Recent Advances in the Chemistry of Glycoconjugate Amphiphiles
Abstract
1. Introduction
2. Glycolipids Synthesis Overview
3. Functionalized Carbohydrate Scaffolds
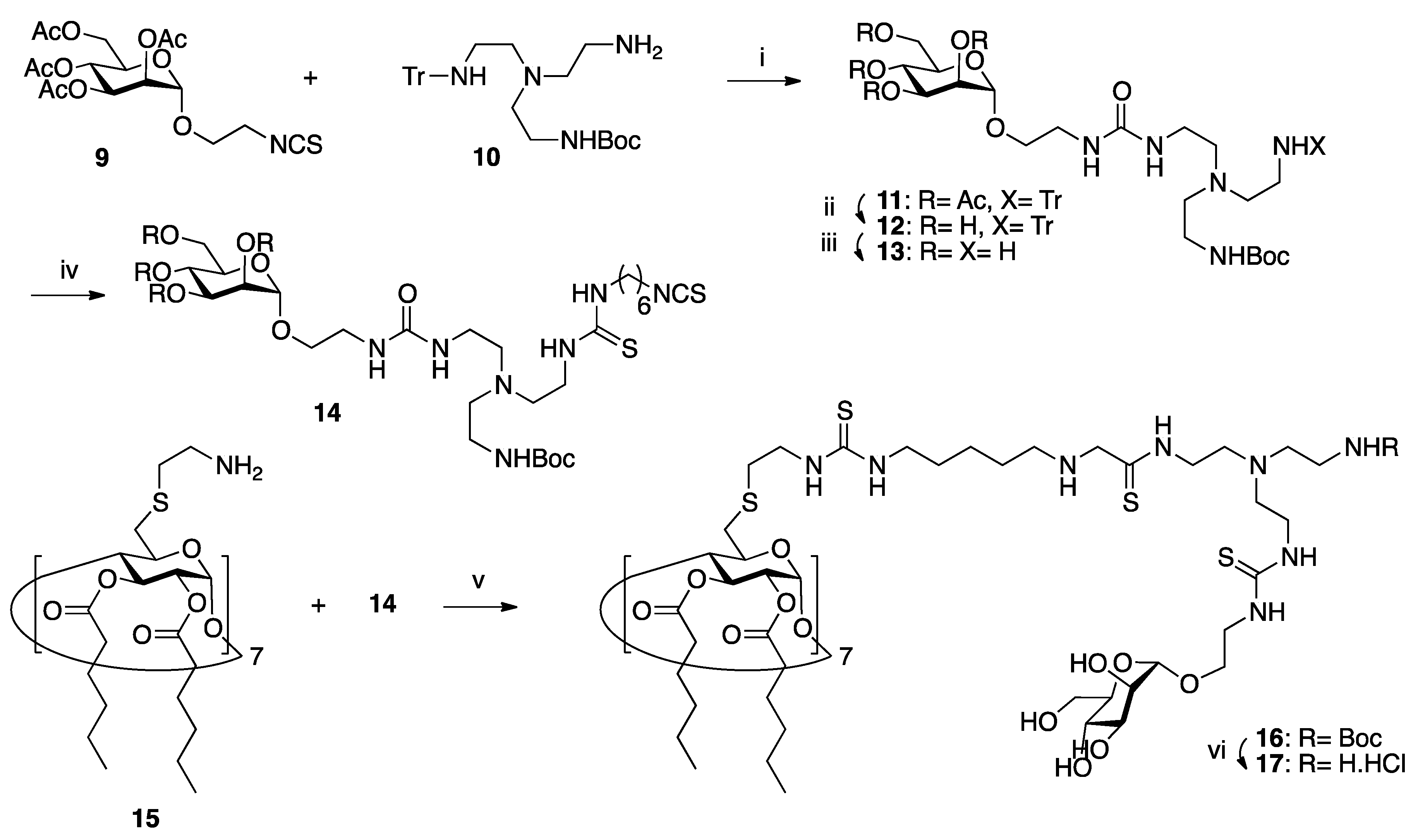
3.1. Glycocyclodextrins
3.2. Glycocalixarenes
3.3. Glycodendrimers
4. Vesicles, Liposomes
5. Gels
5.1. Glycoconjugate Based Gels
5.2. Mechanical Characterization of Gels
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P.H. Carbohydrates in Supramolecular Chemistry. Chem. Rev. 2016, 116, 1693–1752. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.-J.; André, S.; Jiménez-Barbero, J.; Romero, A.; Solís, D. From Lectin Structure to Functional Glycomics: Principles of the Sugar Code. Trends Biochem. Sci. 2011, 36, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, C.R.; Kiessling, L.L. Chemical glycobiology. Science 2001, 291, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- Taylor, M.E.; Drickamer, K. Introduction to Glycobiology, 1st ed.; Oxford University Press: New York, NY, USA, 2003; pp. 122–154. [Google Scholar]
- Lepenies, B.; Yin, J.; Seeberger, P.H. Applications of Synthetic Carbohydrates to Chemical Biology. Curr. Opin. Chem. Biol. 2010, 14, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Roy, R. Syntheses and Some Applications of Chemically Defined Multivalent Glycoconjugates. Curr. Opin. Struct. Biol. 1996, 6, 692–702. [Google Scholar] [CrossRef]
- Bhat, R.; Belardi, B.; Mori, H.; Kuo, P.; Tam, A.; Hines, W.C.; Le, Q.-T.; Bertozzi, C.R.; Bissell, M.J. Nuclear Repartitioning of Galectin-1 by an Extracellular Glycan Switch Regulates Mammary Morphogenesis. Proc. Natl. Acad. Sci. USA 2016, 113, E4820–E4827. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.M.; Wandel, M.P.; Muhlinen, N.; von Foeglein, A.; Randow, F. Galectin 8 Targets Damaged Vesicles for Autophagy to Defend Cells against Bacterial Invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, J.J.; Toone, E.J. The Cluster Glycoside Effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Drickamer, K. Contribution to Ligand Binding by Multiple Carbohydrate-Recognition Domains in the Macrophage Mannose Receptor. J. Biol. Chem. 1992, 267, 1719–1726. [Google Scholar] [PubMed]
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking Complex Biological Membranes and Their Programmable Glycan Ligands with Dendrimersomes and Glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, N.; Maiti, K.; Naresh, K. Multivalent Glycoliposomes and Micelles to Study Carbohydrate–Protein and Carbohydrate–Carbohydrate Interactions. Chem. Soc. Rev. 2013, 42, 4640–4656. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Kim, J.M. Amphiphilic P-Tert-Butylcalix[4]arene Scaffolds Containing Exposed Carbohydrate Dendrons. Angew. Chem. Int. Ed. 1999, 38, 369–372. [Google Scholar] [CrossRef]
- Yokota, S.; Kitaoka, T.; Sugiyama, J.; Wariishi, H. Cellulose I Nanolayers Designed by Self-Assembly of its Thiosemicarbazone on a Gold Substrate. Adv. Mater. 2007, 19, 3368–3370. [Google Scholar] [CrossRef]
