1. Introduction
Infectious diseases have traditionally been a major enemy of human life and health, and have caused tens of millions of deaths in the past hundred years. For example, severe acute respiratory syndrome coronavirus 2 (abbreviated as SARS-CoV-2), which started its epidemic at the end of 2019, has caused 6.5 million deaths in the world [
1]. In the larger family of infectious diseases, vector-borne infectious diseases occupy a large proportion, caused by viruses, bacteria, protozoa, or rickettsia, and are primarily transmitted by disease-transmitting biological agents (anthropoids), called vectors, which carry the disease without getting it themselves. Typical vector-borne diseases include dengue fever virus, West Nile virus, rabies, malaria, and yellow fever virus, to name a few, which have taken away many lives since ancient times.
Mathematical models of vector-borne diseases began with the pioneering work of Dr. Ross [
2] and then modified by MacDonald [
3]. Since then, various dynamical models of vector-borne infectious diseases have emerged. For instance, Abdelrazec et al. [
4] developed a compartment model, which is described by some ordinary differential equations to study the impacts of corvids and non-jays in the transmission of West Nile virus during the single-season mosquito–bird cycle; analyzed the existence and stability of the equilibria; and discussed the existence of backward bifurcation and the roles of corvids and non-corvids in the spread of virus. Garba et al. [
5] presented a dengue fever virus transmission dynamical model that allows for transmission by exposed humans and mosquitoes, and studied the local asymptotic stability of a disease-free equilibrium and the phenomenon of backward bifurcation. Moreover, Simoy et al. [
6] developed an SIS-SI model with active/inactive vectors to compare with the dynamics of the Ross–Macdonald model. Other relevant studies have been continuing [
7,
8,
9,
10,
11,
12,
13].
As we all know, nothing in the world develops overnight. Just as it takes a certain amount of time for a pathogen to go from invading a vivo to showing symptoms and being able to infect other hosts (or vectors), it also takes some time. This period is called the incubation delay of this disease. Of course, there are also other forms of delays in the evolution of disease transmission, for example, immune delay, recovery delay, relapse delay, and so on. From the perspective of mathematical modeling, the delay differential equations give a precise description of these delay phenomena. In recent years, a few mathematical models with delays have been introduced to consider the effects of delays on the transmission of infectious diseases [
14,
15,
16,
17,
18]. In the case of vector-borne diseases, it is more practical to consider the delays, as many vectors carrying the virus reach the end of their lives before they can infect the host. In Ref. [
19], Cai et al. extend the Ross–Macdonald model to a multi-delay model to describe the extrinsic and intrinsic incubation delays, and showed that Hopf bifurcation did not occur in their model when the delays varied. Ding et al. [
20] introduced a dynamic Schistosomiasis model incorporating five delays to analyze the effects of multi-delays on the stability of disease-free equilibrium. By constructing suitable Lyapunov functionals, Tian et al. [
21] obtained the global asymptotical stability of the disease-free and endemic equilibria of a vector-borne disease model with two distributed delays and nonlinear incidence rate. Zhang et al. [
22] proposed a Schistosomiasis model with three delays, and obtained the basic reproduction number and the global dynamics of this model. Here are just a few examples.
Recently, the rapid development of the Internet has enabled media coverage to play an increasingly important role in the transmission and control of infectious diseases. People learn about the epidemic situation, prevention and control methods, diagnostic criteria, and other related contents through the media, which makes the behavior of susceptible or infected people change to some extent, thus affecting the rate of human-to-human contact and the rate of transmission of pathogens [
23,
24,
25,
26,
27,
28,
29]. In order to portray the effects of media coverage on infectious disease, some references have been made by introducing a new state variable
M. For instance, Pawelek et al. [
30] assumed that the transmission rate of pathogens
is reduced by a factor
due to a change in public behavior after reading tweets about influenza, where
denotes how effectively disease messages affect transmission rate. Song et al. [
31] constructed a SEIS-M model with two delays, and investigated the local and global bifurcations by taking the summation of two delays as the bifurcation parameter, and showed that the higher level of media influences the lower the number of infected individuals. Very recently, considering the influence of media on individual behaviors, Hu et al. [
32] proposed a novel COVID-19 transmission model with personal protective awareness, investigated the existence and stability of equilibria, and discussed the influence of protective awareness on the spread and control of this disease.
