Abstract
The economic burden of HIV extends beyond the individual level and affects communities and countries. HIV can lead to decreased economic growth due to lost productivity and increased healthcare costs. In some countries, the HIV epidemic has led to a reduction in life expectancy, which can impact the overall quality of life and economic prosperity. Therefore, it is significant to investigate the intricate dynamics of this viral infection to know how the virus interacts with the immune system. In the current research, we will formulate the dynamics of HIV infection in the host body to conceptualize the interaction of T-cells and the immune system. The recommended model of HIV infection is presented with the help of fractional calculus for more precious outcomes. We introduce numerical methods to demonstrate how the input parameters affect the output of the system. The dynamical behavior and chaotic nature of the system are visualized with the variation of different input factors. The system’s tracking path has been numerically depicted and the impact of the viruses on T-cells has been demonstrated. In addition to this, the key factors of the system has been predicted through numerical findings. Our results predict that the strong non-linearity of the system is responsible for the chaos and oscillation, which are so closely related. The chaotic parameters of the system are highlighted and are recommended for the control of the chaos of the system.
Keywords:
HIV dynamics; immune system; fractional calculus; numerical scheme; time series analysis; chaos MSC:
92B05; 37N25; 92D25
1. Introduction
With HIV infection, the virus attacks and weaken the immune system, which is the body’s defense against infections and diseases. The virus targets CD4 T-cells, which are a type of white blood cell that plays a central role in the immune response. As HIV continues to replicate and destroy CD4 T-cells, the immune system becomes weaker and less able to fight off infections and diseases. This can lead to a wide range of opportunistic infections and cancers that would not normally affect healthy individuals. Without treatment, HIV can progress to AIDS, which is a condition characterized by severe immune system damage and a high risk of developing life-threatening infections and cancers. Effective treatment for HIV involves antiretroviral therapy (ART), which uses a combination of medications to suppress the virus and prevent disease progression. With effective treatment, people living with HIV can achieve an undetectable viral load, which means that the virus is no longer detectable in their blood and they are much less likely to transmit HIV to others. However, even with effective treatment, HIV can still have long-term effects on the immune system, and people living with HIV may be at increased risk for certain infections and cancers.
The infection of HIV infects the host immune system and damage vital organs like the heart, kidneys, and brain, which finally results in death. As there is no effective treatment for this infection, but some medications can significantly improve the patient’s health, while excessive use of these medications has side effects on the host. ART is being used by more people than ever before. People have been seen to occasionally experience symptoms that resemble sore throats, rashes, headaches, fevers, and influenza. Sometimes, the symptoms are dangerous, such as fever, weight gain, diarrhea, coughing and swollen lymph nodes. If left untreated, these can progress to dangerous conditions. Since the first incidence of HIV was discovered in the early 1980s, millions of people globally have become infected, with HIV becoming the most deadly infection in the world [1,2,3]. HIV continues to be a major cause of death globally, with sub-Saharan Africa suffering the worst effect prevalence range: 12 to 42% [2]. The poorest communities bear the heaviest burden of HIV/AIDS and have the least access to medications and other preventive measures. Therefore, a more effective strategy is needed for the prevention, control, and management of this viral infection, which includes raising awareness and providing the optimum ART drug combinations.
Over the years, in vivo studies for the dynamics of HIV infection have been conducted with the goal of better visualization of how the HIV virus and the cells of the body interact. Such knowledge has shown to be extremely helpful, particularly in the creation of ARTs and in the treatment of HIV. In contrast to the past, when research relied on clinical trials, currently, mathematical models of varying degrees of complexity are utilized to simulate and analyze such interactions [4]. As a result, numerous experts in the field of epidemiological modeling [5,6,7,8] have started to create novel models that could be applied to examine the infection. Numerous models that have been created describe and shine a light on different elements that arise from the interaction between HIV and T-cells. Mathematical models for HIV and the immune system are used to understand the dynamics of the virus and the immune response, and to explore strategies for managing the disease. The models typically involve differential equations that describe the interactions between the virus and the immune cells, as well as the effects of treatments and other interventions [9]. Most of these fundamental models comprise at least three state variables, including HIV virion and uninfected and infected CD4 T-cells for the purpose of providing the most basic knowledge on regulating the viral propagation. For example, Ref. [10] created and examined the interaction of healthy T-cells and infected T-cells and the concentration of HIV viruses.
