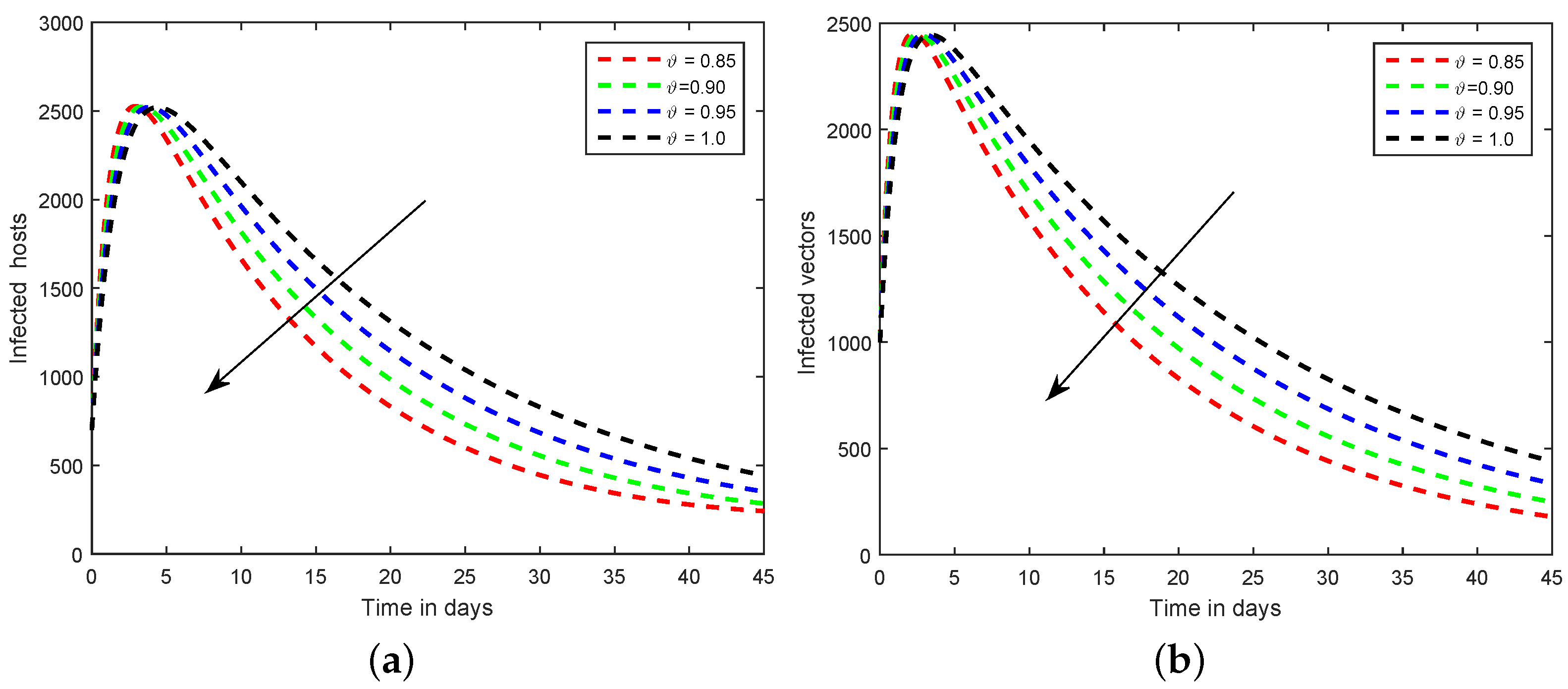
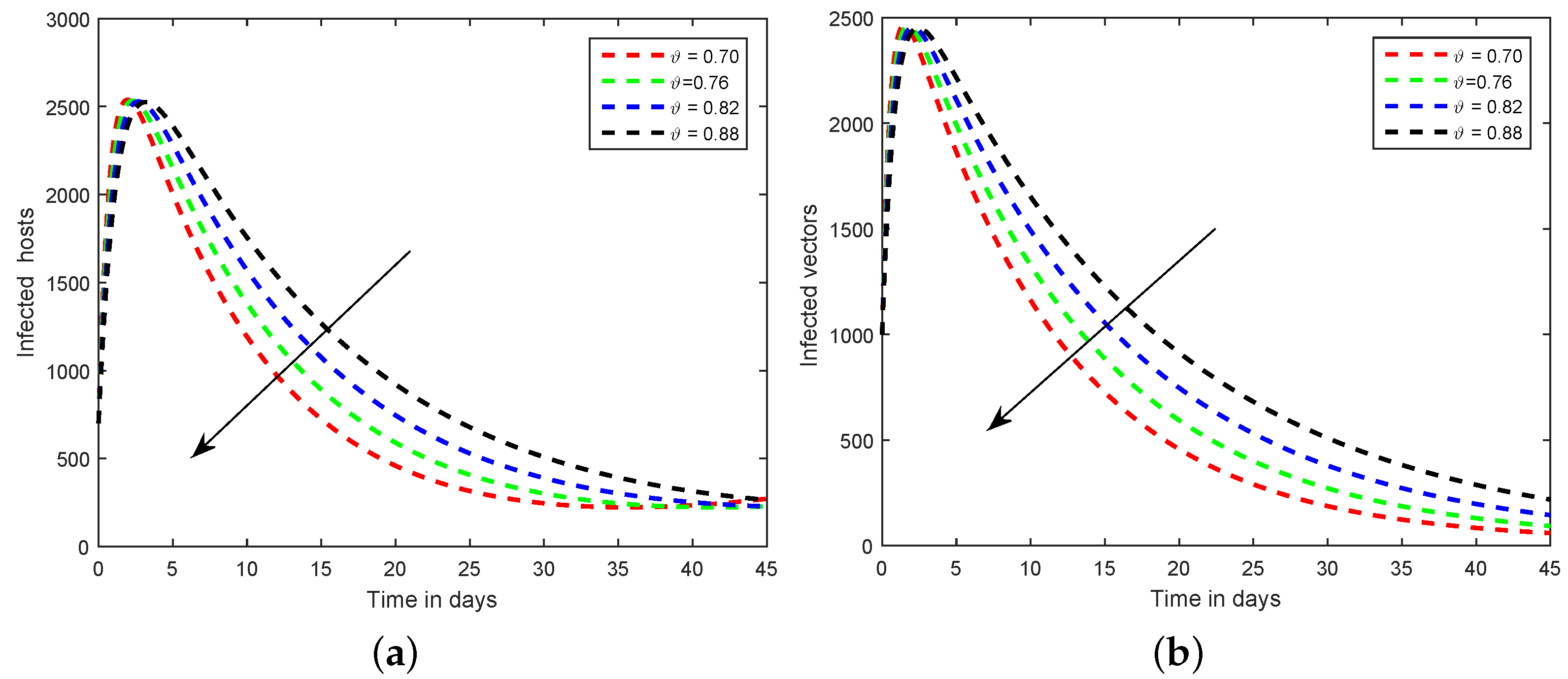
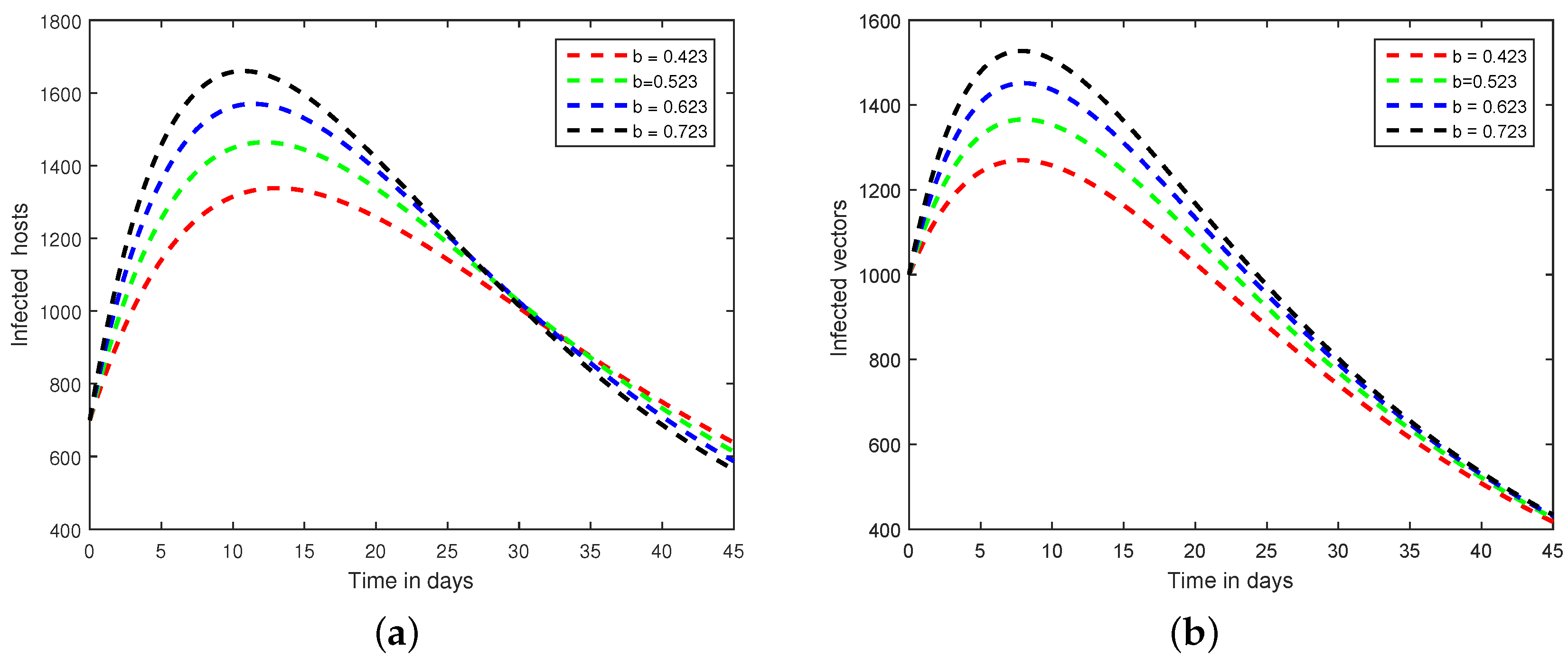
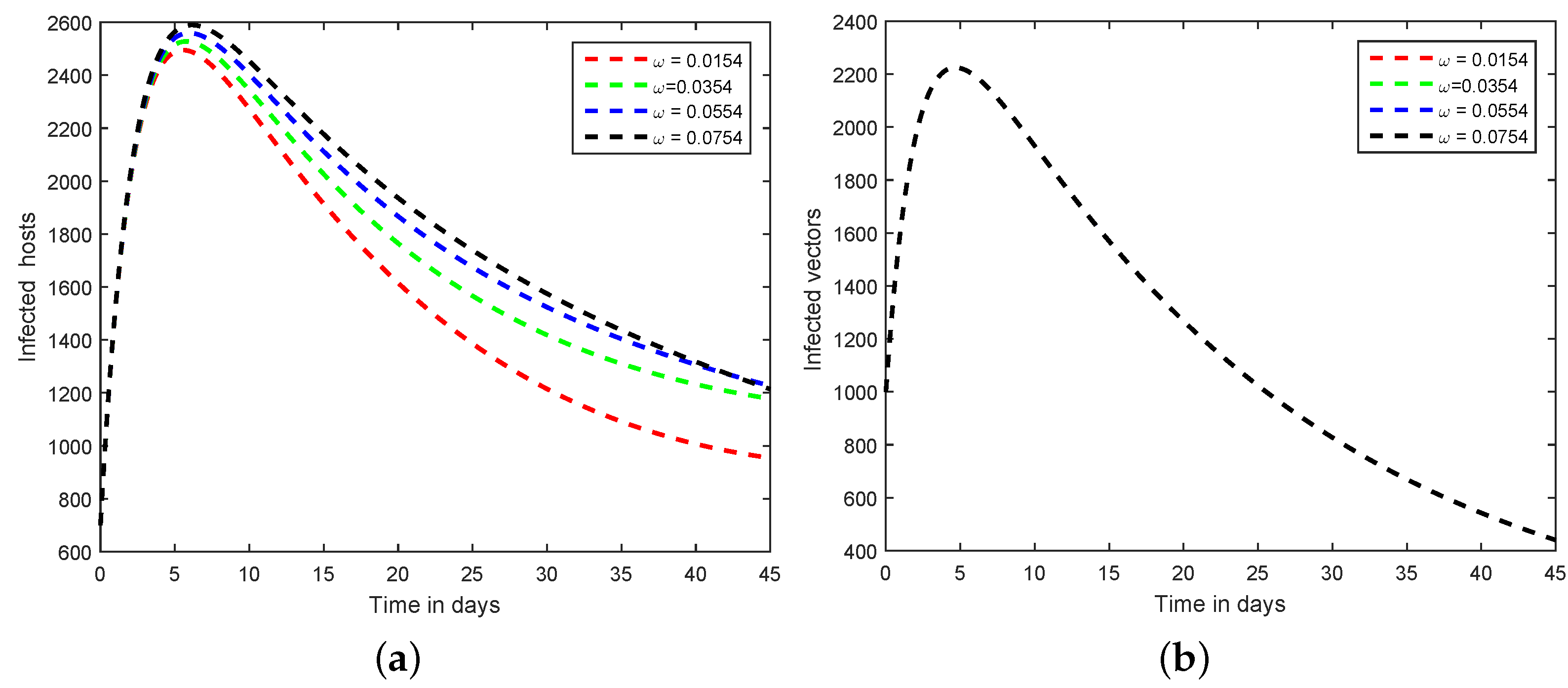
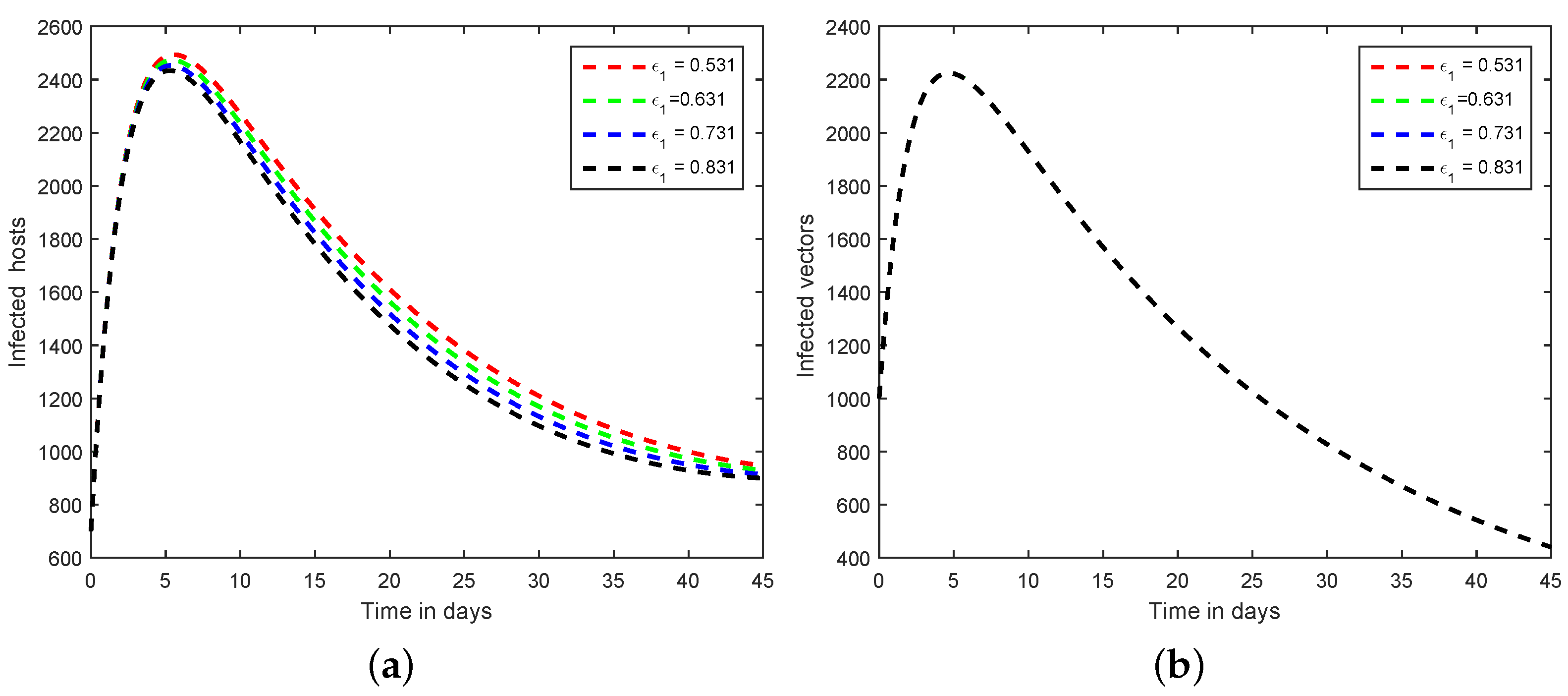
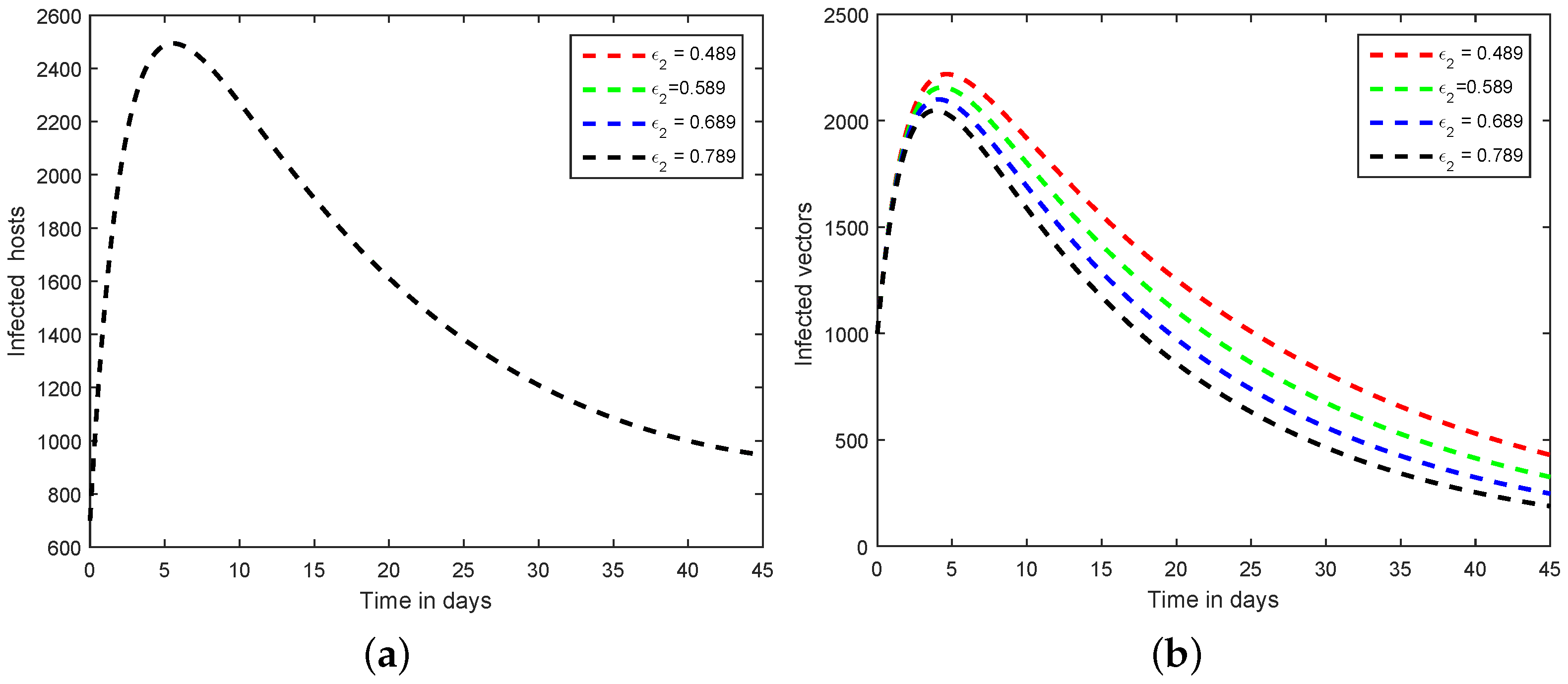
6. Numerical Scheme for the Dynamics
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, S. Plasmodium—A brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthropol. 2021, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.V.; Noronha, K.; Diniz, B.P.; Guedes, G.; Carvalho, L.R.; Silva, V.A.; Calazans, J.A.; Santos, A.S.; Silva, D.N.; Castro, M.C. The economic burden of malaria: A systematic review. Malar. J. 2022, 21, 283. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Wickremasinghe, R.; Newby, G.; Caldera, A.; Fernando, D.; Mendis, K. Malaria control, elimination, and prevention as components of health security: A review. Am. J. Trop. Med. Hyg. 2022, 107, 747. [Google Scholar] [CrossRef]
- Olaniyi, S.; Abimbade, S.F.; Ajala, O.A.; Chuma, F.M. Efficiency and economic analysis of intervention strategies for recurrent malaria transmission. Qual. Quant. 2024, 58, 627–645. [Google Scholar] [CrossRef]
- Smith, D.L.; Battle, K.E.; Hay, S.I.; Barker, C.M.; Scott, T.W.; McKenzie, F.E. Ross, Macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 2012, 8, e1002588. [Google Scholar] [CrossRef]
- Jan, R.; Hinçal, E.; Hosseini, K.; Razak, N.N.A.; Abdeljawad, T.; Osman, M.S. Fractional view analysis of the impact of vaccination on the dynamics of a viral infection. Alex. Eng. J. 2024, 102, 36–48. [Google Scholar] [CrossRef]
- Tchoumi, S.Y.; Diagne, M.L.; Rwezaura, H.; Tchuenche, J.M. Malaria and COVID-19 co-dynamics: A mathematical model and optimal control. Appl. Math. Model. 2021, 99, 294–327. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Razak, N.N.A.; Alyobi, S.; Khan, Z.; Hosseini, K.; Park, C.; Salahshour, S.; Paokanta, S. Fractional Dynamics of Chronic Lymphocytic Leukemia with the Effect of Chemoimmunotherapy Treatment. Fractals 2024, 32, 1–16. [Google Scholar] [CrossRef]
- Romero-Leiton, J.P.; Ibargüen-Mondragón, E. Stability analysis and optimal control intervention strategies of a malaria mathematical model. Appl. Sci. 2019, 21, 184–218. [Google Scholar]