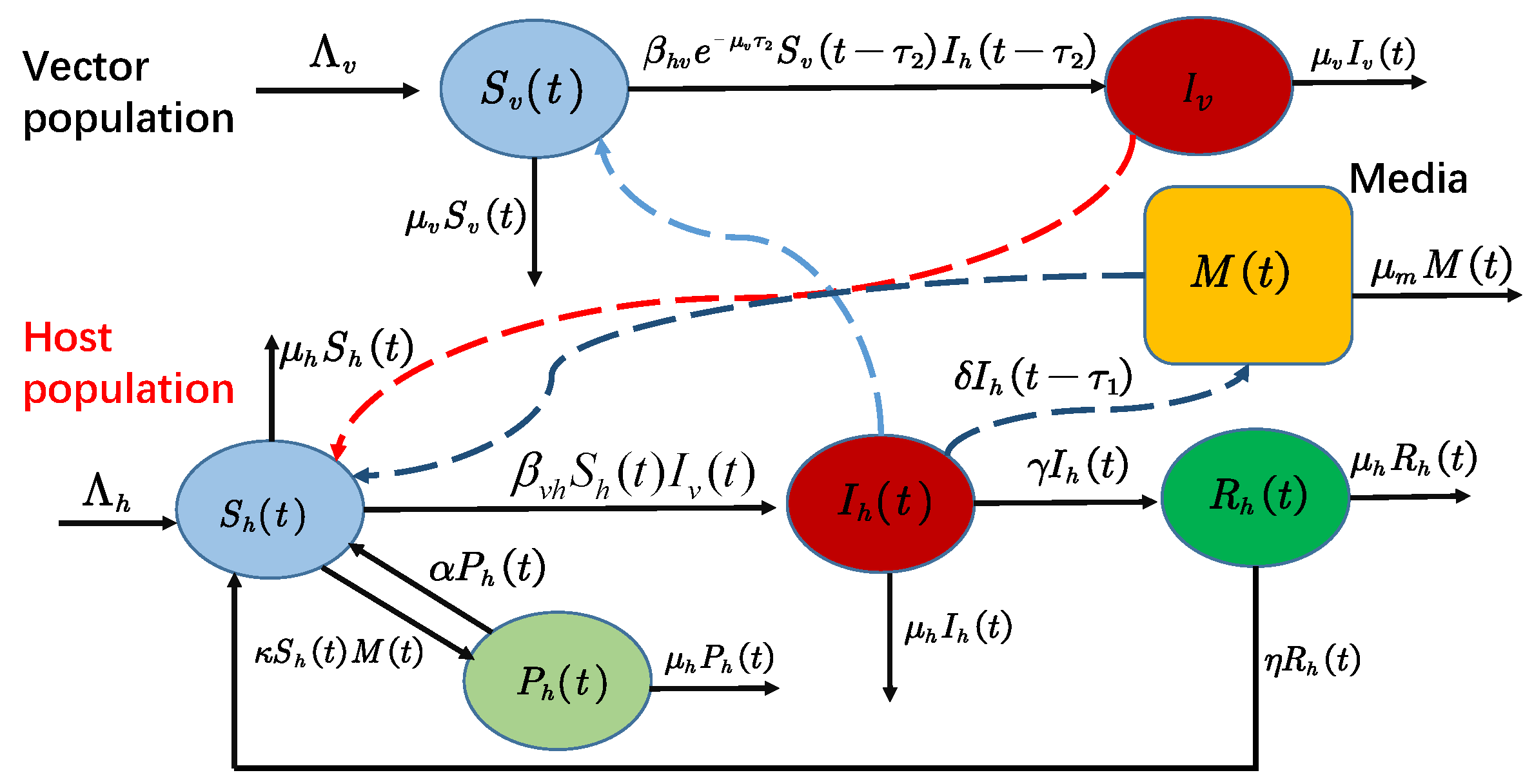
Based on the above discussion, we propose, in this paper, a novel vector-borne model with two delays and the awareness of personal protection, and discuss the impacts of these factors on the control and distribution of disease. The paper is organized as follows: in
Section 2, a model is formulated, and the basic properties of this model are given. In
Section 3, the basic reproduction number, the stability of the disease-free equilibrium, and the existence of the endemic equilibrium are obtained. In
Section 4, the local asymptotical stability of the endemic equilibrium and the existence of the Hopf bifurcation are discussed by using the Hurwitz bifurcation theory. Further, the direction and stability of the Hopf bifurcation is studied in
Section 5. Some numerical examples are presented in
Section 6 to explain the main theoretical results, and a brief conclusion is presented in the last section.
2. Model Formulation and Preliminaries
Let
,
,
, and
denote the quantities of unconsciously susceptible, infected, recovered, and consciously susceptible hosts at time
t, respectively. For the vector population,
and
represent the quantities of susceptible and infected individuals at time
t, respectively. Therefore, the total sizes of host and vector populations at time
t are
, and
, respectively.
and
are the recruitment rates of hosts and vectors,
and
denote the natural death rates of them, respectively;
and
are the probabilities that a bite by an infected vector results in transmission to the host, and a bite results in transmission of the infected host to a susceptible vector;
denotes the recover rate of from infected hosts to recovered hosts;
is the rate at which the resistant become unconscious susceptible again. In order to emphasize the impact of media coverage on the spread of the infectious disease, we use the method in [
30,
31], that is, let
represent the cumulative density of various media at time
t,
is the implementation rate of media coverage, and the media influence dissipates at the rate of
. As a result of media, some unconscious susceptible will increase their awareness of self-protection and transform into conscious susceptible at the rate of
(that is,
), while conscious susceptible transfers to unconscious susceptible at the rate of
due to memory loss or other factors (that is,
). In addition, delay
is introduced to describe the delay between media reports and infectious disease screening and reporting of cases, and delay
is given to describe the incubation period of the virus in vectors on disease transmission. Based on the classical vector-borne disease models (see [
5,
6,
7,
13,
14,
19,
33] for more detail), the relationship between pathogens, hosts, vectors, and media coverage is shown in
Figure 1. Based on the above assumption, a model reads as
The initial condition of Model (1) is
with
being non-negative continuous functions from
to
and
.
The following Lemma 1 is on the existence of global positive solutions of Model (1), which is necessary for our discussion of its dynamics.
Lemma 1. The solution of Model (1)
starting from the initial value (2)
is existent, unique, and non-negative on , which satisfiesFurther, if all initial values are positive, then the solution remains positive on . Proof. Model (1) admits a unique solution
with the initial condition (2) on
by the basic theory of the functional differential equations [
34], where
. Now, we verify, firstly, all components of the solution are positive on interval
, while all initial values are positive. If the conclusion is invalid, then there exists a positive constant
such that
and
,
,
,
,
,
,
for all
. Then, there are seven possible cases:
;
;
;
;
;
;
and
. Now, we discuss only Cases
,
, and
, the others are similar to these three.
- (i)
If
, then one has
due to
for
. However, from the
S-equation of Model (1), it follows that
due to the fact that
and
. This is a contradiction. Therefore,
for
.
- (ii)
If
, this yields the
-equation of Model (1) that
This contradicts ; that is, for .
- (v)
If
, then one gets
due to
for
. However, from the
M-equation, we can obtain
due to
for
. This also contradicts
. Thus,
for
.