It is well known that the models have undergone significant improvements, and several more advanced models have been developed in the literature for biological processes [11,12,13]. A mathematical model of the entire dynamics of HIV infection was developed in [14]. In [4], the model was made to examine how HIV dynamics changed early on in infection, where the findings demonstrated that the initial stage of HIV infection was marked by extremely high viral persistence. A mathematical modeling approach was used by the authors in [15] to demonstrate how migration affects the spread of HIV and AIDS cases. In [16], the researchers investigated an optimal treatment strategy, known as target-oriented treatment (TOT), for HIV infections using a mathematical model. Specifically, they focused on the scenario where a specified number of CD4 T-cells must be achieved during the treatment period. Although antiretroviral therapy can effectively suppress HIV, it often does not fully restore the immune system to its pre-infection state [17]. Thus, further research is required to focus on ways to improve immune reconstitution in individuals with HIV. Other studies have attempted to understand the function of killer T-cells in blocking viral replication in the human host. In [18], the researchers considered T-cells with HIV viruses and showed how the immune system is influenced by the viruses. Some other dynamics considered three-dimensional dynamics by taking viruses and T-cells with the help of mathematical models. The model’s failure to take the HIV virion into account was a significant flaw. The authors in [7] employed a five-dimensional model of ODE’s to illustrate how T-cells, virion, defense cells, and ARTs are related to one another. The findings highlighted how crucial T-cells are in battling HIV virion during acute infection. It is known that the inability of ARTs to reach all cells and exponential viral multiplication caused the disease to spread more widely. In this study, we opt to conceptualize the intricate phenomena of T-cells, T-cells, and virus cells during HIV infection to show how it affects the immune system. Furthermore, we aim to highlight the main characteristics of the phenomenon and provide effective collaboration with the corresponding departments for the prevention of the infection.
This study is organized as follows. In Section 2, we introduce the HIV dynamics to illustrate how the HIV virus interacts with the immune cells. In Section 3, the basic results of fractional calculus are listed for the analysis of the recommended model of HIV infection. For more precise results, the recommended system of HIV infection is presented through fractional derivative in Section 4. In Section 5, the dynamical behavior of the system is investigated through numerical skills. In Section 6, we provide a conclusion.
2. Formulation of HIV Dynamics
In this section, we will introduce a mathematical model for the infection of HIV to represent the interaction of viruses and immune cells. It is well known that numerous mathematical models have been established in the literature for this viral infection [19,20,21]. The authors presented the dynamics of HIV with different assumptions for the input of the system. According to [22], the HIV transmission phenomenon is as follows:
where s represents the rate of recruitment of T-cells into the host body and r is the growth rate of the population of healthy T-cells, the terms , , and are used to indicate the death rate of T-cells, infected T-cells, and the mortality of HIV viruses, respectively, k indicates the infection rate of healthy T-cells, and N is the number of cells that infected T-cells reproduce. The HIV model from Perelson and Nelson [23] is given by the following system:
In [22], a variable source term was introduced instead of the constant term for T-cells. This new source term reflects the number of new healthy T-cells generated by the thymus, which is dependent on the concentration of the viral load. As the size of the viral load increases, the production of healthy T-cells is observed to decrease. Thus, the source term is now considered to be a variable instead of a constant. Then, according to [24], we have:
where the rate of cellular infection and is the effectiveness of a protease inhibitor, respectively. In our model, we assume the concentration of and activated T-cells by and , respectively. The thymus continuously produces T-cells at a rate of and naturally eliminates them at a rate of . In the presence of HIV virion, T-cell activation proceeds at a different rate. Furthermore, T-cells are transformed into activated T-cells in the presence of HIV virion, which are produced at a rate of and naturally depleted at a rate of . Thus, the dynamics of HIV infection is given by:
where and the initial condition is given as , , , and . The above model (4) will be investigated to visualize the interaction of immune system and HIV viruses.
3. Fundamentals of Fractional Calculus
In this section, we will presented the basic results of fractional theory for the analysis of our system. We will use the well-known Liouville–Caputo and Atangana–Baleanu operators in this study (see [25,26,27]). The basic results related to these operators are given below.
Definition 1.