- Cunha, C.R.A.; Oliveira, A.D.P.R.; Firmino, T.V.C.; Tenório, D.P.L.A.; Pereira, G.; Carvalho, L.B., Jr.; Santos, B.S.; Correia, M.T.S.; Fontes, A. Biomedical applications of glyconanoparticles based on quantum dots. Biochim. Biophys. Acta. 2017, 1862, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, X.; Ruan, H.; Cui, J.; Li, H. Synthesis and Applications of Glyconanoparticles. Curr. Org. Chem. 2016, 20, 1502–1511. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, S.; Kumar, P.; Narain, R. Glyconanoparticles: Synthesis and biomedical applications. In RSC Polymer Chemistry Series, Glycopolymer Code: Synthesis of Glycopolymers and their Applications; Becer, C.R., Hartmann, L., Eds.; Royal Society of Chemistry: London, UK, 2015; Volume 15, pp. 196–220. ISBN 978-1-84973-978-8. [Google Scholar]
- Sunasee, R.; Adokoh, C.K.; Darkwa, J.; Narain, R. Therapeutic potential of carbohydrate-based polymeric and nanoparticle systems. Expert Opin. Drug Deliv. 2014, 11, 867–884. [Google Scholar] [CrossRef] [PubMed]
- Breinbauer, R.; Köhn, M. Azide-Alkyne Coupling: A Powerful Reaction for Bioconjugate Chemistry. ChemBioChem 2003, 4, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. A Hydrophilic Azacyclooctyne for Cu-Free Click Chemistry. Org. Lett. 2008, 10, 3097–3099. [Google Scholar] [CrossRef] [PubMed]
- Jewett, J.C.; Bertozzi, C.R. Cu-Free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Song, S.X.; Zhang, H.L.; Kim, C.G.; Sheng, L.; He, X.P.; Long, Y.T.; Li, J.; Chen, G.R. Expeditious preparation of triazole-linked glycolipids via microwave accelerated click chemistry and their electrochemical and biological assessments. Tetrahedron 2010, 66, 9974–9980. [Google Scholar] [CrossRef]
- Goyard, D.; Shiao, T.C.; Fraleigh, N.L.; Vu, H.-Y.; Lee, H.; Diaz-Mitoma, F.; Le, H.T.; Roy, R. Expedient synthesis of functional single-component glycoliposomes using thiol-yne chemistry. J. Mater. Chem. B 2016, 4, 4227–4233. [Google Scholar] [CrossRef]
- Salman, S.M.; Heidelberg, T.; Tajuddin, H.A.B. N-linked glycolipids by Staudinger coupling of glycosylated alkyl diazides with fatty acids. Carbohydr. Res. 2013, 375, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ogunsina, M.; Samadder, P.; Idowu, T.; Arthur, G.; Schweizer, F. Design, synthesis and evaluation of cytotoxic properties of bisamino glucosylated antitumor ether lipids against cancer cells and cancer stem cells. Med. Chem. Commun. 2016, 7, 2100–2110. [Google Scholar] [CrossRef]
- Gatard, S.; Nasir, M.N.; Deleu, M.; Klai, N.; Legrand, V.; Bouquillon, S. Bolaamphiphiles Derived from Alkenyl L-Rhamnosides and Alkenyl D-Xylosides: Importance of the Hydrophilic Head. Molecules 2013, 18, 6101–6112. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Gervay-Hague, J. Synthesis of glycolipid antigens. In Chemical Glycobiology; ACS Symposium Series; Chen, X., Halcomb, R.L., Wang, P.G., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; Volume 990, pp. 153–166. ISBN 978-0-8412-7440-2. [Google Scholar]