To sum up the above discussion, we can find that all components of the solution are positive on interval , while all initial values are positive. Further, from the continuous dependence of the solution on the initial value, we can see that the solution , , , , , , of Model (1) starting from the initial value (2) is existent, unique, and non-negative on .
Finally, we focus on the global existence of solutions of Model (1). Adding the hosts equations and the last two equations of Model (1), respectively, one has
Then, and are bounded in , and the bounds can be chosen to be and . Further, it yields from the M-equation that . This implies that is bound in interval . Thus, again, utilizing the basic theory of functional differential equations, the solution is existent, unique, non-negative, and bounded on . The proof is finished. □
According to the limit theory of dynamical systems [
35], the dynamics of Model (1) are equivalent to the following model:
From the proof of Lemma 1,
is a positively invariant, where
Therefore, in the rest of this paper, we study the dynamics of Model (3) on .
3. Stability of Disease-Free Equilibrium
It is obvious that Model (3) admits the disease-free equilibrium
. Define the basic reproduction number as
Further, the endemic equilibrium
of (3) satisfies
By direct calculation,
where
is the positive root of the quadratic equation
where
Let
be two roots of (4). From the expressions, we have that
,
. Further,
if
, and
if
. It is easy to see that
and
are negative if
, and
is positive and
is negative if
. From the relationship between the roots of Equation (4) and the equilibrium of Model (3), the following result is valid.
Theorem 1. There is always the disease-free equilibrium to Model (3); however, if , Model (3) admits a unique endemic equilibrium in addition to .
In order to investigate the local stability of any equilibrium
of Model (3), we linearize this model and obtain the characteristic equation at
as
where
It is clear that the characteristic equation at
becomes
where
. Note that three roots of (6) are
,
,
. So, the stability of
is given by the real part of roots of the following equation
Obviously,
and (7) reads as, for
,
The roots of (8) have negative real parts if
. Further, since the roots of (7) depend on
continuously (see for [
36] more details), then the real part of the root only becomes positive by crossing through the imaginary axis in the complex plane. Note that
is not a root, amd the roots of (7) can cross the imaginary axis, that is,
. Now, substituting
into (8) and separating the real and imaginary parts, one has
Squaring and taking the sum of the above two equations, one can easily obtain
From Expression (9), it is found that the equation has no positive roots for . Therefore, all of the roots of (7) are in the left-half plane for all if . That is, the disease-free equilibrium of Model (3) is local asymptotically stable for this case. However, for , Equation (9) has a positive root, which implies is unstable. By the above discussion, the following result is valid.
Theorem 2. The disease-free equilibrium of Model (3) is locally asymptotically stable if , and which is unstable for .
4. Stability of the Endemic Equilibrium and Bifurcatioan Analysis
From (5), the characteristic equation of Model (3) at
reads as
where
, and
Here, for the two different delays and , the stability of the endemic equilibrium can be categorized as follows: ; and ; and ; and ; and .
From the Jacobian matrix (5) in this case, the characteristic Equation (10) is equivalent to
with
Obviously,
and
. As the application of Routh–Hurwitz criteria, if
then all roots of (11) have negative real parts; that is, the endemic equilibrium
is locally asymptotically stable.
Summarizing the above discussion, Theorem 3 is obvious.
Theorem 3. If and (12) hold, the endemic equilibrium of Model (3) is locally asymptotically stable.
If
and
, it yields from (5) that the characteristic Equation (10) is
Suppose
is a root of Equation (13), then this separates the real and imaginary parts such that
and
Squaring and adding both (14) and (15) leads to the following equation:
where
Set
in Equation (16), then we obtain
If , then and , Equation (18) has at least one positive root.
Theorem 4. Suppose that the conditions of Theorem 3 hold and . If , then the endemic equilibrium of Model (3) is asymptotically stable for . Furthermore, if for , then Model (3) undergoes the Hopf bifurcation for , where is the positive root of Equation (13)
and Proof. If
, then Equation (18) has at least one positive root
. So, Equation (16) admits a positive root
. Then, the characteristic Equation (13) has a pair of pure imaginary roots
. Further, from Equations (14) and (15),
is a function of
, and
Define
. If the conditions of Theorem 3 hold and
,
keeps its stability from the Butler Lemma (see [
37] for more details). In the following, we verify the transversality condition
. Differentiating Equation (13), we obtain
with
From (13) and (16), it yields that
If
for
, then
that is, the transversality condition holds. So, Model (3) undergoes the Hopf bifurcation at
for
. □
Theorem 5. Suppose that the conditions of Theorem 3 hold and . If , then the endemic equilibrium of Model (3)
is locally asymptotically stable for . Furthermore, if for , then Model (3)
undergoes the Hopf bifurcation for andwhere is a root of the characteristic equationand The proof is the same as that of Theorem 4, so we omit it.
Theorem 6. Suppose that the conditions of Theorem 3 hold and . If , then there exists such that the endemic equilibrium of Model (3)
is asymptotically stable for . Furthermore, if for , then Model (3)
admits the Hopf bifurcation for , andwhere is a positive root of (24)
; , , , , , and are given in (27)
; and . Proof. Suppose that
is fixed in
and
. Let
be a root of Equation (10), and separate the real and imaginary parts
and
The stability at
changes when Equation (10) has pure imaginary roots. Let
, Equations (20) and (21) become
and
We square and add the above equations to produce the following equation:
where
If
, then
and
, and Equation (24) has at least one positive root
. Therefore, (22) and (23) become
and
with
Since
is a positive root of Equations (24), the characteristic Equation (10) has a pair of pure imaginary roots
at
. Now, from Equations (25) and (26), we obtain
where
and
, and
. If the conditions of Theorem 3 hold, and
,
remains stable from the Butler lemma [
37].
