Long-Term Bifurcation and Stochastic Optimal Control of a Triple-Delayed Ebola Virus Model with Vaccination and Quarantine Strategies
Abstract
1. Introduction
2. Stochastic Long-Run Dynamics of Ebola Model
- 1.
- The stationary case ()—that is, ecosystem (3)—admits a single ergodic limiting distribution . In other words, Ebola epidemic persists.
- 2.
- The eradication case ()—that is, the Ebola epidemic—will disappear with full probability.
3. Stochastic Optimal Control Strategies
- A1:
- The isolation of the vulnerable population as well as of infectives is represented by the control measure . This control strategy aims to minimize the value of the transmission coefficient .
- A2:
- The rate of quarantine of the infected population is controlled via the function . This strategy aims to reduce the number of infectives by shifting them into the quarantine class.
4. Parameters Estimation and Curve Fitting
5. Numerical Verification
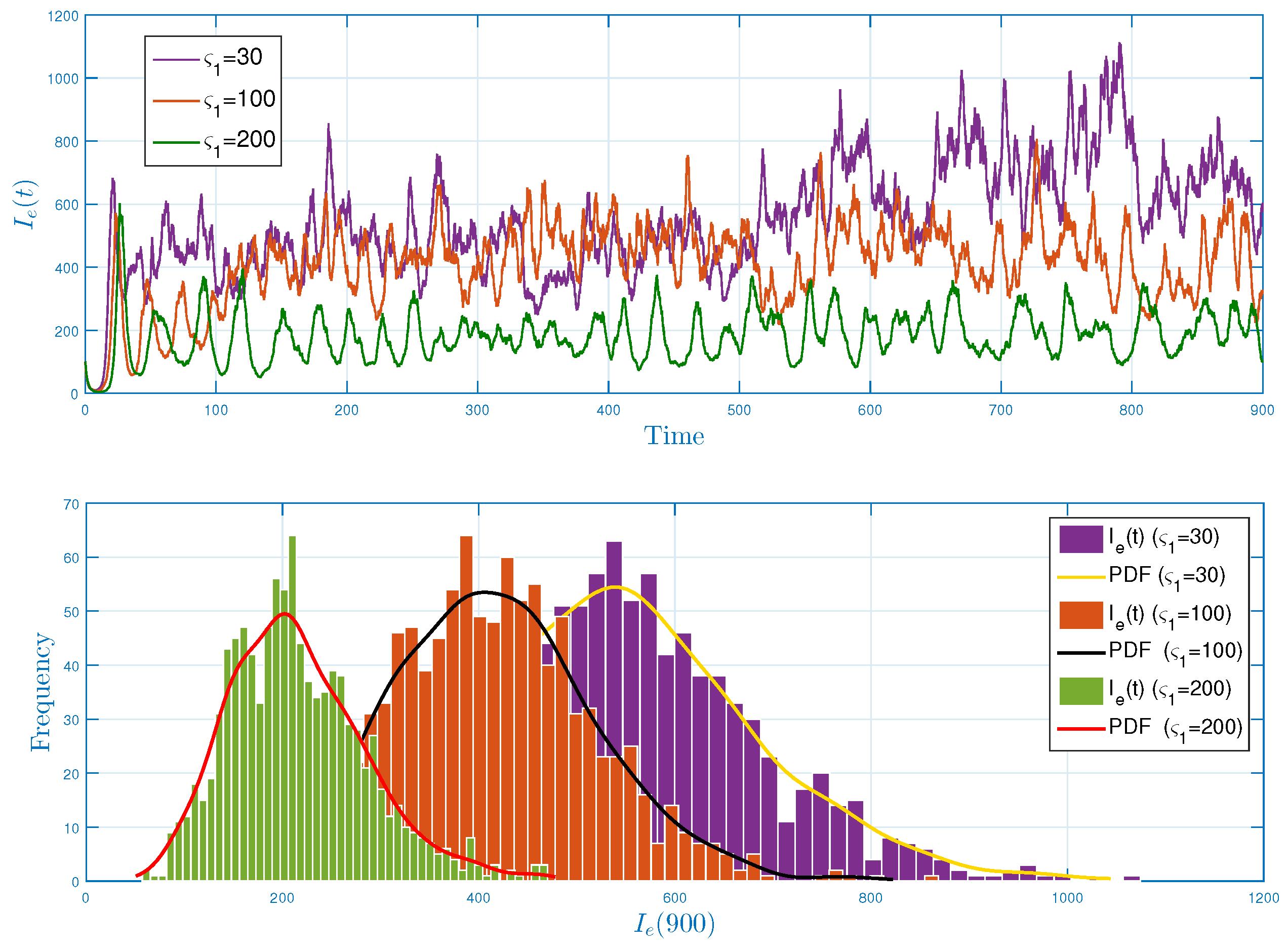
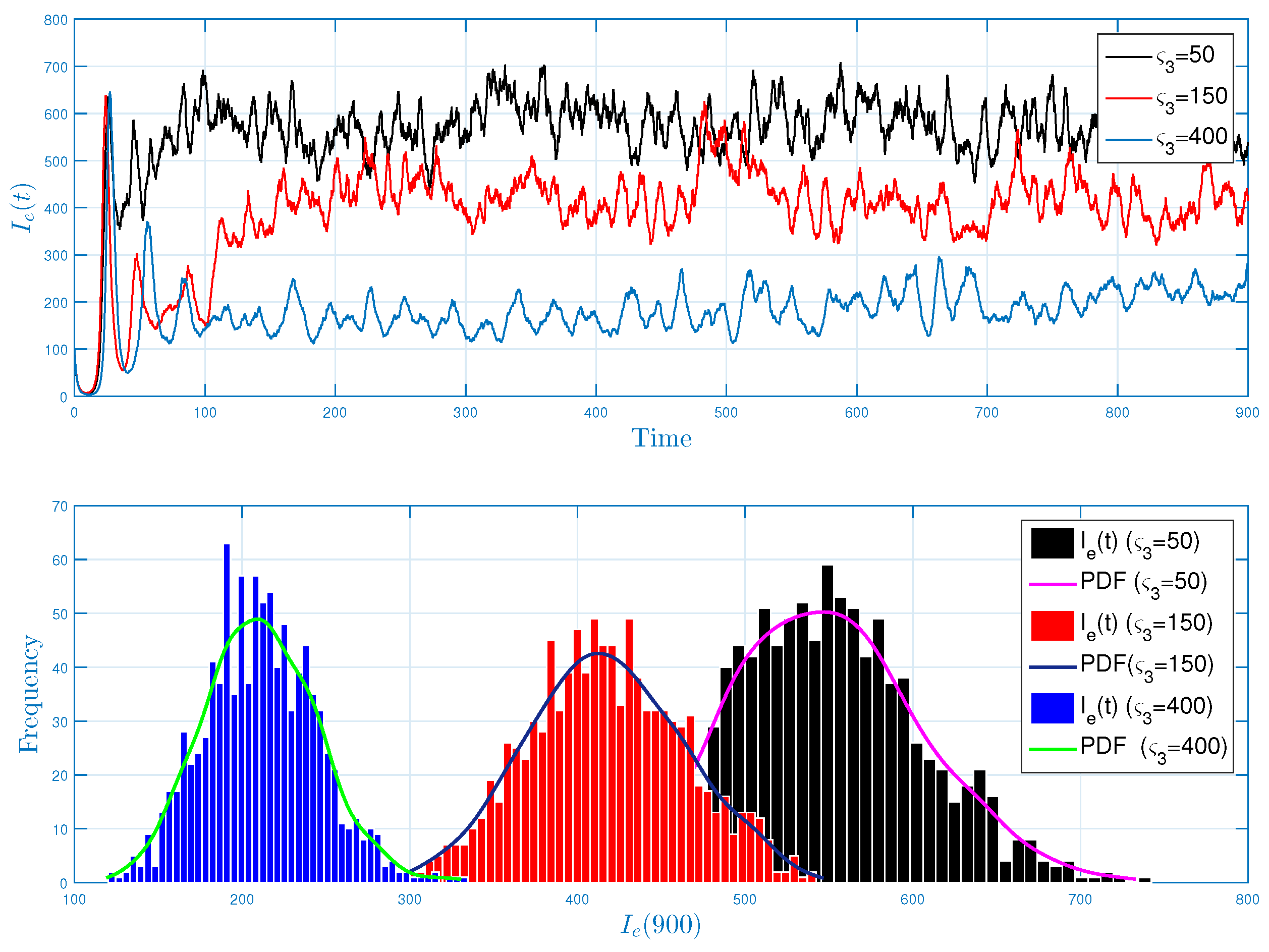
5.1. Stochastic Long-Run Behavior of Ebola Virus Model
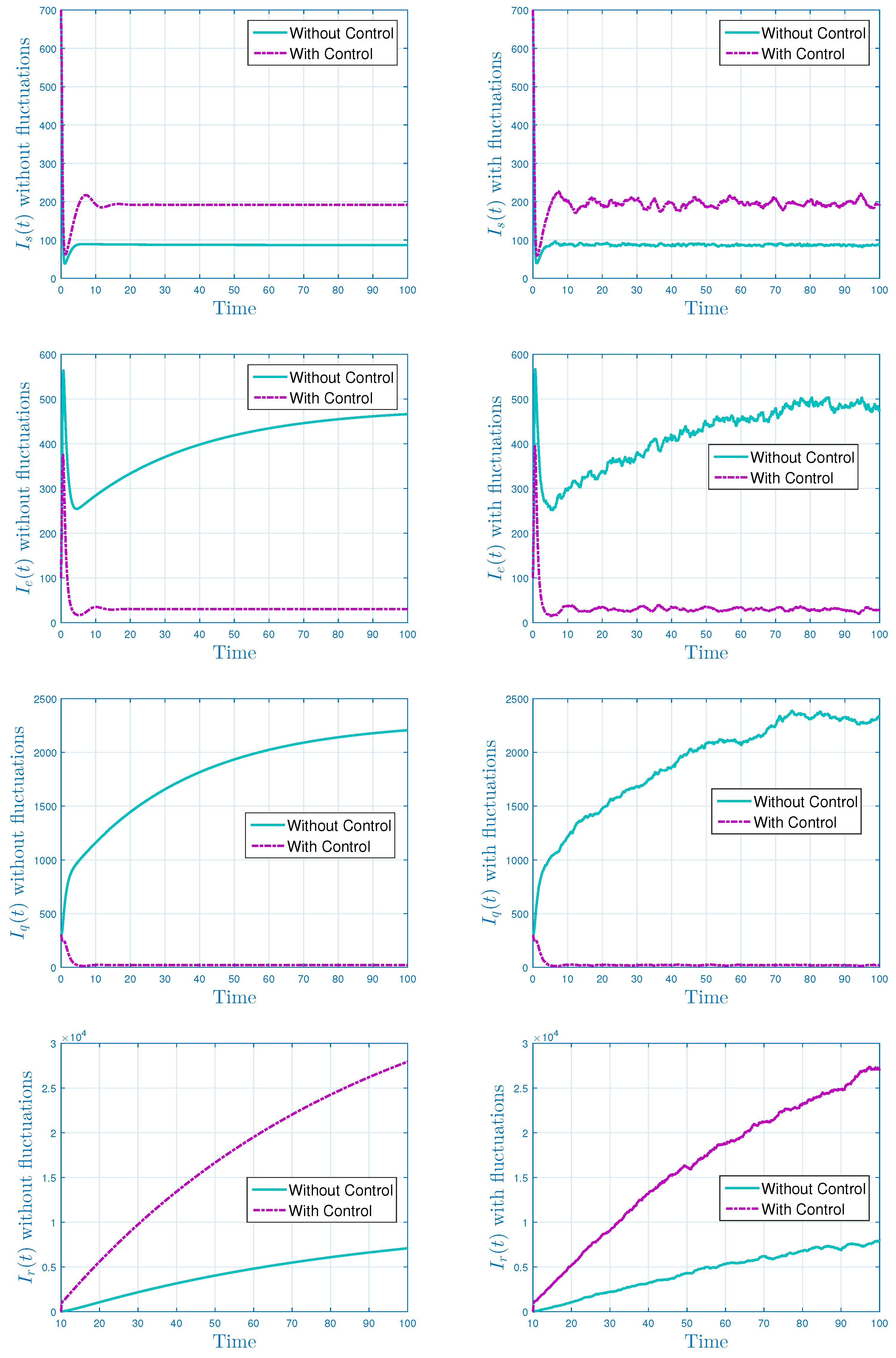
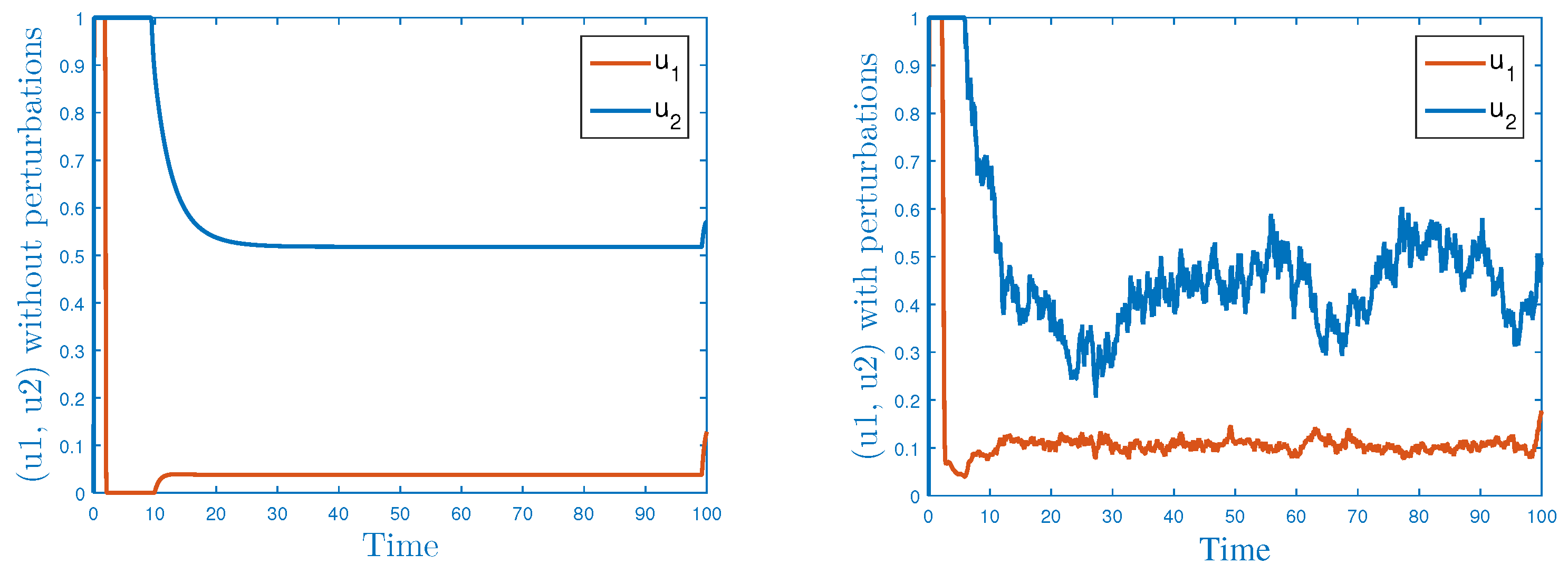
5.2. Stochastic Optimal Control Strategies
6. Conclusions
- In Theorem 1, we have proved the main result related to the stationarity and extinction of the Ebola virus. It is worthy to mention that the analysis of these long-time properties is very significant for the underlying perturbed systems. Especially, in the case of epidemiological models, the ergodicity offers a general idea of the infection permanence.
- For controlling the rapid spread of the disease, we assumed two control parameters, and these were incorporated into the stochastic model. The stochastic model was analyzed with the help of Pontryagin principle, and the required optimality conditions were derived therein. To check the validity of the theoretical results and effectiveness of the control parameters, we plotted the models by simulating the same in MATLAB. Through simulations, the obtained results for persistence/extinction of the Ebola infection are verified. It was concluded that by using both the control variables, one can easily eliminate the Ebola from the community with minimum cost.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christopher, D. Ebola virus disease in West Africa—The first 9 months. N. Engl. J. Med. 2015, 372, 189. [Google Scholar]
- Bell, B.P. Overview, Control Strategies, and Lessons Learned in the CDC Response to the 2014–2016 Ebola Epidemic. MMWR Suppl. 2016, 65 (Suppl. S3), 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ebola (Ebola Virus Disease). The Centers for Disease Control and Prevention. Available online: http://www.cdc.gov/ebola/resources/virus-ecology.html (accessed on 1 August 2014).