- Zhang, W.; Zheng, X.; Xia, C.; Perali, R.S.; Yao, Q.; Liu, Y.; Zheng, P.; Wang, P.G. Alpha-lactosylceramide as a novel “sugar-capped” CD1d ligand for natural killer T cells: Biased cytokine profile and therapeutic activities. ChemBioChem 2008, 9, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Long, X.; Goff, R.D.; Zhou, D.; Cantu, C.; Mattner, J.; Saint Mezard, P.; Teyton, L.; Bendelac, A.; Savage, P.B. Alpha anomers of iGb3 and Gb3 stimulate cytokine production by natural killer T cells. ACS Chem. Biol. 2009, 4, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y. Process Chemistry: The Science, Business, Logic, and Logistics. Chem. Rev. 2006, 106, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Hudlicky, T. Design Constraints in Practical Syntheses of Complex Molecules: Current Status, Case Studies with Carbohydrates and Alkaloids, and Future Perspectives. Chem. Rev. 1996, 96, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Verma, V.A.; Paxton, T.J.; Pillow, T.H. Function-Oriented Synthesis, Step Economy, and Drug Design. Acc. Chem. Res. 2008, 41, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Miller, B.L. Synthesis at the molecular frontier. Nature 2009, 460, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.W.; Schombs, M.W.; Witschi, M.A.; Gervay-Hague, J. Regioselective silyl/acetate exchange of disaccharides yields advanced glycosyl donor and acceptor precursors. J. Org. Chem. 2013, 78, 9677–9688. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.W.; Schombs, M.W.; Gervay-Hague, J. Integrating ReSET with glycosyl iodide glycosylation in step-economy syntheses of tumor-associated carbohydrate antigens and immunogenic glycolipids. J. Org. Chem. 2014, 79, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrand, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed]
- Danishefsky, S.J.; Shue, Y.-K.; Chang, M.N.; Wong, C.-H. Development of Globo-H cancer vaccine. Acc. Chem. Res. 2015, 48, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.I.; Lee, H.Y.; Chang, S.H.; Wang, C.H.; Tu, Y.C.; Lin, Y.C.; Hwang, D.R.; Wu, C.Y.; Wong, C.H. Effective sugar nucleotide regeneration for the large-scale enzymatic synthesis of Globo H and SSEA4. J. Am. Chem. Soc. 2013, 135, 14831–14839. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Labrada, K.; Brouard, I.; Méndez, I.; Pérez, C.S.; Gavín, J.A.; Rivera, D.G. Combined Ugi-4CR/CuAAC approach to triazole-based neoglycolipids. Eur. J. Org. Chem. 2014, 3671–3683. [Google Scholar] [CrossRef]
- Jebali, A.; Nayeri, E.K.; Roohana, S.; Aghaei, S.; Ghaffari, M.; Daliri, K.; Fuente, G. Nano-carbohydrates: Synthesis and application in genetics, biotechnology, and medicine. Adv. Colloid Interface Sci. 2017, 240, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Conklin, J.; Hogan, V.; Raz, A. Carbohydrate-binding proteins in cancer, and their ligands as therapeutic agents. Trends Mol. Med. 2002, 8, 187–192. [Google Scholar] [CrossRef]
- Brewer, C.F.; Miceli, M.C.; Baum, L.G. Clusters, bundles, arrays and lattices: Novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 2002, 12, 616–623. [Google Scholar] [CrossRef]