Differentiating Equations (22) and (23) with respect to
, we obtain
with
From (28) and (29), there is
So the transversality condition holds, Model (3) has the Hopf bifurcation when . □
Following a similar discussion as in Case , we can obtain the following result.
Theorem 7. Suppose that the conditions of Theorem 3 hold and . If , then there exists such that the endemic equilibrium of Model (3)
is locally asymptotically stable for . Furthermore, if for , then Model (3)
exists the Hopf bifurcation for , andwhere is a positive root of the equationwithand The proof of Theorem 7 is the same as that of Theorem 6, we omit it.
5. Direction and Stability of Hopf Bifurcation
In the last section, the existence of the Hopf bifurcation is obtained under some technical conditions. Following the ideas given in [
38], we search for, in this section, the direction and stability of these bifurcations by the normal form method and the center manifold theory. Throughout this section, it is considered that Model (3) undergoes a Hopf bifurcation at equilibrium
for
. Without loss of generality, assume that
.
The transformations
,
,
,
,
and
,
. Still denoting
, then takes Model (3) to
where
, which is the Banach space of continuous functions. In (35),
and
, respectively, are given by
and
with
Therefore, by Reisz representation theorem, matrix components with a bounded variation function
exist such that
For any
, define that
and
Applying the above notations, Model (35) can be rewritten as
where
for
. For
, define
and a bilinear inner product
where
. Then,
and
are adjoint operators. From
Section 4, assume that
are eigenvalues of
and the real parts of the other eigenvalues are strictly negative real parts. So, these are also the eigenvalues of
. Now, we compute the eigenvectors of
and
corresponding to
and
, respectively.
If
is the eigenvector of
corresponding to
, that is,
and
where
and
. Further, it follows that
with
Similarly, if
is the eigenvector of
corresponding to
, then we have
From
,
and (41), one has
Therefore, select that
as
Next, we compute, firstly, the center manifold
at
. To this end, define
On the center manifold
, it yields that
where
z and
are the local coordinates for
in the direction of
and
. For the solution
of System (35) with
, it follows that
The above differential equation can be rewritten as
where
Due to (42) and (43), define that
one can obtain
and
It follows from (44) that
Comparing the coefficients with (44) yields
From (40) and (42), we have
where
This follows from (46) that
for
. Comparing the coefficients with (47), one can obtain that
By Equations (46) and (47) and the definition of
W, comparing the coefficients, one has
This yields from (48) and (49) that
Solving for
, it follows that
And similarly,
where
and
can be computed by the following equations, respectively,
where
,
and
With the above analysis, one can see
in (45) is determined. And from (50) and (51), we have the following values
By using the bifurcation theorem in [
38], the following result is arrived.
Theorem 8. The Hopf bifurcation is supercritical , if ; the bifurcation periodic orbits are stable if . The period of the bifurcation periodic solution increases , if .
6. Numerical Simulation and Discussion
We present, in this section, some numerical examples to explain the main theoretical results and discuss the effects of some important parameters on the distribution of vector-borne disease.
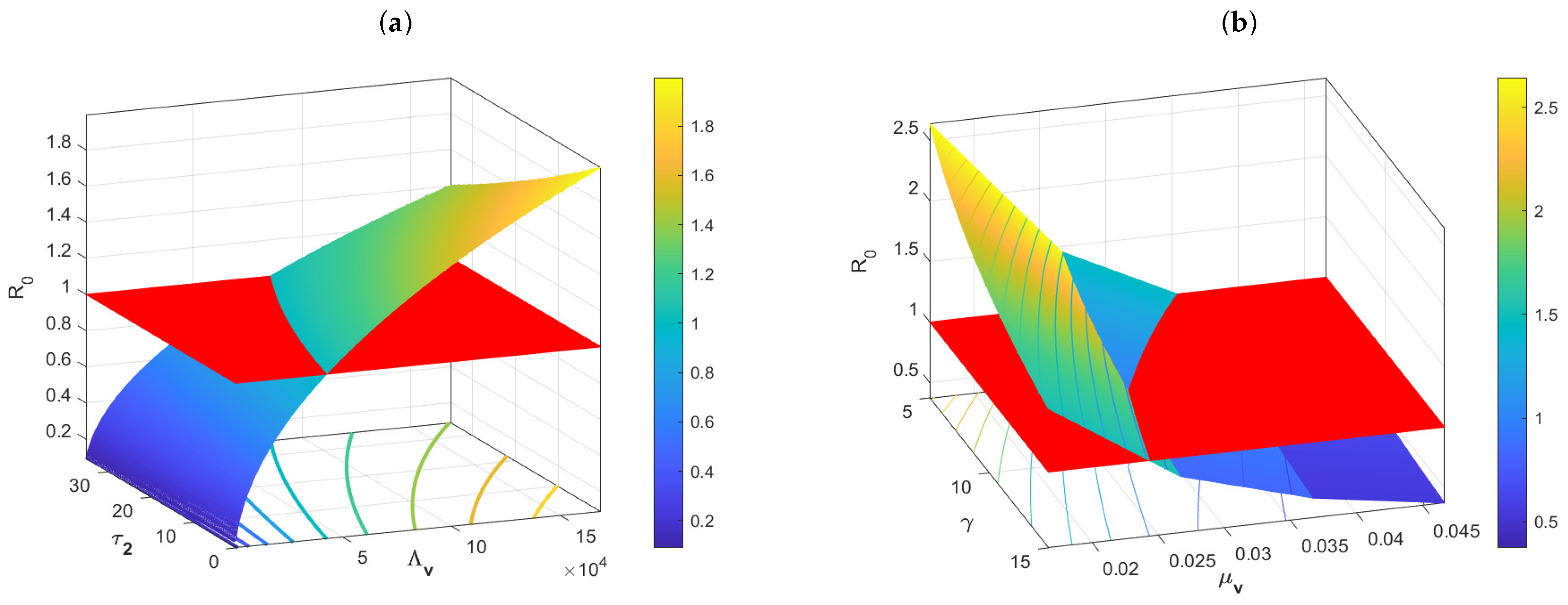
Example 1. The impacts of the main parameters on the basic reproduction number .