- Legrand, J.; Grais, R.F.; Boelle, P.Y.; Valleron, A.J.; Flahault, A. Understanding the dynamics of Ebola epidemics. Epidemiol. Infect. 2007, 135, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Naim, M.; Sabbar, Y.; Zeb, A. Stability characterization of a fractional-order viral system with the non-cytolytic immune assumption. Math. Model. Numer. Simul. Appl. 2022, 2, 164–176. [Google Scholar] [CrossRef]
- Farman, M.; Akgül, A.; Abdeljawad, T.; Naik, P.A.; Bukhari, N.; Ahmad, A. Modeling and analysis of fractional order Ebola virus model with Mittag-Leffler kernel. Alex. Eng. J. 2022, 61, 2062–2073. [Google Scholar] [CrossRef]
- Gourronc, F.A.; Rebagliati, M.; Kramer-Riesberg, B.; Fleck, A.M.; Patten, J.J.; Geohegan-Barek, K.; Klingelhutz, A.J. Adipocytes are susceptible to Ebola Virus infection. Virology 2022, 573, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, P. Ebola and COVID-19 in DR Congo and Guinea. Lancet Infect. Dis. 2021, 21, 461. [Google Scholar] [CrossRef]
- Bausch, D.G. The need for a new strategy for Ebola vaccination. Nat. Med. 2021, 27, 580–581. [Google Scholar] [CrossRef]
- Almuqrin, M.A.; Goswami, P.; Sharma, S.; Khan, I.; Dubey, R.S.; Khan, A. Fractional model of Ebola virus in population of bats in frame of Atangana-Baleanu fractional derivative. Results Phys. 2021, 26, 104295. [Google Scholar] [CrossRef]
- Bibby, K.; Casson, L.W.; Stachler, E.; Haas, C.N. Ebola virus persistence in the environment: State of the knowledge and research needs. Environ. Sci. Technol. Lett. 2015, 2, 2–6. [Google Scholar] [CrossRef]
- World Health Organization. WHO Ebola Virus Disease. 2018. Available online: http://www.who.int/mediacentre/factsheets/fs103/en/ (accessed on 8 April 2018).
- World Health Organization. WHO Ebola Situation Reports: Democratic Republic of the Congo. 2018. Available online: http://www.who.int/ebola/situation-reports/drc-2018/en/ (accessed on 22 October 2018).
- Fasina, F.O.; Shittu, A.; Lazarus, D.; Tomori, O.; Simonsen, L.; Viboud, C.; Chowell, G. Transmission dynamics and control of Ebola virus disease outbreak in Nigeria, July to September 2014. Eurosurveillance 2014, 19, 20920. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20920 (accessed on 1 August 2018). [CrossRef]
- Althaus, C.L. Estimating the reproduction number of Ebola virus (EBOV) during the 2014 outbreak in West Africa. PLoS Curr. 2014, 6. [Google Scholar] [CrossRef]
- Leroy, E.M.; Rouquet, P.; Formenty, P.; Souquière, S.; Kilbourne, A.; Froment, J.M.; Rollin, P.E. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 2004, 303, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D.; Khoo, E.; Tuite, A. Early epidemic dynamics of the West African 2014 Ebola outbreak: Estimates derived with a simple two-parameter model. PLoS Curr. 2014, 6. [Google Scholar] [CrossRef]
- World Health Organization. WHO Ebola Data and Statistics World Health Organization. 2018. Available online: http://apps.who.int/gho/data/node.ebola-sitrep (accessed on 12 April 2018).
- Wang, X.S.; Zhong, L. Ebola outbreak in West Africa: Real-time estimation and multiple-wave prediction. arXiv 2015, arXiv:1503.06908. [Google Scholar] [CrossRef] [PubMed]
- Ivorra, B.; Ngom, D.; Ramos, Á.M. Be-codis: A mathematical model to predict the risk of human diseases spread between countries—Validation and application to the 2014–2015 ebola virus disease epidemic. Bull. Math. Biol. 2015, 77, 1668–1704. [Google Scholar] [CrossRef] [PubMed]
- Mubayi, A.; Zaleta, C.K.; Martcheva, M.; Castillo-Chávez, C. A cost-based comparison of quarantine strategies for new emerging diseases. Math. Biosci. Eng. 2010, 7, 687. [Google Scholar] [PubMed]
- Ross, R. Yellow fever in the old world. Trans. R. Soc. Trop. Med. Hyg. 1911, 4, 233–237. [Google Scholar] [CrossRef]