- Wang, J.L.; Gray, R.M.; Haudek, K.C.; Patterson, R.J. Nucleocytoplasmic lectins. Biochim. Biophys. Acta 2004, 1673, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Bojarova, P.; Kren, V. Sugared biomaterial binding lectins: Achievements and perspectives. Biomater. Sci. 2016, 4, 1142–1160. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Murphy, P.V.; Gabius, H.J. Multivalent Carbohydrate-Lectin Interactions: How Synthetic Chemistry Enables Insights into Nanometric Recognition. Molecules 2016, 21, 629. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bolaños, J.G.; Maya, I.; Oliete, A. Multivalent glycoconjugates in medicinal chemistry. In Carbohydrate Chemistry; Rauter, A.P., Lindhorst, T., Eds.; The Royal Society of Chemistry: Cambrige, UK, 2012; Volume 38, pp. 303–337. ISBN 978-1-84973-439-4. [Google Scholar]
- Roy, R.; Shiao, T.C.; Rittenhouse-Olson, K. Glycodendrimers: Versatile tools for nanotachnology. Braz. J. Pharm. Sci. 2013, 49, 85–108. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, N.; Bienvenu, C.; Charrat, C.; Jimenez Blanco, J.L.; Diaz-Moscoso, A.; Ortiz Mellet, C.; Garcia Fernandez, J.M.; Vierling, P.; Di Giorgio, C. Cell uptake mechanisms of glycosylated cationic pDNA-cyclodextrin nanoparticles. RSC Adv. 2015, 5, 29135–29144. [Google Scholar] [CrossRef]
- Diaz-Moscoso, A.; Guilloteau, N.; Bienvenu, C.; Méndez-Ardoy, A.; Jiménez Blanco, J.L.; Benito, J.M.; Le Gourriérec, L.; Di Giorgio, C.; Vierling, P.; Defaye, J.; et al. Mannosyl-coated nanocomplexes from amphiphilic cyclodextrins and pDNA for site-specific gene delivery. Biomaterials 2011, 32, 7263–7273. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, C.D. Calixarenes, 2nd ed.; The Royal Society of Chemistry: Cambrige, UK, 2008; ISBN 978-0854042586. [Google Scholar]
- Sansone, F.; Rispoli, G.; Casnati, A.; Ungaro, R. Multivalent Glycocalixarenes. In Synthesis and Biological Applications of Glycoconjugates; Renaudet, O., Spinelli, N., Eds.; Bentham Science Publishers Ltd.: Sharjah, UAE, 2011; pp. 36–63. ISBN 978-1-60805-537-1. [Google Scholar]
- Bernardi, S.; Fezzardi, P.; Rispoli, G.; Sestito, S.E.; Peri, F.; Sansone, F.; Casnati, A. Clicked and long spaced galactosyl- and lactosylcalix[4]arenes: New multivalent galectin-3 ligands. Beilstein J. Org. Chem. 2014, 10, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, C.D. Synthesis of calixarenes and thiacalixarenes. In Calixarenes 2001; Asfari, Z., Böhmer, V., Harrowfield, J., Vicens, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2001; pp. 1–25. ISBN 0-7923-6960-2. [Google Scholar]
- Hu, Y.; Beshr, G.; Garvey, C.J.; Tabor, R.F.; Titz, A.; Wilkinson, B.L. Photoswitchable Janus Glycodendrimer Micelles as Multivalent Inhibitors of LecA and LecB from Pseudomonas aeruginosa. Colloids Surf. B 2017, 159, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.P. “Janus” dendrimers: Synthesis and properties. New J. Chem. 2012, 36, 217–226. [Google Scholar] [CrossRef]