If h is taken such that h in a way that , the integral in fractional Liouville–Caputo structure is as follows:
where Υ is used for the gamma function and β is the order of the operator.
Definition 2.
If h is taken such that , the Liouville–Caputo fractional derivative is introduced as given below:
where , , and is closer to as beta gets closer to one.
Secondly, we illustrated the operator of Atangana–Baleanu. It captures the occurrence of biological processes with a non-local and non-singular kernel. The results obtained with this novel derivative have shown to be more reliable than those obtained with other alternatives. The fundamental concepts of this operator are given below.
Definition 3.
Take h such that , where β is in the range [0, 1] and , then the Atangana–Baleanu operator is given by:
Definition 4.
The fractional integral of the Atangana–Baleanu operator for h is as follows:
as the fractional order approaches 0, the original function is attained.
Theorem 1
(see [28]). If you assume that such that , then the following is true:
Additionally, the Lipschitz requirement for this ABC derivative is met:
4. Fractional Dynamics of HIV Infection
These days, the theory of fractional calculus is frequently used by researchers to formulate epidemic models for infectious diseases due to its accurate findings. These models have the ability to represent the crossover and non-local nature of real-world problems. More detailed results have been obtained recently for infections diseases through fractional-order modeling. Thus, the dynamics of HIV infection will be formulated in the structure of fractional calculus to obtain more precious results in this section. First, we illustrate the dynamics of HIV infection with the help of the Liouville–Caputo derivative and then with the operator of Atangana–Baleanu in the Liouville–Caputo structure.
4.1. HIV Dynamics via the Liouville–Caputo Operator
In this subsection, we will illustrate the intricate phenomena of HIV infection through the Liouville–Caputo operator. The main aim is to conceptualize the dynamics of HIV and the immune system. We will also present a numerical scheme to highlight the tracking path of the recommended model with the variability of different factors. Our model (4) according to the Liouville–Caputo framework is as follows:
where stands for the Liouville–Caputo fractional operator. To show the dynamical behavior of the recommended model (5), we represent a numerical scheme for the fractional Liouville–Caputo non-linear ordinary differential system [28]. For the scheme, we take the following system:
following the application of the fundamental theorem, we have:
here, we take the time , , and obtain:
and
Then, is approximated in the following manner:
Following the simplification, we obtain:
Furthermore, we have:
Next, we have:
Simplifying even more, we obtain:
Equations (16) and (17) were combined into Equation (10), and the resulting approximation is shown below:
Through this numerical method, we represent the system (4) for HIV infection. More specifically, the tracking path of the system will be represented in the Liouville–Caputo system through the above numerical scheme.
4.2. HIV Dynamics via the ABC Operator
Here, we will illustrate the recommended model (4) using the Atangana–Baleanu fractional derivative. The impact of various factors on the HIV system will then be visualized using a numerical method for the ABC derivative. Our model (4) is first represented by the Atangana–Baleanu derivative as:
where , and denote the initial conditions and indicates the ABC fractional derivative. In the next step, we will represent a numerical scheme to illustrate the above dynamics of HIV infection. To do so, we take the following general fractional system:
through mathematical skills, we obtain the following:
At this moment, , , the result is as follows:
To obtain the estimated value in this case, we utilize the interpolation polynomial on the function on the interval as follows:
incorporating this into (21), we have:
The results of the aforementioned calculation are as follows:
We arrive at the following approximation to the proposed model of HIV infection:
This numerical method is employed to determine the ABC fractional system solution. This technique will be used to research the fractional system (19) of HIV infection’s transmission phenomena.
5. Numerical Discussions
The interaction between the HIV virus and the immune system is a dynamic process that involves complex interactions between the virus and various components of the immune system. Researchers are continually working to better understand these interactions and to develop new treatments and therapies to manage HIV infection and improve the health outcomes of those living with the virus. Here, our main concern is to conceptualize the dynamical behavior of the system and to examine the solution pathways with the variation of different input factors of the system numerically. Other analyses of the system will be considered in our future research. In this study, we showed the phase portrait, chaos, and the tracking paths in order to comprehend how various elements influence the system. To do this, we perform numerous simulations to understand the role of input factors in the system.