- Ozodiegwu, I.D.; Ambrose, M.; Galatas, B.; Runge, M.; Nandi, A.; Okuneye, K.; Dhanoa, N.P.; Maikore, I.; Uhomoibhi, P.; Bever, C.; et al. Application of mathematical modelling to inform national malaria intervention planning in Nigeria. Malar. J. 2023, 22, 137. [Google Scholar] [CrossRef]
- Olaniyi, S.; Ajala, O.A.; Abimbade, S.F. Optimal control analysis of a mathematical model for recurrent malaria dynamics. In Operations Research Forum; Springer International Publishing: Cham, Switzerland, 2023; Volume 4, p. 14. [Google Scholar]
- Tchoumi, S.Y.; Njintang, N.Y.; Kamgang, J.C.; Tchuenche, J.M. Malaria and malnutrition in children: A mathematical model. Frankl. Open 2023, 3, 100013. [Google Scholar] [CrossRef]
- Chiyaka, C.; Garira, W.; Dube, S. Effects of treatment and drug resistance on the transmission dynamics of malaria in endemic areas. Theor. Popul. Biol. 2009, 75, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Tumwiine, J.; Hove-Musekwa, S.D.; Nyabadza, F. A mathematical model for the transmission and spread of drug sensitive and resistant malaria strains within a human population. Int. Sch. Res. Not. 2014, 2014, 636973. [Google Scholar] [CrossRef]
- Okosun, K.O.; Ouifki, R.; Marcus, N. Optimal control analysis of a malaria disease transmission model that includes treatment and vaccination with waning immunity. Biosystems 2011, 106, 136–145. [Google Scholar] [CrossRef]
- Luz, P.M.; Codeco, C.T.; Medlock, J.; Struchiner, C.J.; Valle, D.; Galvani, A.P. Impact of insecticide interventions on the abundance and resistance profile of Aedes aegypti. Epidemiol. Infect. 2009, 137, 1203–1215. [Google Scholar] [CrossRef]
- Aldila, D.; Nuraini, N.; Soewono, E.; Supriatna, A.K. March. Mathematical model of temephos resistance in Aedes aegypti mosquito population. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; Volume 1589, pp. 460–463. [Google Scholar]
- Alphey, N.; Coleman, P.G.; Donnelly, C.A.; Alphey, L. Managing insecticide resistance by mass release of engineered insects. J. Econ. Entomol. 2014, 100, 1642–1649. [Google Scholar] [CrossRef]
- Gourley, S.A.; Liu, R.; Wu, J. Slowing the evolution of insecticide resistance in mosquitoes: A mathematical model. Proc. R. Soc. A Math. Phys. Eng. Sci. 2011, 467, 2127–2148. [Google Scholar] [CrossRef]
- Bloland, P.B.; World Health Organization. Drug Resistance in Malaria; No. WHO/CDS/CSR/DRS/2001.4; World Health Organization: Geneva, Switzerland, 2001.