- Angeli, A.; Li, M.; Dupin, L.; Vergoten, G.; Noël, M.; Madaoui, M.; Wang, S.; Meyer, A.; Géhin, T.; Vidal, S.; et al. Design and Synthesis of Galactosylated Bifurcated Ligands with Nanomolar Affinity for Lectin LecA from Pseudomonas aeruginosa. ChemBioChem 2017, 18, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- L’Haridon, L.; Mallet, J.-M. Carbohydrate-based dendrimers. In Carbohydrate Chemistry; Rauter, A.P., Lindhorst, T., Queneau, Y., Eds.; The Royal Society of Chemistry: Cambrige, UK, 2014; Volume 40, pp. 257–269. ISBN 978-1-84973-965-8. [Google Scholar]
- Sharma, R.; Naresh, K.; Chabre, Y.M.; Rej, R.; Saadeh, N.K.; Roy, R. “Onion peel” dendrimers: A straightforward synthetic approach towards highly diversified architectures. Polym. Chem. 2014, 5, 4321–4331. [Google Scholar] [CrossRef]
- Thomas, B.; Pifferi, C.; Daskhan, G.C.; Fiore, M.; Berthet, N.; Renaudet, O. Divergent and convergent synthesis of GalNAc-conjugated dendrimers using dual orthogonal ligations. Org. Biomol. Chem. 2015, 13, 11529–11538. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Mukhopadhyay, B. Use of ‘click chemistry’ for the synthesis of carbohydrate-porphyrin dendrimers and their multivalent approach toward lectin sensing. Tetrahedron Lett. 2016, 57, 1775–1781. [Google Scholar] [CrossRef]
- McSweeney, L.; Dénès, F.; Scanlan, E.M. Thiyl-Radical Reactions in Carbohydrate Chemistry: From Thiosugars to Glycoconjugate Synthesis. Eur. J. Org. Chem. 2016, 2080–2095. [Google Scholar] [CrossRef]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and Analytical Applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.C.; Micheletto, Y.M.S.; da Silveira, N.P.; da Silva Pinto, L.; Giacomelli, F.C.; de Lima, V.R.; Frizon, T.E.A.; Dal-Bó, A.G. Self-assembled carbohydrate-based vesicles for lectin targeting. Colloids Surf. B 2016, 148, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Dal Bo, A.G.; Soldi, V.; Giacomelli, F.C.; Jean, B.; Pignot-Paintrand, I.; Borsali, R.; Fort, S. Self-assembled carbohydrate-based micelles for lectin targetting. Soft Matter 2011, 7, 3453–3461. [Google Scholar] [CrossRef]
- Micheletto, Y.M.S.; da Silveira, N.P.; Barboza, D.M.; Dos Santos, M.C.; de Lima, V.R.; Giacomelli, F.C.; Martinez, J.C.V.; Frizon, T.E.A.; Dal-Bó, A.G. Investigation of self-association between new glycosurfactant N-acetyl-β-d-glucosaminyl-PEG-docosanate and soybean phosphatidylcholine into vesicles. Colloids Surf. A 2015, 467, 166–172. [Google Scholar] [CrossRef]
- Dal-Bó, A.G.; Soldi, V.; Giacomelli, F.C.; Travelet, C.; Borsali, R.; Fort, S. Synthesis, micellization and lectin binding of new glycosurfactants. Carbohydr. Res. 2014, 397, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.B. Thiol-yne ‘click’ coupling chemistry and recent applications in polymer and materials synthesis and modification. Polymer 2014, 55, 5517–5549. [Google Scholar] [CrossRef]
- Saxon, E.; Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Gololobov, Y.G.; Kasukhin, L.F. Recent advances in the Staudinger reaction. Tetrahedron 1992, 48, 1353–1406. [Google Scholar] [CrossRef]