In Figure 1 and Figure 2, the impact of the fractional parameter on the dynamics of HIV infection has been visualized. The fractional values are taken to be 0.95 and 0.75 in these Figures, respectively. We noticed that this parameter has an attractive impact on the dynamics of the system of the HIV infection. The chaotic nature of the system has been highlighted in Figure 3, Figure 4, Figure 5 and Figure 6. In Figure 3, we conceptualize the chaotic nature of the system by changing the value of the input factor from 1.0 to and . The chaos of the system is also highlighted in Figure 4 by assuming the input parameter , , while in Figure 5, we considered , , and . These results are obtained by changing the initial values of the state-variable. The chaos in the system is characterized by sensitive dependence on initial conditions, which means that small differences in initial conditions can lead to large differences in the behavior of the system over time. These graphs show that the system exhibits a strong chaotic phenomena, which can be managed using these input settings. The oscillatory and chaotic characteristics of the suggested system are intimately linked by the non-linearity of the system. Policymakers are urged to utilize the fractional parameter, since it has been shown to have a significant impact on the system. The importance of input factors on the output of the system has been shown in Figure 7, Figure 8 and Figure 9. In these simulations, we conceptualize the tracking path behavior with the variation of input factors. We noticed how these factors affect the solution pathways of the system.
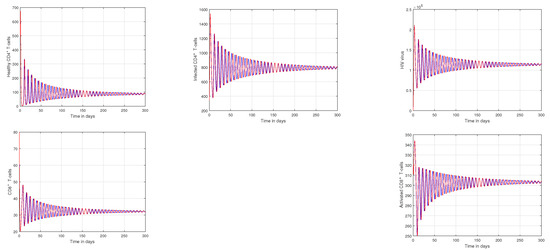
Figure 1.
Plotting the tracking paths of healthy CD4 T-cells, infected CD4 T-cells, HIV virus, CD8 T-cells, and activated CD8 T-cells of HIV infection for different fractional parameter values, where the blue curve signifies and the red curve signifies .
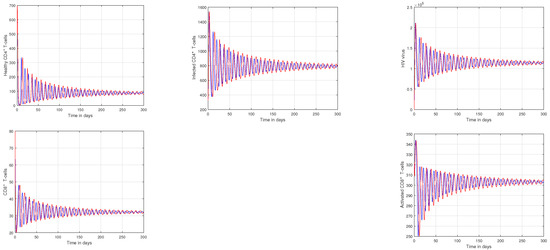
Figure 2.
Plotting the tracking paths of healthy CD4 T-cells, infected CD4 T-cells, HIV virus, CD8 T-cells, and activated CD8 T-cells of HIV infection for different fractional parameter values, where the blue curve signifies and the red curve signifies .
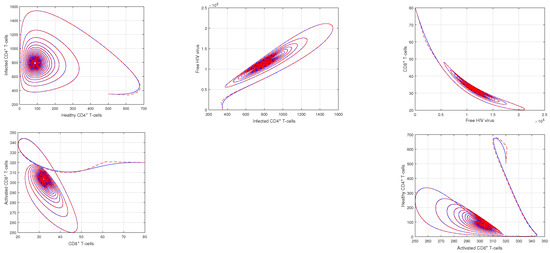
Figure 3.
Illustration of the the dynamical behavior of different cells to show the phase portrait of healthy CD4 T-cells vs. infected CD4 T-cells, infected CD4 T-cells vs. HIV virus, HIV virus vs. CD8 T-cells, CD8 T-cells vs. activated CD8 T-cells, and activated CD8 T-cells vs. healthy CD4 T-cells with the input values and .
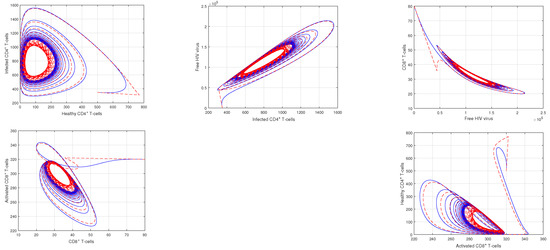
Figure 4.
Illustration of the the dynamical behavior of different cells to show the phase portrait of healthy CD4 T-cells vs. infected CD4 T-cells, infected CD4 T-cells vs. HIV virus, HIV virus vs. CD8 T-cells, CD8 T-cells vs. activated CD8 T-cells, and activated CD8 T-cells vs. healthy CD4 T-cells with the input values , and .