The plots in
Figure 2a imply that the incubation period
of the pathogen in the vectors has an inhibitory effect on the spread of the disease, and that ignoring this delay overestimates
of the disease; whereas
is positively correlated with the recruitment rate
of the vector. In addition,
Figure 2b also shows that
is negatively correlated with the death rate of hosts
and the recovery rate
of hosts. This also implies that we can make
less than 1 by decreasing
, increasing
and
. These numerical simulations overlap exactly with the expression for
.
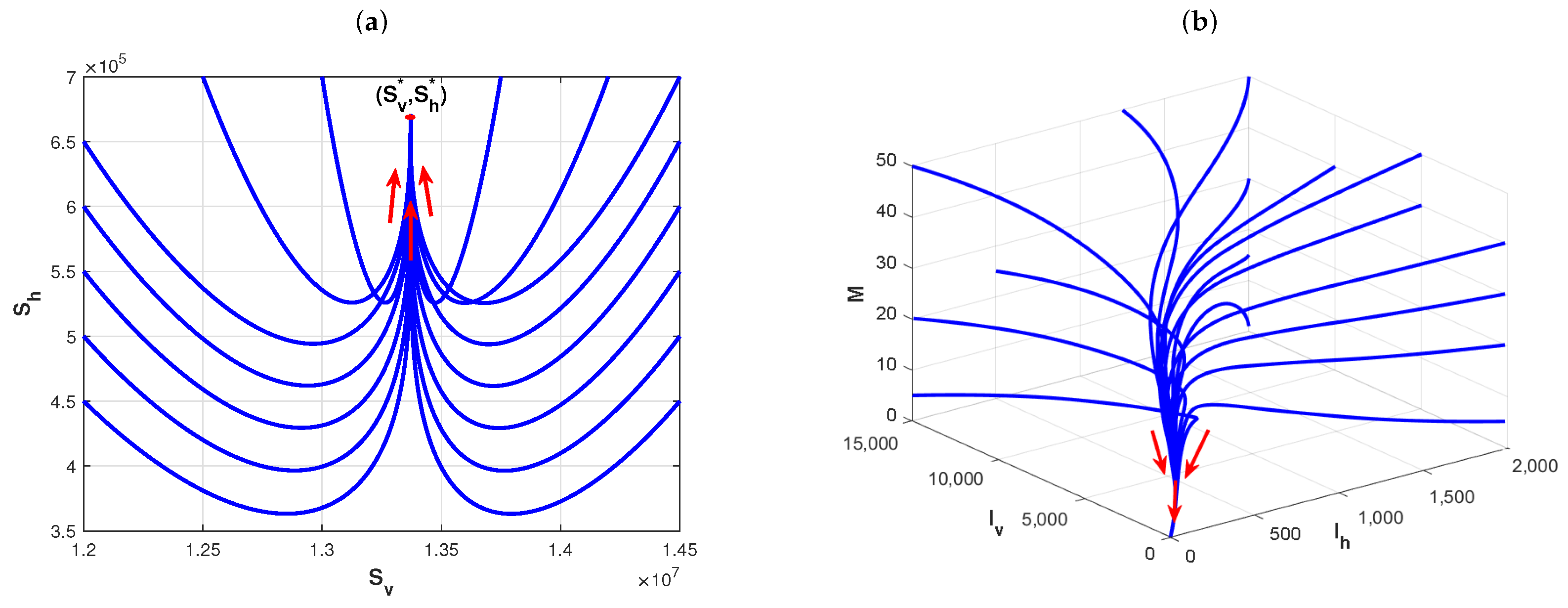
Example 2. The existence and stability of disease-free and endemic equilibria of Model (1).
According to [
7,
14,
19,
20], the basic model parameters are fixed as follows:
,
,
,
,
. If we choose that
,
,
,
,
,
,
, and
. By direct calculation, the basic reproduction number is
in this case. Therefore, from Theorem 2, the disease-free equilibrium
is asymptotically stable, which is shown in
Figure 3a,b. This implies that the disease is ultimately eliminated. However, we’re just changing the values of
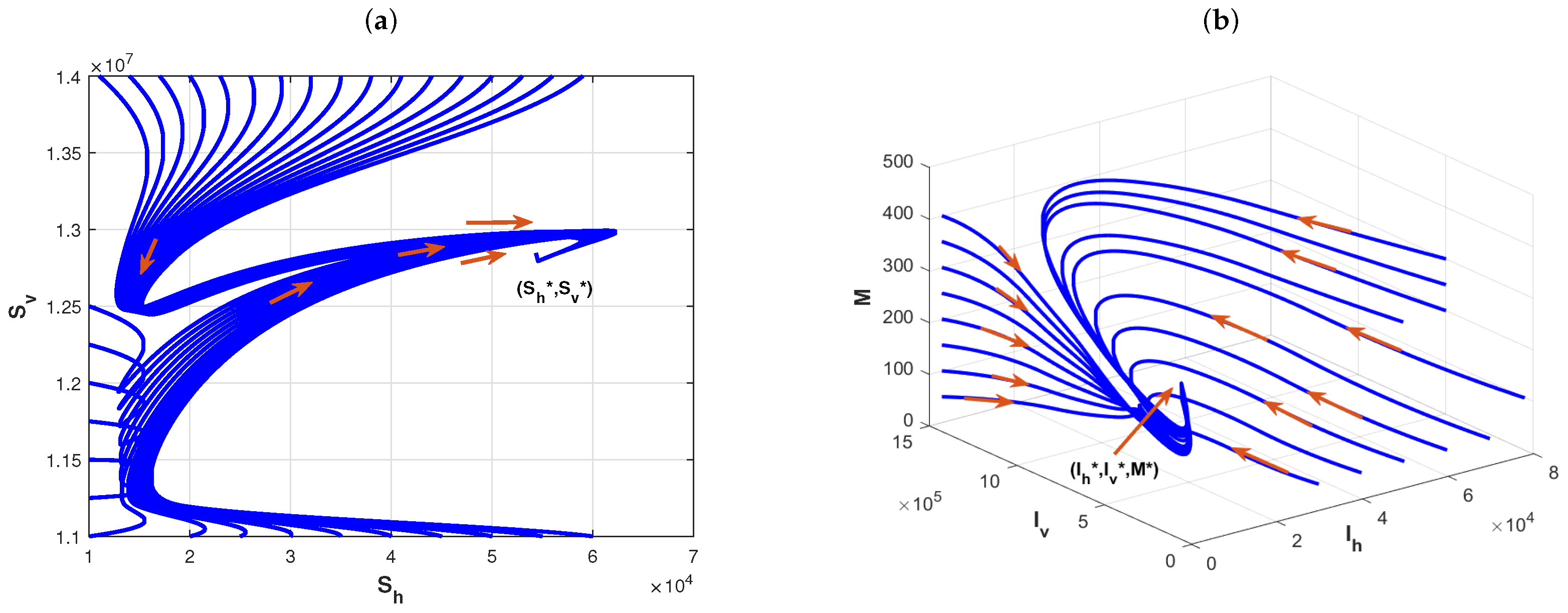
and
as
and
, respectively, and other parameters are fixed as in
Figure 3. By direct calculation, one has