- Köhn, M.; Breinbauer, R. The Staudinger Ligation—A Gift to Chemical Biology. Angew. Chem. Int. Ed. 2004, 43, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Edgar, K.J. Staudinger Reactions for Selective Functionalization of Polysaccharides: A Review. Biomacromolecules 2015, 16, 2556–2571. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.L.; Kiessling, L.L.; Raines, R.T. Staudinger Ligation: A Peptide from a Thioester and Azide. Org. Lett. 2000, 2, 1939–1941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Y.; Sun, X.-L. Chemically-selective surface glyco-functionalization of liposomes through Staudinger ligation. Chem. Commum. 2009, 0, 3032–3034. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, H.; Gruzdys, V.; Sun, X.L. Azide-Reactive Liposome for Chemoselective and Biocompatible Liposomal Surface Functionalization and Glyco-Liposomal Microarray Fabrication. Langmuir 2011, 27, 13097–13103. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiang, R.; Zhang, H.; Gruzdys, V.; Sun, X.-L. Chemoselectively surface funtionalizable tethered bilayer lipid membrane for versatile membrane mimetic systems fabrication. J. Mater. Chem. 2012, 22, 6148–6155. [Google Scholar] [CrossRef]
- Vabbilisetty, P.; Sun, X.-L. Liposome surface functionalization based on different anchoring lipids via Staudinger ligation. Org. Biomol. Chem. 2014, 12, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Krieg, E.; Bastings, M.M.C.; Besenius, P.; Rybtchinski, B. Supramolecualr Polymers in Aqueous Media. Chem. Rev. 2016, 116, 2414–2477. [Google Scholar] [CrossRef] [PubMed]
- Ramos Sasselli, I.; Halling, P.J.; Ulijn, R.V.; Tuttle, T. Supramolecular Fibers in Gels Can Be at Thermodynamic Equilibrium: A Simple Packing Model Reveals Preferential Fibril Formation versus Crystallization. ACS Nano 2016, 10, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xuewen Du, J.L.; Xu, B. Supramolecular biofunctional materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Xin, Z.; Chao, M.; Huan, Y.; Zceshan, A.; Xianbo, M.; Song, L.; Yan, D.; Nongyue, H. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Jeon, O.; Krebs, M.D.; Schapira, D.; Alsberg, E. Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials 2014, 35, 6278–6286. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.C.I.M.; Ahmad, N.; Pandey, M.; Abeer, M.M.; Mohamad, N. Recent advances in the role of supramolecular hydrogels in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Oo, N.N.L.; Lee, J.P.; Li, Z.; Loh, X.J. Recent development of synthetic nonviral systems for sustained gene delivery. Drug Discov. Today 2017, 22, 1318–1335. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Lo, I.M.C. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res. 2016, 106, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.J.; Fitremann, J.; Mauzac, M.; Serrano, J.L.; Oriol, L. Synthesis and Characterization of Maltose-Based Amphiphiles as Supramolecular Hydrogelators. Langmuir 2011, 27, 15236–15247. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.J.; Romero, P.; Serrano, J.L.; Fitremann, J.; Oriol, L. Supramolecular Hydrogels Based on Glycoamphiphiles: Effect of the Disaccharide Polar Head. Chem. Mater. 2012, 24, 3847–3858. [Google Scholar] [CrossRef]