- Barbarossa, M.V.; Dénes, A.; Kiss, G.; Nakata, Y.; Röst, G.; Vizi, Z. Transmission dynamics and final epidemic size of Ebola virus disease outbreaks with varying interventions. PLoS ONE 2015, 10, e0131398. [Google Scholar] [CrossRef]
- Chowell, G.; Hengartner, N.W.; Castillo-Chavez, C.; Fenimore, P.W.; Hyman, J.M. The basic reproductive number of Ebola and the effects of public health measures: The cases of Congo and Uganda. J. Theor. Biol. 2004, 229, 119–126. [Google Scholar] [CrossRef]
- Zhang, L.; Addai, E.; Ackora-Prah, J.; Arthur, Y.D.; Asamoah, J.K.K. Fractional-Order Ebola-Malaria Coinfection Model with a Focus on Detection and Treatment Rate. Comput. Math. Methods Med. 2022, 2022, 6502598. [Google Scholar] [CrossRef] [PubMed]
- Nathaniel, O.O.; Ezugwu, A.E.l.; Mohamed, T.I.A.; Abualigah, L. Ebola optimization search algorithm: A new nature-inspired metaheuristic optimization algorithm. IEEE Access 2022, 10, 16150–16177. [Google Scholar]
- Abdalla, S.; Chirove, F.; Govinder, K. A systematic review of mathematical models of the Ebola virus disease. Int. J. Model. Simul. 2022, 42, 814–830. [Google Scholar] [CrossRef]
- Pan, W.; Li, T.; Ali, S. A fractional order epidemic model for the simulation of outbreaks of Ebola. Adv. Differ. Equations 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Moremedi, G.M.; Kaondera-Shava, R.; Lubuma, J.M.; Morris, N.; Tsanou, B. A Simple Mathematical Model for Ebola in Africa. Biomath Commun. 2015, 2, 42–74. [Google Scholar]
- Dénes, A.; Gumel, A.B. Modeling the impact of quarantine during an outbreak of Ebola virus disease. Infect. Dis. Model. 2019, 4, 12–27. [Google Scholar] [CrossRef]
- Din, A.; Khan, A.; Baleanu, D. Stationary distribution and extinction of stochastic coronavirus (COVID-19) epidemic model. Chaos Solitons Fractals 2020, 139, 110036. [Google Scholar] [CrossRef] [PubMed]
- Din, A.; Li, Y.; Yusuf, A.; Liu, J.; Aly, A.A. Impact of information intervention on stochastic hepatitis B model and its variable-order fractional network. Eur. Phys. J. Spec. Top. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Din, A.; Li, Y.; Yusuf, A. Delayed hepatitis B epidemic model with stochastic analysis. Chaos Solitons Fractals 2021, 146, 110839. [Google Scholar] [CrossRef]
- Lu, H.; Ding, Y.; Gong, S.; Wang, S. Mathematical modeling and dynamic analysis of SIQR model with delay for pandemic COVID-19. Math. Biosci. Eng. 2021, 18, 3197–3214. [Google Scholar] [CrossRef] [PubMed]
- Berge, T.; Ouemba Tassé, A.J.; Tenkam, H.M.; Lubuma, J. Mathematical modeling of contact tracing as a control strategy of Ebola virus disease. Int. J. Biomath. 2018, 11, 1850093. [Google Scholar] [CrossRef]
- Bonyah, E.; Badu, K.; Asiedu-Addo, S.K. Optimal control application to an Ebola model. Asian Pac. J. Trop. Biomed. 2016, 6, 283–289. [Google Scholar] [CrossRef]
- Rachah, A.; Torres, D.F. Dynamics and optimal control of Ebola transmission. Math. Comput. Sci. 2016, 10, 331–342. [Google Scholar] [CrossRef]
- Area, I.; Ndairou, F.; Nieto, J.J.; Silva, C.J.; Torres, D.F. Ebola model and optimal control with vaccination constraints. arXiv 2017, arXiv:1703.01368. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Xie, N.; Fu, W.; Wang, K.; Ding, X. Mathematical analysis and simulation of a Hepatitis B model with time delay: A case study for Xinjiang, China. Math. Biosci. Eng. 2020, 17, 1757–1775. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ma, W.; Takeuchi, Y. Global analysis for delay virus dynamics model with Beddington–DeAngelis functional response. Appl. Math. Lett. 2011, 24, 1199–1203. [Google Scholar] [CrossRef]
- Hattaf, K.; Lashari, A.; Louartassi, Y.; Yousfi, N. A delayed SIR epidemic model with a general incidence rate. Electron. J. Qual. Theory Differ. Equations 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Din, A.; Li, Y. Mathematical analysis of a new nonlinear stochastic hepatitis B epidemic model with vaccination effect and a case study. Eur. Phys. J. Plus 2022, 137, 1–24. [Google Scholar] [CrossRef]