in this case. The plots in
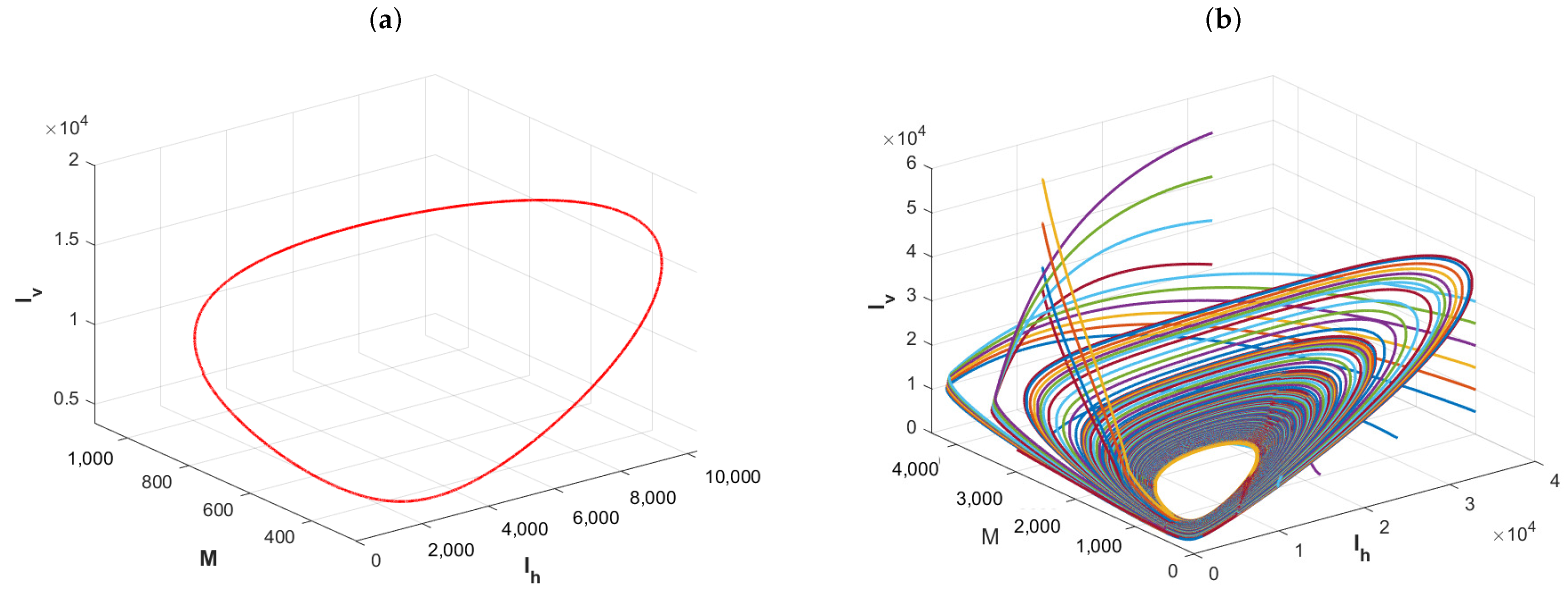
Figure 4a,b show that the endemic equilibrium
of Model (1) is asymptotically stable.
Example 3. The effects of the delay for the distribution of infectious disease.
We fix the basic parameters of the model as follows:
,
,
and
, which determine the sizes of the hosts and vector population. We choose, firstly,
,
,
,
,
,
,
,
and
. In this case,
by the expression of the basic reproduction number. The plot in
Figure 5a shows that Model (1) has a periodic solution, which is verified to be asymptotically stable by the plots of
Figure 5b. Although it yields from the expression for the basic reproduction number
that delay
is independent of
, it can cause the disease to exhibit a cyclic oscillation phenomenon. Further, one changes,
,
, ,
,
,
,
,
,
,
, and
. By the direct calculation, it is clear that
in this case. The plot in
Figure 6a shows that Model (1) admits a periodic solution and the plots in
Figure 6b imply that the periodic solution is asymptotically stable. Numerical simulations imply that the delay
in media coverage and the incubation period
of pathogens in vectors are important factors, and ignoring them underestimates the complexity of vector-borne disease transmission.