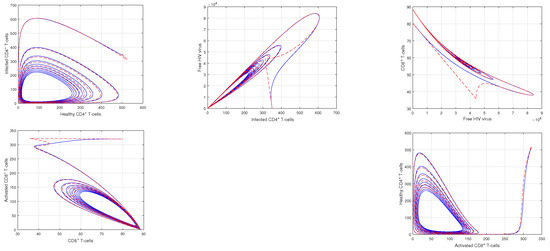
Figure 5.
Illustration of the dynamical behavior of different cells to show the phase portrait of healthy CD4 T-cells vs. infected CD4 T-cells, infected CD4 T-cells vs. HIV virus, HIV virus vs. CD8 T-cells, CD8 T-cells vs. activated CD8 T-cells, and activated CD8 T-cells vs. healthy CD4 T-cells with the input values , and .
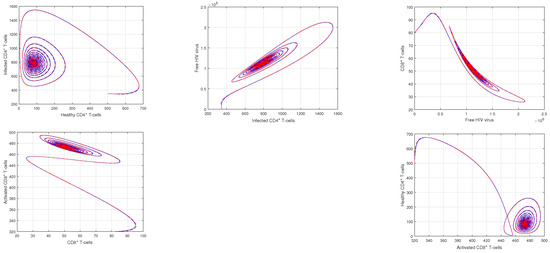
Figure 6.
Illustration of the the dynamical behavior of different cells to show the phase portrait of healthy CD4 T-cells vs. infected CD4 T-cells, infected CD4 T-cells vs. HIV virus, HIV virus vs. CD8 T-cells, CD8 T-cells vs. activated CD8 T-cells, and activated CD8 T-cells vs. healthy CD4 T-cells with the input values , and .

Figure 7.
Representation of the time series of the CD8 T-cells and activated CD8 T-cells with the variation of input factor , where the curves for , and 1600 are indicated by black, red, green, and blue, respectively.

Figure 8.
Representation of the tracking path of the CD8 T-cells and activated CD8 T-cells with the variation of , where the curves , and are indicated by black, red, green, and blue, respectively.
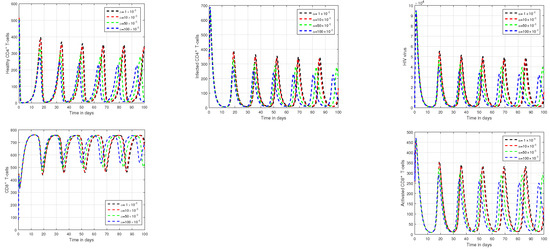
Figure 9.
Illustration of the tracking paths of healthy CD4 T-cells, infected CD4 T-cells, HIV virus, CD8 T-cells, and activated CD8 T-cells of HIV infection with the variation of input factor , i.e., , and .
Chaos theory focuses on non-linear processes that are challenging to predict or control. The chaotic nature lead to the system instability, and therefore, it is valuable to find out the critical factors responsible for the chaos of the system. It has been observed that the initial conditions of state variables and the non-linearity of the system are the main reasons of this irregular nature of the system. This is vital because they offer important knowledge on the HIV infection mechanism. The system’s chaotic behavior serves as an example of how even minor disturbances may cause substantial systemic alterations. These disorganized charts illustrate the system’s instability and vulnerability to initial circumstances, which renders it unpredictable. According to our research, the non-linearity of the system considerably affects oscillation and chaos, which are both significantly amplified.
6. Concluding Remarks
Mathematical models for HIV and the immune system can help researchers and clinicians to better understand the dynamics of the disease, predict the impact of interventions, and develop new strategies for the management of the infection. In this study, we constructed a novel model for the in vivo dynamics of HIV infection. The interaction of HIV viruses with both HIV-negative and HIV-positive T-cells was considered in our system. The recommended system is discussed in terms of Liouville–Caputo and Atangana–Baleanu fractional operators for more accurate findings. The solution routes and chaotic behavior of HIV infection are displayed with the help of numerical methods. We performed numerous simulations to mimic the system’s most crucial parameters. The solution pathways of the system has been shown numerically. The fractional parameter is found to be sensitive to the system outputs. We have shown a strong relationship between chaos and oscillatory behavior. The impact of input factors has been conceptualized with the help of numerical results and findings. In a future study, we will enhance the model and demonstrate how delay affects the dynamics of HIV infection. In order to demonstrate the effects of immunization and treatment on the system, the model will also be upgraded to include the effect of the therapeutic approach that can restore immune system health and reduce the risk of opportunistic infections and other complications.