- Sabbar, Y.; Kiouach, D.; Rajasekar, S.P. Acute threshold dynamics of an epidemic system with quarantine strategy driven by correlated white noises and Lévy jumps associated with infinite measure. Int. J. Dyn. Control 2022, 1–14. [Google Scholar] [CrossRef]
- Sabbar, Y.; Kiouach, D. New method to obtain the acute sill of an ecological model with complex polynomial perturbation. Math. Methods Appl. Sci. 2022. [Google Scholar] [CrossRef]
- Sabbar, Y.; Kiouach, D.; Rajasekar, S.P.; El-Idrissi, S.E.A. The influence of quadratic Lévy noise on the dynamic of an SIC contagious illness model: New framework, critical comparison and an application to COVID-19 (SARS-CoV-2) case. Chaos Solitons Fractals 2022, 159, 112110. [Google Scholar] [CrossRef] [PubMed]
- Kiouach, D.; Sabbar, Y. Stability and threshold of a stochastic SIRS epidemic model with vertical transmission and transfer from infectious to susceptible individuals. Discret. Dyn. Nat. Soc. 2018, 2018, 7570296. [Google Scholar] [CrossRef]
- Sabbar, Y.; Khan, A.; Din, A. Probabilistic Analysis of a Marine Ecological System with Intense Variability. Mathematics 2022, 10, 2262. [Google Scholar] [CrossRef]
- Khan, A.; Sabbar, Y.; Din, A. Stochastic modeling of the Monkeypox 2022 epidemic with cross-infection hypothesis in a highly disturbed environment. Math. Biosci. Eng. 2022, 19, 13560–13581. [Google Scholar] [CrossRef]
- Driss, K.; Sabbar, Y. Modeling the impact of media intervention on controlling the diseases with stochastic perturbations. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2074. [Google Scholar]
- Zhao, Y.; Jiang, D.; O’Regan, D. The extinction and persistence of the stochastic SIS epidemic model with vaccination. Phys. A Stat. Mech. Its Appl. 2013, 392, 4916–4927. [Google Scholar] [CrossRef]
- Mao, X. Stochastic Differential Equations and Applications; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Özköse, F.; Şenel, M.T.; Habbireeh, R. Fractional-order mathematical modelling of cancer cells-cancer stem cells-immune system interaction with chemotherapy. Math. Model. Numer. Simul. Appl. 2021, 1, 67–83. [Google Scholar] [CrossRef]
- Din, A.; Abidin, M.Z. Analysis of fractional-order vaccinated Hepatitis-B epidemic model with Mittag-Leffler kernels. Math. Model. Numer. Simul. Appl. 2022, 2, 59–72. [Google Scholar] [CrossRef]
- Sinan, M.; Leng, J.; Anjum, M.; Fiaz, M. Asymptotic behavior and semi-analytic solution of a novel compartmental biological model. Math. Model. Numer. Simul. Appl. 2022, 2, 88–107. [Google Scholar] [CrossRef]
- Allegretti, S.; Bulai, I.M.; Marino, R.; Menandro, M.A.; Parisi, K. Vaccination effect conjoint to fraction of avoided contacts for a Sars-Cov-2 mathematical model. Math. Model. Numer. Simul. Appl. 2021, 1, 56–66. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, D. Stationary distribution and extinction of a stochastic SIR model with nonlinear perturbation. Appl. Math. Lett. 2017, 73, 8–15. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, D.; Hayat, T.; Alsaedi, A. Stationary distribution of a regime-switching predator–prey model with anti-predator behaviour and higher-order perturbations. Phys. A Stat. Mech. Its Appl. 2019, 515, 199–210. [Google Scholar] [CrossRef]
- Zhou, B.; Han, B.; Jiang, D. Ergodic property, extinction and density function of a stochastic SIR epidemic model with nonlinear incidence and general stochastic perturbations. Chaos Solitons Fractals 2021, 152, 111338. [Google Scholar] [CrossRef]
- Lesniewski, A. Epidemic control via stochastic optimal control. arXiv 2020, arXiv:2004.06680. [Google Scholar]
- Din, A. The stochastic bifurcation analysis and stochastic delayed optimal control for epidemic model with general incidence function. Chaos Interdiscip. J. Nonlinear Sci. 2021, 31, 123101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, Z. Dynamics and optimal control of a stochastic coronavirus (COVID-19) epidemic model with diffusion. Nonlinear Dyn. 2022, 109, 91–120. [Google Scholar] [CrossRef]