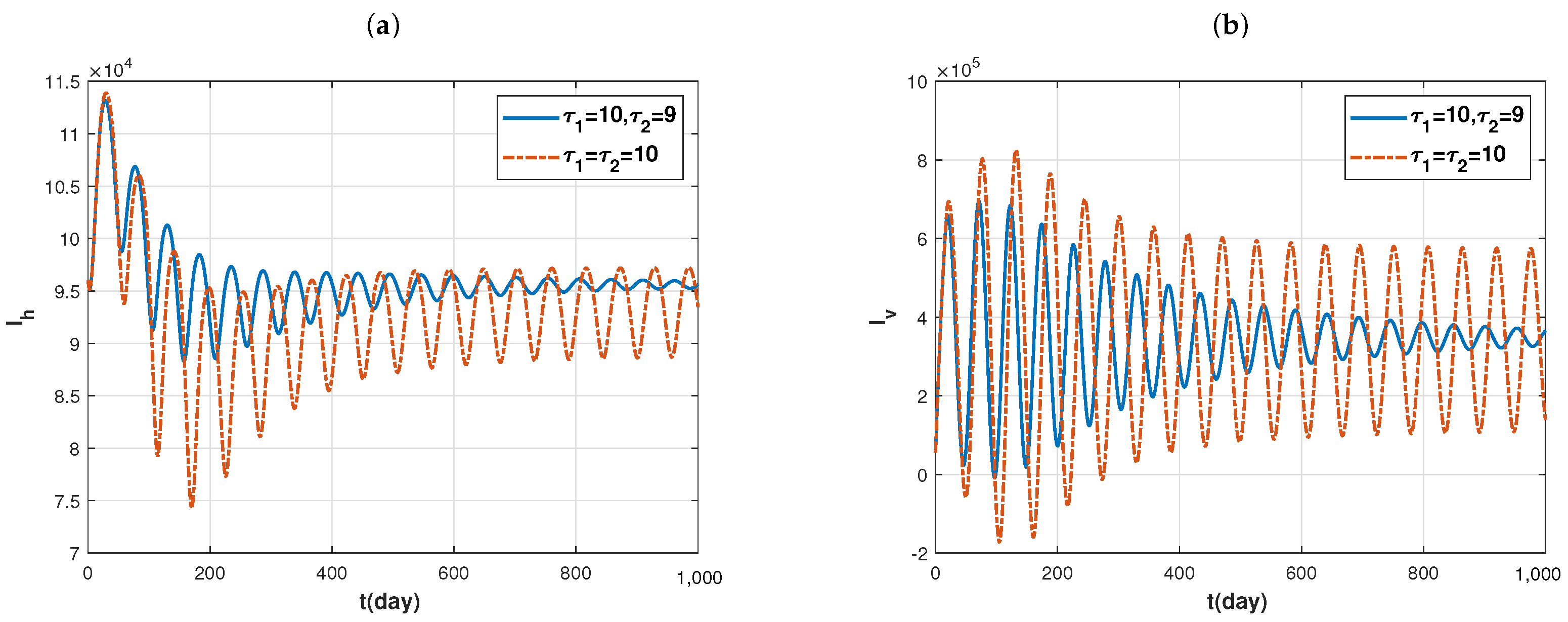
Now, we discuss the distribution of disease under the combined effects of the two delays
and
. To this end, we choose two sets of delay parameters:
and
;
, and other parameters are fixed as in
Figure 6. By direct calculation, we find that the basic reproduction number
is approximately equal to 7.661706 in both cases. However, the trajectories of
and
, that is, infected individuals, are shown by the blue solid lines and red dotted lines in
Figure 7a,b, respectively. It is easy to see that the distributions of infected individuals have a very significant change under both sets of delay parameters. In other words, a small change in the delay will lead to a drastic fluctuation in the number of infected individuals. This is just like the old Chinese saying: every inch of the difference is a thousand miles away. Therefore, it is unreasonable to ignore the delay in the modeling of vector-borne diseases, and the accurate measurement of the incubation period of pathogens in vectors has an important impact on the prevention and control of diseases.