Author Contributions
Conceptualization, A.J., H.M.S., A.K., R.J. and Y.S.H.; Data curation, H.M.S.; Funding acquisition, Y.S.H.; Investigation, A.J., H.M.S., A.K., P.O.M., R.J. and Y.S.H.; Methodology, A.J.; Project administration, P.O.M.; Resources, A.J.; Software, A.K.; Supervision, H.M.S. and R.J.; Validation, A.K. and P.O.M.; Visualization, Y.S.H.; Writing—original draft, A.J., A.K. and P.O.M.; Writing—review and editing, R.J. and Y.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The researchers would like to acknowledge the Deanship of Scientific Research, Taif University for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mbogo, W.R.; Luboobi, L.S.; Odhiambo, J.W. Stochastic Model for In-Host HIV Dynamics with Therapeutic Intervention. Int. Sch. Res. Not. 2013, 2013, 103708. [Google Scholar]
- United Nations International Children’s Emergency Fund, Joint United Nations Programme on HIV and AIDS and World Health Organization (2011) Global HIV/ AIDS Response: Epidemic Update and Health Sector Progress towards Universal Access: Progress Report 2011; World Health Organization: Geneva, Switzerland, 2011.
- World Health Organization. World Health Statistics 2010; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Jones, E.; Roemer, P.; Raghupathi, M.; Pankavich, S. Analysis and Simulation of the Three-Component Model of HIV Dynamics. SIAM Undergrad. Res. Online 2013, 7, 89–106. [Google Scholar] [CrossRef]
- Adams, B.; Banks, H.; Davidian, M.; Kwon, H.D.; Tran, H.; Wynne, S.; Rosenberg, E. HIV Dynamics: Modeling, Data Analysis, and Optimal Treatment Protocols. J. Comput. Appl. Math. 2015, 184, 10–49. [Google Scholar] [CrossRef]
- Alizon, S.; Magnus, C. Modelling the Course of an HIV Infection: Insights from Ecology and Evolution. Viruses 2012, 4, 1984–2013. [Google Scholar] [CrossRef]
- Arruda, E.F.; Dias, C.M.; De Magalhaes, C.V.; Pastore, D.H.; Thomé, R.C.; Yang, H.M. An Optimal Control Approach to HIV Immunology. Appl. Math. 2015, 6, 1115–1130. [Google Scholar] [CrossRef]
- Chandra, P. Mathematical Modeling of HIV Dynamics: In Vivo. Indian Math. Soc. Math.—Stud. India 2009, 78, 7–27. [Google Scholar]
- Rivadeneira, P.S.; Moog, C.H.; Stan, G.B.; Costanza, V.; Brunet, C.; Raffi, F.; Ferrfé, V.; Mhawej, M.J.; Biafore, F.; Ouattara, D.A. Mathematical Modeling of HIV Dynamics after Antiretroviral Therapy Initiation: A Clinical Research Study. AIDS Res. Hum. Retroviruses 2014, 30, 831–834. [Google Scholar] [CrossRef]
- Wodarz, D.; Nowak, M.A. Mathematical Models of HIV Pathogenesis and Treatment. BioEssays 2002, 24, 1178–1187. [Google Scholar] [CrossRef]
- Chen, S.B.; Rajaee, F.; Yousefpour, A.; Alcaraz, R.; Chu, Y.M.; Gómez-Aguilar, J.F.; Bekiros, S.; Aly, A.A.; Jahanshahi, H. Antiretroviral therapy of HIV infection using a novel optimal type-2 fuzzy control strategy. Alex. Eng. J. 2021, 60, 1545–1555. [Google Scholar] [CrossRef]
- Gul, N.; Bilal, R.; Algehyne, E.A.; Alshehri, M.G.; Khan, M.A.; Chu, Y.M.; Islam, S. The dynamics of fractional order Hepatitis B virus model with asymptomatic carriers. Alex. Eng. J. 2021, 60, 3945–3955. [Google Scholar] [CrossRef]
- Chu, Y.M.; Ali, A.; Khan, M.A.; Islam, S.; Ullah, S. Dynamics of fractional order COVID-19 model with a case study of Saudi Arabia. Results Phys. 2021, 21, 103787. [Google Scholar] [CrossRef]