- Chen, Y.; Georgiou, T.T.; Pavon, M. Optimal transport in systems and control. Annu. Rev. Control Robot. Auton. Syst. 2021, 4, 89–113. [Google Scholar] [CrossRef]
- Germain, M.; Pham, H.; Warin, X. Neural networks-based algorithms for stochastic control and PDEs in finance. arXiv 2021, arXiv:2101.08068. [Google Scholar]
- Fleming, W.H.; Rishel, R.W. Deterministic and Stochastic Optimal Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2021; Volume 1. [Google Scholar]
- Liu, L.; Meng, X. Optimal harvesting control and dynamics of two-species stochastic model with delays. Adv. Differ. Equations 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Kiouach, D.; Sabbar, Y.; El Azami El-idrissi, S. New results on the asymptotic behavior of an SIS epidemiological model with quarantine strategy, stochastic transmission, and Lévy disturbance. Math. Methods Appl. Sci. 2021, 44, 13468–13492. [Google Scholar] [CrossRef]
- Peng, S.; Zhu, X. Necessary and sufficient condition for comparison theorem of 1-dimensional stochastic differential equations. Stoch. Process. Their Appl. 2006, 116, 370–380. [Google Scholar] [CrossRef]
- Tong, J.; Zhang, Z.; Bao, J. The stationary distribution of the facultative population model with a degenerate noise. Stat. Probab. Lett. 2013, 83, 655–664. [Google Scholar] [CrossRef]
- Zhao, D.; Yuan, S. Sharp conditions for the existence of a stationary distribution in one classical stochastic chemostat. Appl. Math. Comput. 2018, 339, 199–205. [Google Scholar] [CrossRef]
- Touzi, N.; Tourin, A. Optimal Stochastic Control, Stochastic Target Problems, and Backward SDE; Springer: New York, NY, USA, 2013; Volume 29. [Google Scholar]
- Nakanishi, M.; Cooper, L.G. Parameter estimation for a multiplicative competitive interaction model-least squares approach. J. Mark. Res. 1974, 11, 303–311. [Google Scholar]




| Subset | Definition |
|---|---|
| Parameter | Epidemiological Meaning | Value | Source |
|---|---|---|---|
| Recruitment rate into susceptible class | 10 | Estimated | |
| h | The Ebola transmission rate | Fitted | |
| u | The normal death rate of each class | Fitted | |
| v | The vaccination rate of the susceptible individuals | Estimated | |
| z | The recovery rate of quarantine class | Fitted | |
| q | The quarantine rate of infected class | Fitted | |
| w | The recovery rate of infectious individuals | Fitted | |
| The disease-related death rate of infected class | Fitted | ||
| The disease-related death rate of isolated class | Fitted | ||
| Time delay associated with | 21 | Fitted | |
| Time delay associated with | 21 | Estimated | |
| Time delay associated with | 21 | Estimated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Din, A.; Khan, A.; Sabbar, Y. Long-Term Bifurcation and Stochastic Optimal Control of a Triple-Delayed Ebola Virus Model with Vaccination and Quarantine Strategies. Fractal Fract. 2022, 6, 578. https://doi.org/10.3390/fractalfract6100578
Din A, Khan A, Sabbar Y. Long-Term Bifurcation and Stochastic Optimal Control of a Triple-Delayed Ebola Virus Model with Vaccination and Quarantine Strategies. Fractal and Fractional. 2022; 6(10):578. https://doi.org/10.3390/fractalfract6100578
Chicago/Turabian StyleDin, Anwarud, Asad Khan, and Yassine Sabbar. 2022. "Long-Term Bifurcation and Stochastic Optimal Control of a Triple-Delayed Ebola Virus Model with Vaccination and Quarantine Strategies" Fractal and Fractional 6, no. 10: 578. https://doi.org/10.3390/fractalfract6100578
APA StyleDin, A., Khan, A., & Sabbar, Y. (2022). Long-Term Bifurcation and Stochastic Optimal Control of a Triple-Delayed Ebola Virus Model with Vaccination and Quarantine Strategies. Fractal and Fractional, 6(10), 578. https://doi.org/10.3390/fractalfract6100578