- Goodby, J.; Cowling, S.; Davis, E.; Queneau, Y. Liquid Crystal Glycolipids. In Carbohydrate Chemistry; Rauter, A.P., Lindhorst, T., Queneau, Y., Eds.; The Royal Society of Chemistry: Cambrige, UK, 2014; Volume 40, pp. 312–340. ISBN 978-1-84973-965-8. [Google Scholar]
- Goodby, J.W.; Görtz, V.; Cowling, S.J.; Mackenzie, G.; Martin, P.; Plusquellec, D.; Benvegnu, T.; Boullanger, P.; Lafont, D.; Queneau, Y.; et al. Thermotropic liquid crystalline glycolipids. Chem. Soc. Rev. 2007, 36, 1971–2032. [Google Scholar] [CrossRef] [PubMed]
- Hashim, R.; Sugimura, A.; Minamikawa, H.; Heidelberg, T. Nature-like synthetic alkyl branched-chain glycolipids: A review on chemical structure and self-assembly properties. Liq. Cryst. 2012, 39, 1–17. [Google Scholar] [CrossRef]
- Garidel, P.; Kaconis, Y.; Heinbockel, L.; Wulf, M.; Gerber, S.; Munk, A.; Vill, V.; Brandenburg, K. Self-Organisation, Thermotropic and Lyotropic Properties of Glycolipids Related to their Biological Implications. Open Biochem. J. 2015, 9, 49–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, D.; Marcozzi, A.; Zheng, L.; Su, J.; Pesce, D.; Zajaczkowski, W.; Kolbe, A.; Pisula, W.; Müllen, K.; et al. Thermotropic liquid crystals from biomacromolecules. Proc. Natl. Acad. Sci. USA 2014, 111, 18596–18600. [Google Scholar] [CrossRef] [PubMed]
- Ochi, R.; Kurotani, K.; Ikeda, M.; Kiyonaka, S.; Hamachi, I. Supramolecular hydrogels based on bola-amphiphilic glycolipids showing color change in response to glycosidases. Chem. Commun. 2013, 49, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.; Kumari, H. Low Molecular Weight Supramolecular Gels Under Shear: Rheology as the Tool for Elucidating Structure-Function Correlation. Chem. Eur. J. 2017, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Godeau, G.; Brun, C.; Arnion, H.; Staedel, C.; Barthlémy, P. Glycosyl-nucleoide fluorinated amphiphiles as components of nanostructures hydrogels. Tetrahedron Lett. 2010, 1, 1012–1015. [Google Scholar] [CrossRef]
- Ziane, S.; Schlaubitz, S.; Miraux, S.; Patwa, A.; Lalande, C.; Bilem, I.; Lepreux, S.; Rousseau, B.; Le Meins, J.F.; Latxague, L.; et al. A thermosensitive low molecular weight hydrogel as scaffold for tissue engineering. Eur. Cells Mater. 2012, 23, 147–160. [Google Scholar] [CrossRef]
- Latxague, L.; Ramin, M.A.; Appavoo, A.; Berto, P.; Maisani, M.; Ehret, C.; Chassande, O.; Barthélémy, P. Control of Stem-Cell Behavior by Fine Tuning the Supramolecular Assemblies of Low-Molecular-Weight Gelators. Angew. Chem. Int. Ed. 2015, 54, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
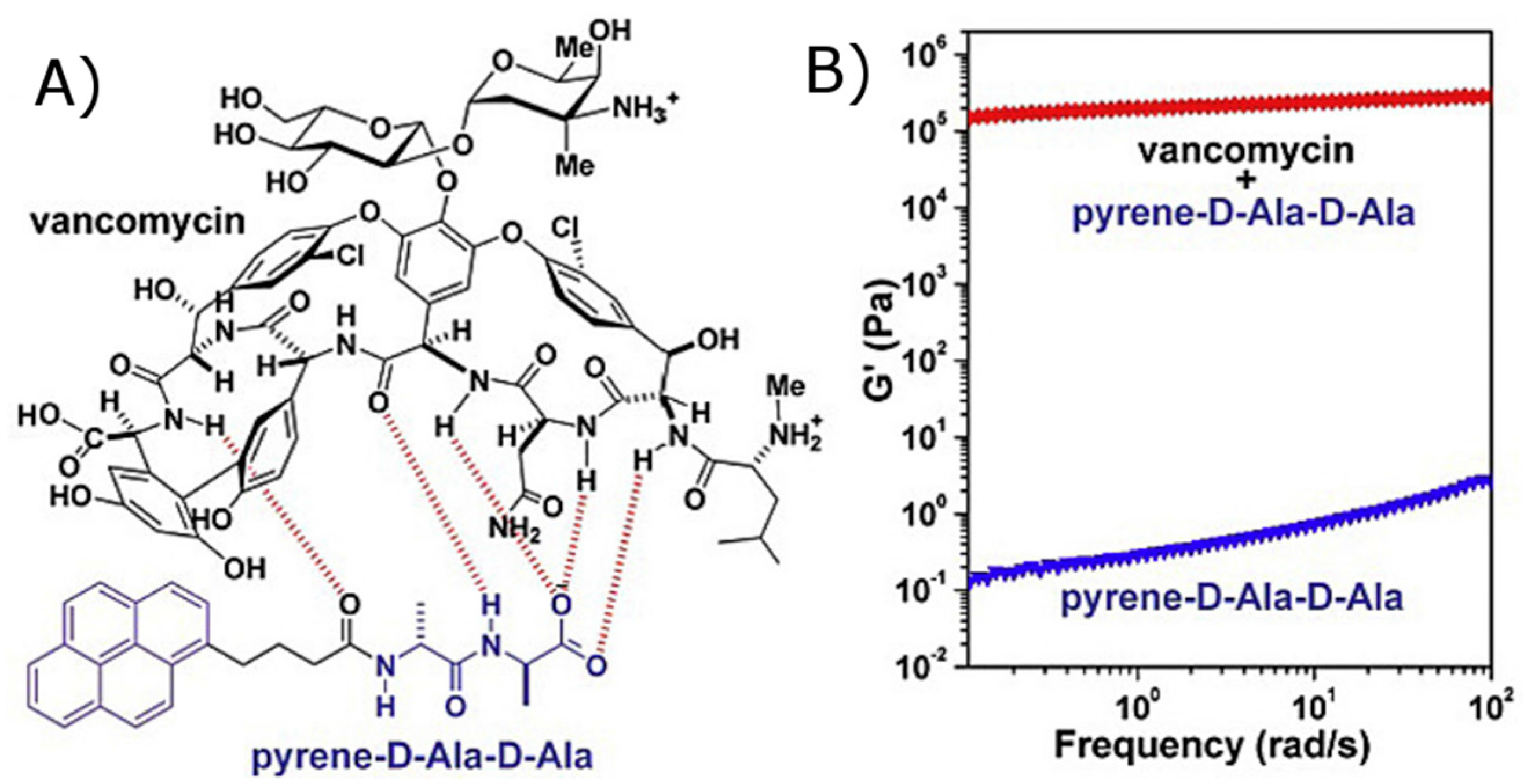