- Duffin, R.P.; Tullis, R.H. Mathematical Models of the Complete Course of HIV Infection and AIDS. Comput. Math. Meth. Med. 2002, 4, 215–221. [Google Scholar] [CrossRef]
- Apenteng, O.O.; Osei, P.P.; Ismail, N.A.; Chiabai, A. Analysing the impact of migration on HIV/AIDS cases using epidemiological modelling to guide policy makers. Infect. Dis. Model. 2022, 7, 252–261. [Google Scholar] [CrossRef]
- Mallick, U.K.; Rahman, A.; Biswas, M.H.A.; Samsuzzoha, M.; Roy, S.K. An Optimal Immunotherapeutic Treatment of HIV Infections to Regain the Targeted CD4+ T Cell Count: A Boundary Value Problem Approach. J. Appl. Nonlinear Dyn. 2023, 12, 39–51. [Google Scholar] [CrossRef]
- Gandhi, R.T.; Bedimo, R.; Hoy, J.F.; Landovitz, R.J.; Smith, D.M.; Eaton, E.F.; Lehmann, C.; Springer, S.A.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society–USA Panel. JAMA 2023, 329, 63–84. [Google Scholar] [CrossRef]
- Culshaw, R.V.; Ruan, S.; Spiteri, R.J. Optimal HIV Treatment by Maximising Immune Response. J. Math. Biol. 2004, 48, 545–562. [Google Scholar] [CrossRef]
- Zhou, X.; Song, X.; Shi, X. A differential equation model of HIV infection of CD4+ T-cells with cure rate. J. Math. Anal. 2008, 342, 1342–1355. [Google Scholar] [CrossRef]
- Mobisa, B.; Lawi, G.O.; Nthiiri, J.K. Modelling In Vivo HIV dynamics under combined antiretroviral treatment. J. Appl. Math. 2018, 2018, 8276317. [Google Scholar] [CrossRef]
- Arshad, S.; Baleanu, D.; Bu, W.; Tang, Y. Effects of HIV infection on CD4+ T-cell population based on a fractional-order model. Adv. Differ. Equ. 2017, 2017, 92. [Google Scholar] [CrossRef]
- Vazquez-Leal, H.; Hernandez-Martinez, L.; Khan, Y.; Jimenez-Fernandez, V.M.; Filobello-Nino, U.; Diaz-Sanchez, A.; Herrera-May, A.L.; Castaneda-Sheissa, R.; Marin-Hernandez, A.; Rabago-Bernal, F.; et al. Multistage HPM applied to path tracking damped oscillations of a model for HIV infection of CD4+ T cells. Br. J. Math. Stat. Psychol. 2014, 4, 1035–1047. [Google Scholar] [CrossRef]
- Perelson, A.S.; Nelson, P.W. Mathematical analysis of HIV-1 dynamics in vivo. SIAM Rev. 1999, 41, 3–44. [Google Scholar] [CrossRef]
- Perelson, A.S. Modeling the interaction of the immune system with HIV. In Mathematical and Statistical Approaches to AIDS Epidemiology; Lecture Notes in Biomathematics; Springer: Berlin/Heidelberg, Germany, 1989; Volume 83. [Google Scholar]
- Pinto, C.M.A.; Carvalho, A.R.M.; Baleanu, D.; Srivastava, H.M. Efficacy of the post-exposure prophylaxis and of the HIV latent reservoir in HIV infection. Mathematics 2019, 7, 515. [Google Scholar] [CrossRef]
- Srivastava, H.M. Some parametric and argument variations of the operators of fractional calculus and related special functions and integral transformations. J. Nonlinear Convex Anal. 2021, 22, 1501–1520. [Google Scholar]
- Srivastava, H.M. An introductory overview of fractional-calculus operators based upon the Fox-Wright and related higher transcendental functions. J. Adv. Engrg. Comput. 2021, 5, 135–166. [Google Scholar] [CrossRef]
- Atangana, A.; Baleanu, D. New fractional derivatives with nonlocal and non-singular kernel: Theory and application to heat transfer model. J. Therm. Sci. 2016, 20, 763–769. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).