- Agusto, F.B. Malaria drug resistance: The impact of human movement and spatial heterogeneity. Bull. Math. Biol. 2014, 76, 1607–1641. [Google Scholar] [CrossRef]
- Gao, D.; Ruan, S. A multipatch malaria model with logistic growth populations. SIAM J. Appl. Math. 2012, 72, 819–841. [Google Scholar] [CrossRef]
- Weiss, G.H. On the spread of epidemics by carriers. Biometrics 1965, 21, 481–490. [Google Scholar] [CrossRef]
- Bichara, D.; Iggidr, A. Multi-patch and multi-group epidemic models: A new framework. J. Math. Biol. 2018, 77, 107–134. [Google Scholar] [CrossRef] [PubMed]
- Bock, W.; Jayathunga, Y. Optimal control and basic reproduction numbers for a compartmental spatial multipatch dengue model. Math. Methods Appl. Sci. 2018, 41, 3231–3245. [Google Scholar] [CrossRef]
- Chaves, L.F.; Harrington, L.C.; Keogh, C.L.; Nguyen, A.M.; Kitron, U.D. Blood feeding patterns of mosquitoes: Random or structured? Front. Zool. 2010, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Vinauger, C.; Buratti, L.; Lazzari, C.R. Learning the way to blood: First evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. I. Appetitive learning. J. Exp. Biol. 2011, 214, 3032–3038. [Google Scholar] [CrossRef] [PubMed]
- Ullam, S.M. Problems in Modern Mathematics; Wiley: New York, NY, USA, 1940; Chapter VI. [Google Scholar]
- Khalaf, S.L.; Kadhim, M.S.; Khudair, A.R. Studying of COVID-19 fractional model: Stability analysis. Partial. Differ. Equ. Appl. Math. 2023, 7, 100470. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Abbas, M.; Omame, A. Analysis of a non-integer order compartmental model for cholera and COVID-19 incorporating human and environmental transmissions. Phys. Scr. 2023, 98, 125223. [Google Scholar] [CrossRef]
- Kilbas, A.A.; Srivastava, H.M.; Trujillo, J.J. Theory and Applications of Fractional Differential Equations; Elsevier: Amsterdam, The Netherlands, 2006; Volume 204. [Google Scholar]
- Podlubny, I. Fractional Differential Equations: An Introduction to Fractional Derivatives, Fractional Differential Equations, to Methods of Their Solution and Some of Their Applications; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Granas, A.; Dugundji, J. Elementary fixed point theorems. In Fixed Point Theory; Springer: New York, NY, USA, 2003; pp. 9–84. [Google Scholar]
- Atangana, A.; Owolabi, K.M. Corrigendum: New numerical approach for fractional differential equations. Math. Model. Nat. Phenom. 2021, 16, 47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jan, R.; Boulaaras, S.; Alharbi, A.; Abdul Razak, N.N. Fractional-Calculus Analysis of the Dynamics of a Vector-Borne Infection with Preventive Measures. Fractal Fract. 2024, 8, 691. https://doi.org/10.3390/fractalfract8120691
Jan R, Boulaaras S, Alharbi A, Abdul Razak NN. Fractional-Calculus Analysis of the Dynamics of a Vector-Borne Infection with Preventive Measures. Fractal and Fractional. 2024; 8(12):691. https://doi.org/10.3390/fractalfract8120691
Chicago/Turabian StyleJan, Rashid, Salah Boulaaras, Asma Alharbi, and Normy Norfiza Abdul Razak. 2024. "Fractional-Calculus Analysis of the Dynamics of a Vector-Borne Infection with Preventive Measures" Fractal and Fractional 8, no. 12: 691. https://doi.org/10.3390/fractalfract8120691
APA StyleJan, R., Boulaaras, S., Alharbi, A., & Abdul Razak, N. N. (2024). Fractional-Calculus Analysis of the Dynamics of a Vector-Borne Infection with Preventive Measures. Fractal and Fractional, 8(12), 691. https://doi.org/10.3390/fractalfract8120691