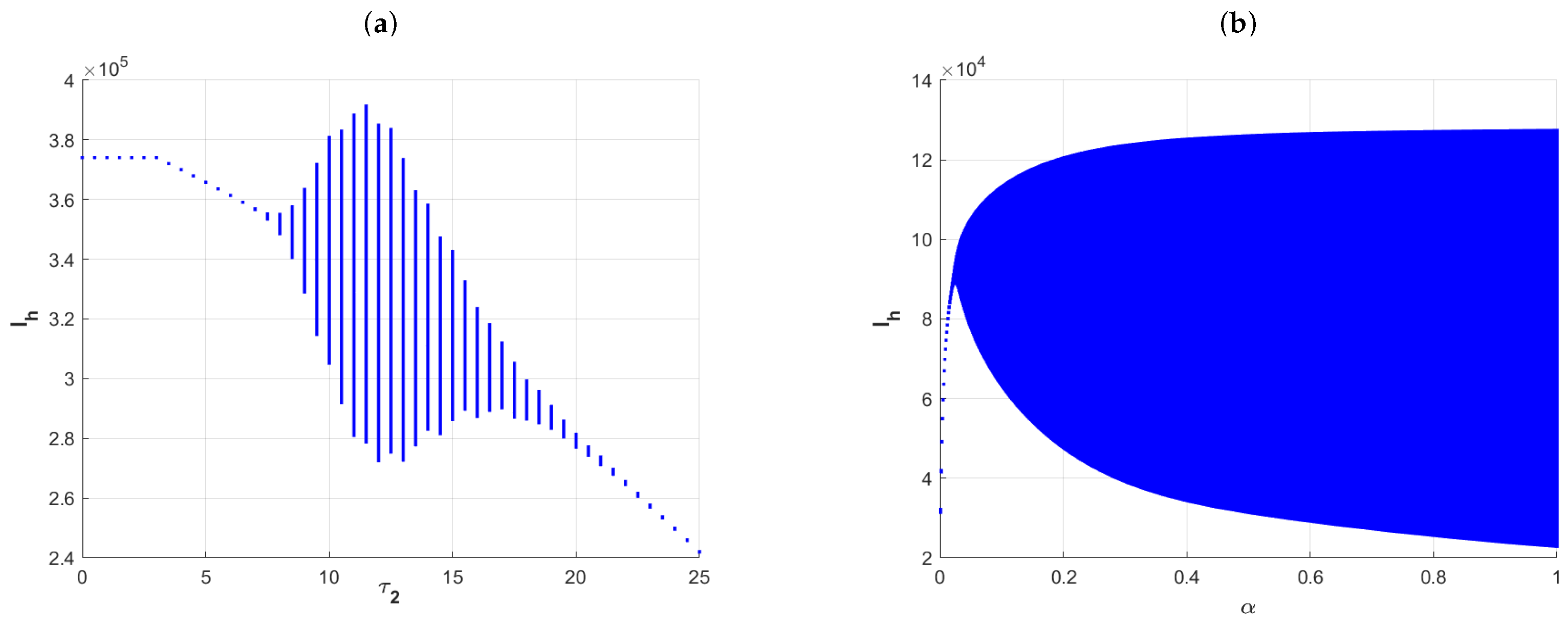
Finally, we consider the effects of the incubation delay of the pathogen
in the vectors and the duration of personal protection awareness (that is,
,
is the rate of conscious susceptible transfers to unconscious susceptible)on the distribution of infected individuals. Here, the parameter
is increased from 0 to 25, the parameter
is increased from 0 to 1, and the other parameters are fixed as in
Figure 7. The plots in
Figure 8a show that Model (1) has an endemic equilibrium while
, whereas when
, the number of infected individuals shows oscillations. This also implies that there is a periodic solution to the model in this case. And when
, the model again has an endemic equilibrium. The plots in
Figure 8b show the effect of
on the distribution of infected individuals, which implies that
has some significant effects on the size of the infected population. Specifically, the model has an endemic equilibrium state when a is very small, i.e., when the duration of personal protection awareness is maintained for a longer period of time, and the size of infected individuals is relatively low. And when
is relatively large, the model has a positive periodic solution, and the amplitude increases with
. Numerical simulations show that rapid media response and a strong personal protection awareness can be effective in curbing the size of disease transmission.
7. Conclusions
As we all know, the incubation period of pathogens in vivo cannot be ignored in the spread of infectious diseases. It is directly related to the size and duration of disease outbreaks. In addition, the degree of harm of infectious diseases is also inextricably linked to the host’s race, living habits, behavioral habits, and knowledge structure. Thanks to the rapid development of the Internet, all kinds of media can more effectively provide people with information on public health and medical science, and disseminate to the public various policies and regulations on health prevention and disease control, so as to improve public health awareness, self-protection awareness and the ability to prevent and control infectious diseases, and curb the spread of diseases. Therefore, quantitatively portraying the incubation period of pathogens and the response speed of the media on disease transmission is one of the current hot issues in the prevention and control of infectious diseases. From the viewpoint of mathematical modeling, we propose a novelty model with multi-delays to discuss the effects of the above factors. In particular, our model also portrays the impact of news media on the personal behavior of susceptible individuals by introducing a compartment of susceptible individuals with personal protection (that is,
, perfect sense of personal protection, which can be found in [
39,
40,
41]), and explores the role of the memory loss for presence awareness in disease prevention and control. This makes our model more consistent with the current characteristics of infectious disease transmission, which is one of the highlights of this article.
The exact expression of the basic reproduction number is obtained, which can be used to determine the existence and stability of disease-free and endemic equilibria. Specifically, when the is less than 1, the model exists only in the disease-free equilibrium, which is locally asymptotically stable, while when is greater than 1, the model exists an unique endemic equilibrium in addition to the disease-free equilibrium. Additionally, we also obtain sufficient conditions for the local asymptotic stability of the endemic equilibrium. It should be especially emphasized here that, with delays as the bifurcation parameter, we also discussed the existence of the Hopf bifurcation, especially the direction and stability of the Hopf bifurcation applying the bifurcation theory. This is the other highlight of this paper.
Some numerical examples are carried out to explain the main theoretical results and investigate the sensitivity of the main parameters to the basic reproduction number. Our numerical simulations also show that the delay
in media coverage has no effect on disease elimination and prevalence, which is consistent with our theoretical results. That is, the basic reproduction number is independent of
. But numerical simulations also show that
affects the size of disease outbreaks (see
Figure 5). In addition, numerical simulations also show that the sizes of the infected vectors and infected hosts fluctuate dramatically even when the basic reproduction number is almost equal under the combined effect of
and
(see
Figure 7). This implies that ignoring or misestimating the magnitude of the delay can affect the accurate prevention and control of vector-borne diseases to varying degrees.
It should be noted that our initial intention is, in this paper, to portray the impact of a timely media response with perfect protection on the distribution of infectious diseases. In particular, in our model, we take into account the effect of forgetting mechanisms on the awareness of personal protection. Modeling vector-borne infections with imperfect protection and the effects of media delays is also an element of our future research. In addition, some factors are also ignored in the mathematical modeling process in order to perform the necessary theoretical analysis. For example, the effect of climate change and uncertainty on vector population size and behavior, the effect of vaccination on disease control, and the effect of spatial heterogeneity and frequent dispersal of host populations in space on the spatiotemporal distribution of disease, to name a few. These are also all worthy of further discussion.