- Zhang, Y.; Yang, Z.; Yuan, F.; Gu, H.; Gao, P.; Xu, B. Molecular recognition remolds the self-assembly of hydrogelators and increases the elasticity of the hydrogel by 106-fold. J. Am. Chem. Soc. 2004, 126, 15028–15029. [Google Scholar] [CrossRef] [PubMed]
- Latxague, L.; Gaubert, A.; Maleville, D.; Baillet, J.; Ramin, M.A.; Barthélémy, P. Carbamate-based bolaamphiphile as low-molecular-weight hydrogelators. Gels 2016, 2, 25. [Google Scholar] [CrossRef]
- Ramin, M.A.; Latxague, L.; Sindhu, K.R.; Chassande, O.; Barthélémy, P. Low molecular weight hydrogels derived from urea based-bolaamphiphiles as new injectable biomaterials. Biomaterials 2017, 145, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Prasad, Y.S.; Miryala, S.; Lalitha, K.; Ranjitha, K.; Barbhaiwala, S.; Sridharan, V.; Maheswari, C.U.; Srinandan, C.S.; Nagarajan, S. Disassembly of Bacterial Biofilms by the SelfAssembled Glycolipids Derived from Renewable Resources. ACS Appl. Mater. Interfaces 2017, 9, 40047–40058. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ochi, R.; Wadaa, A.; Hamachi, I. Supramolecular hydrogel capsule showing prostate specific antigen-responsive function for sensing and targeting prostate cancer cells. Chem. Sci. 2010, 1, 491–498. [Google Scholar] [CrossRef]
- Fitremann, J.; Lonetti, B.; Fratini, E.; Fabing, I.; Payré, B.; Boulé, C.; Loubinoux, I.; Vaysse, L.; Oriol, L. A shear-induced network of aligned wormlike micelles in a sugar-based molecular gel. From gelation to biocompatibility assays. J. Colloid Interface Sci. 2017, 504, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Maisani, M.; Ziane, S.; Ehret, C.; Levesque, L.; Siadous, R.; Le Meins, J.F.; Chevalier, P.; Barthélémy, P.; De Oliviera, H.; Amédée, J.; et al. A new composite hydrogel combining the biological properties of collagen with the mechanical proerties of a supramolecular scaffold for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Patwa, A.; Labille, J.; Bottero, J.Y.; Thiéry, A.; Barthélémy, P. Decontamination of nanoparticles from aqueous samples using supramolecular gels. Chem. Commun. 2015, 51, 2547–2550. [Google Scholar] [CrossRef] [PubMed]
- Tamigney Kenfack, M.; Mazur, M.; Nualnoi, T.; Shaffer, T.L.; Ngassimou, A.; Blériot, Y.; Marrot, J.; Marchetti, R.; Sintiprungrat, K.; Chantratita, N.; et al. Deciphering minimal antigenic epitopes associated with Burkholderia pseudomallei and Burkholderia mallei lipopolysaccharide O-antigens. Nat. Commun. 2017, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Gunay, G.; Sardan Ekiz, M.; Ferhati, X.; Richichi, B.; Nativi, C.; Tekinay, A.B.; Guler, M.O. Antigenic GM3 Lactone Mimetic Molecule Integrated Mannosylated Glycopeptide Nanofibers for the Activation and Maturation of Dendritic Cells. ACS Appl. Mater. Interfaces 2017, 9, 16035–16042. [Google Scholar] [CrossRef] [PubMed]

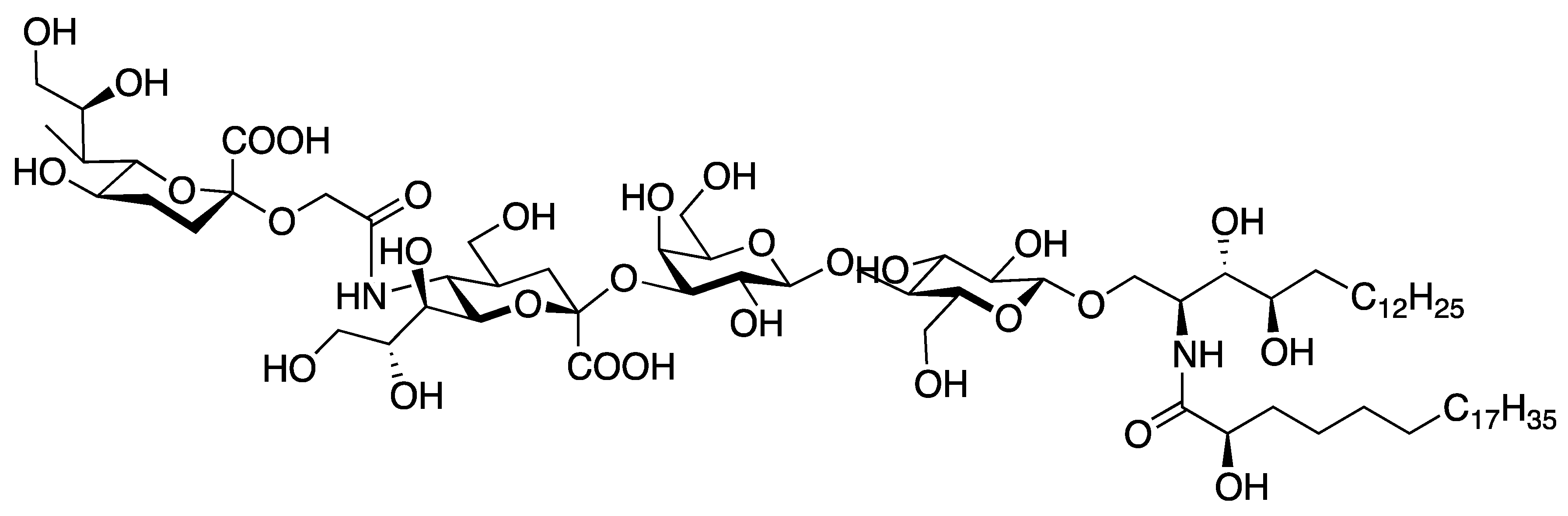
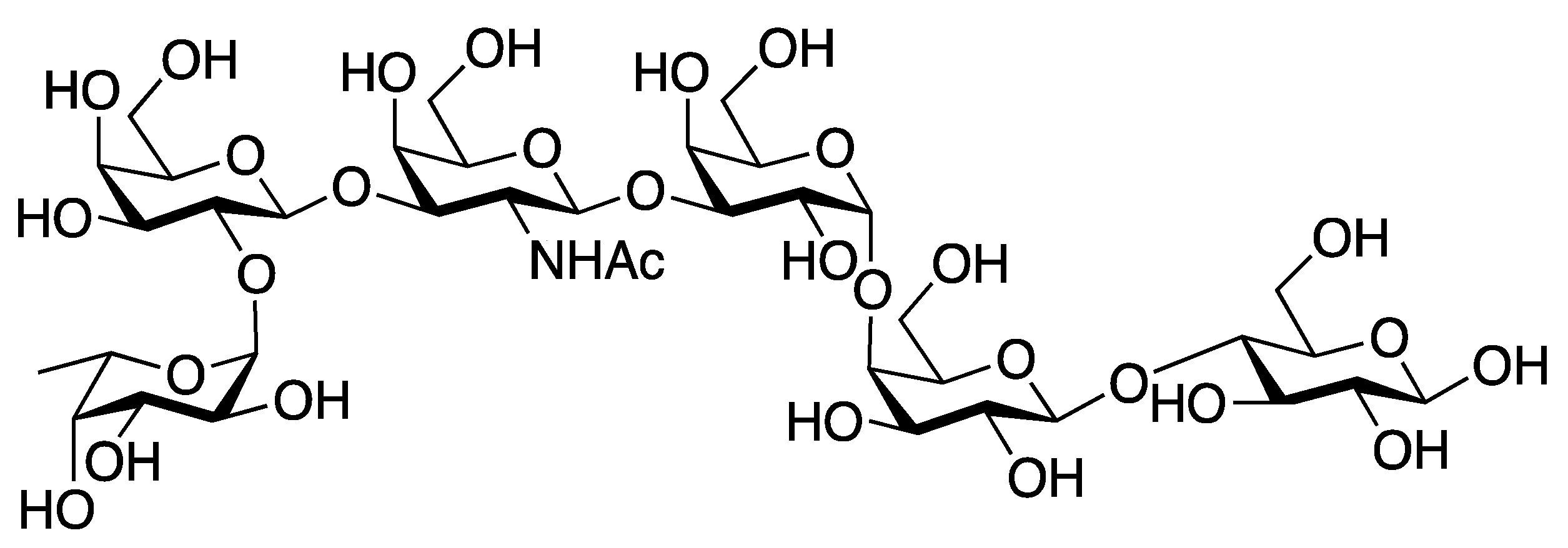
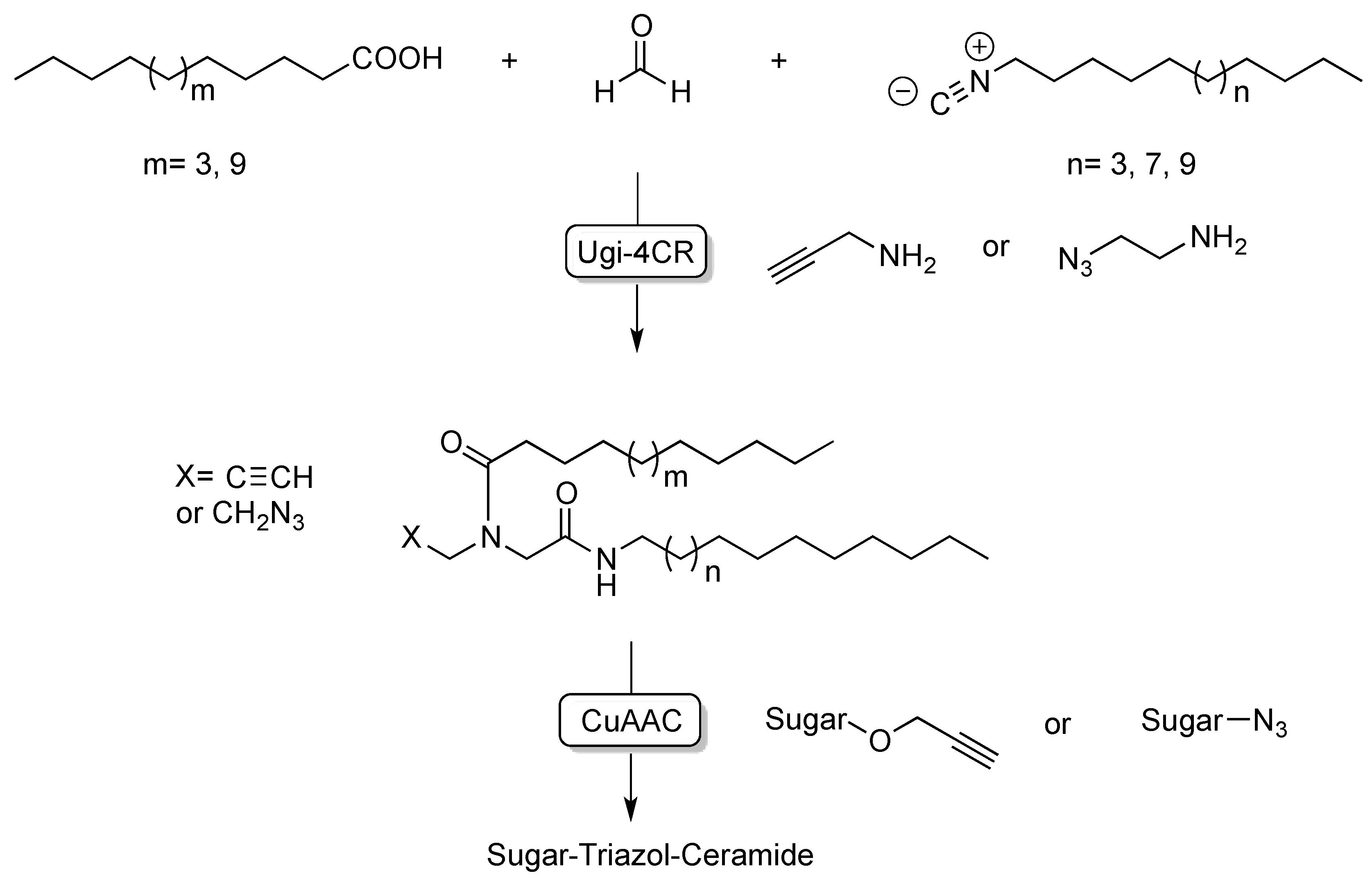
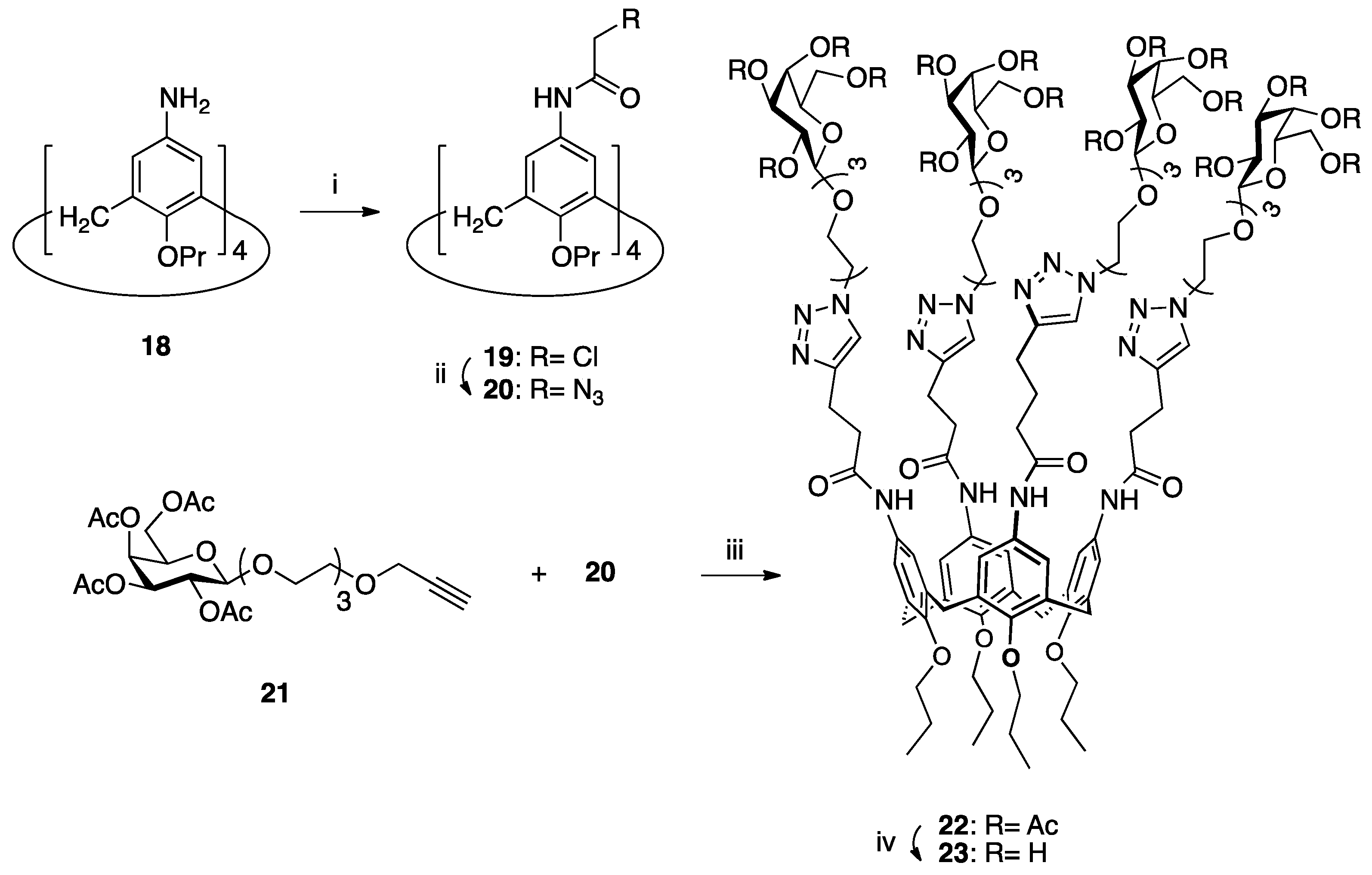
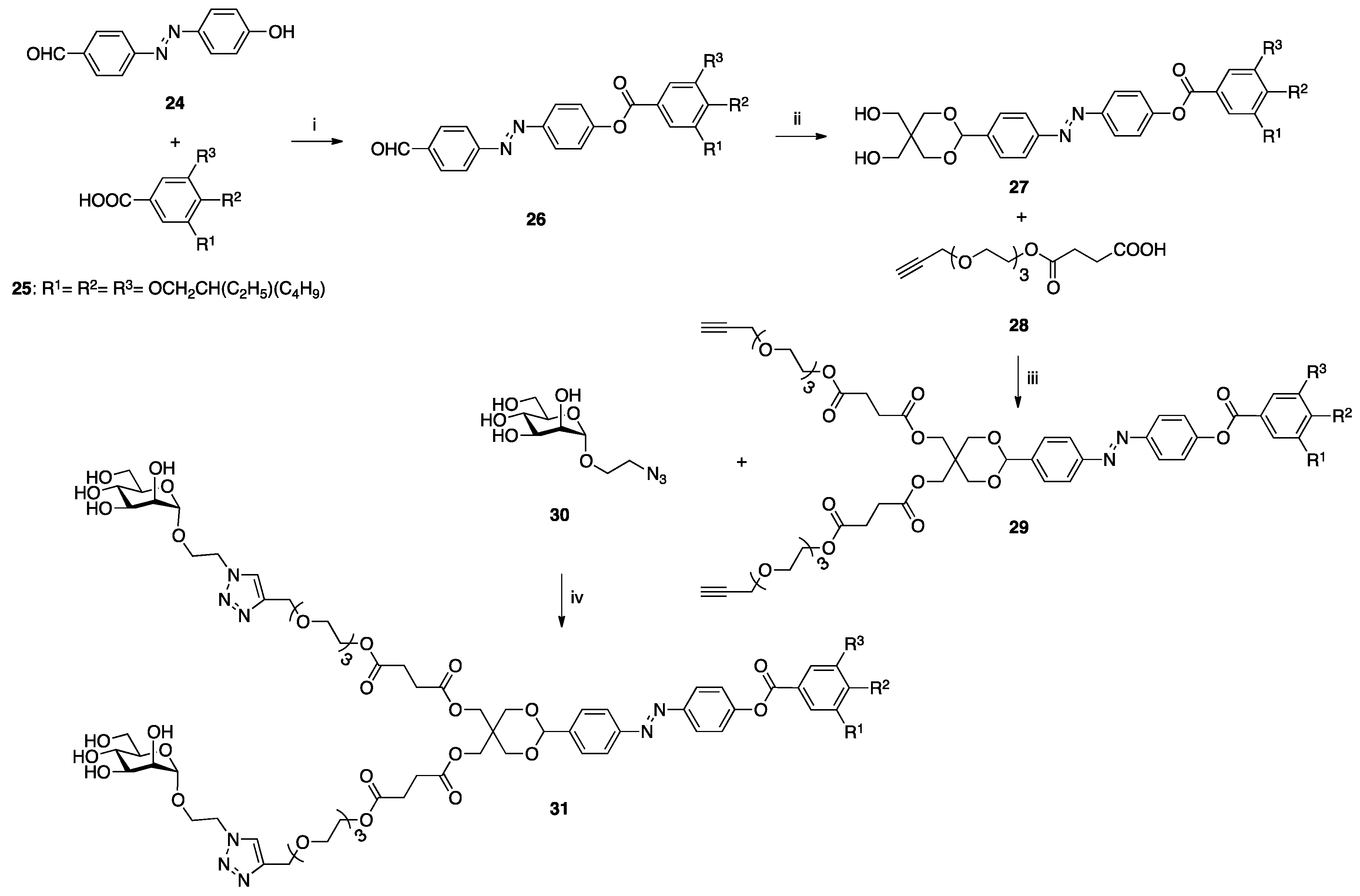




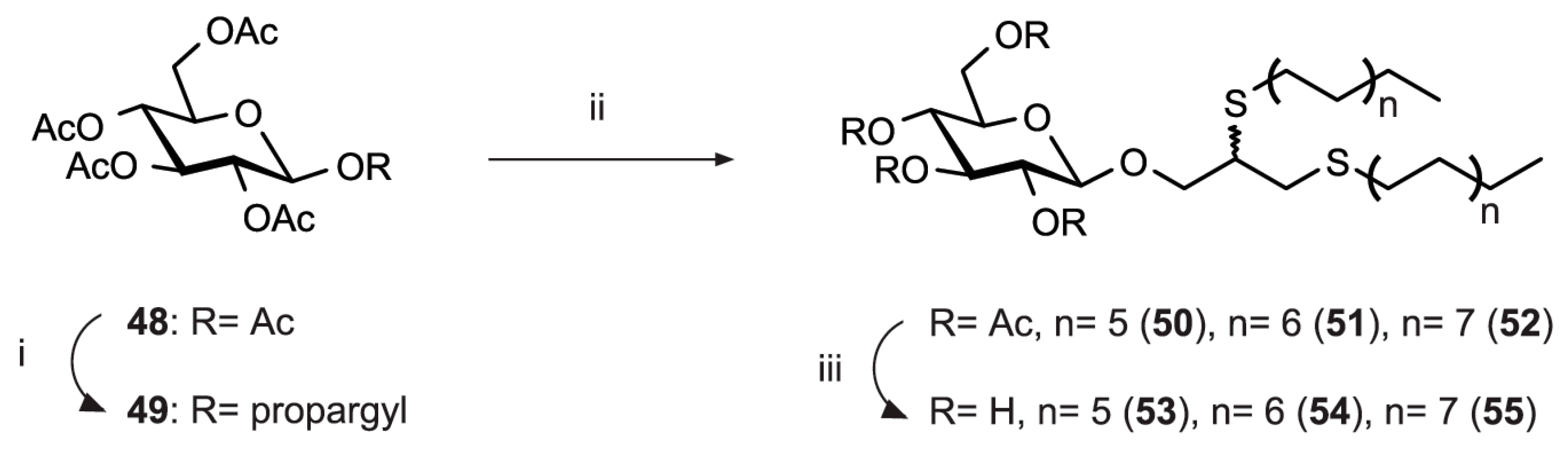
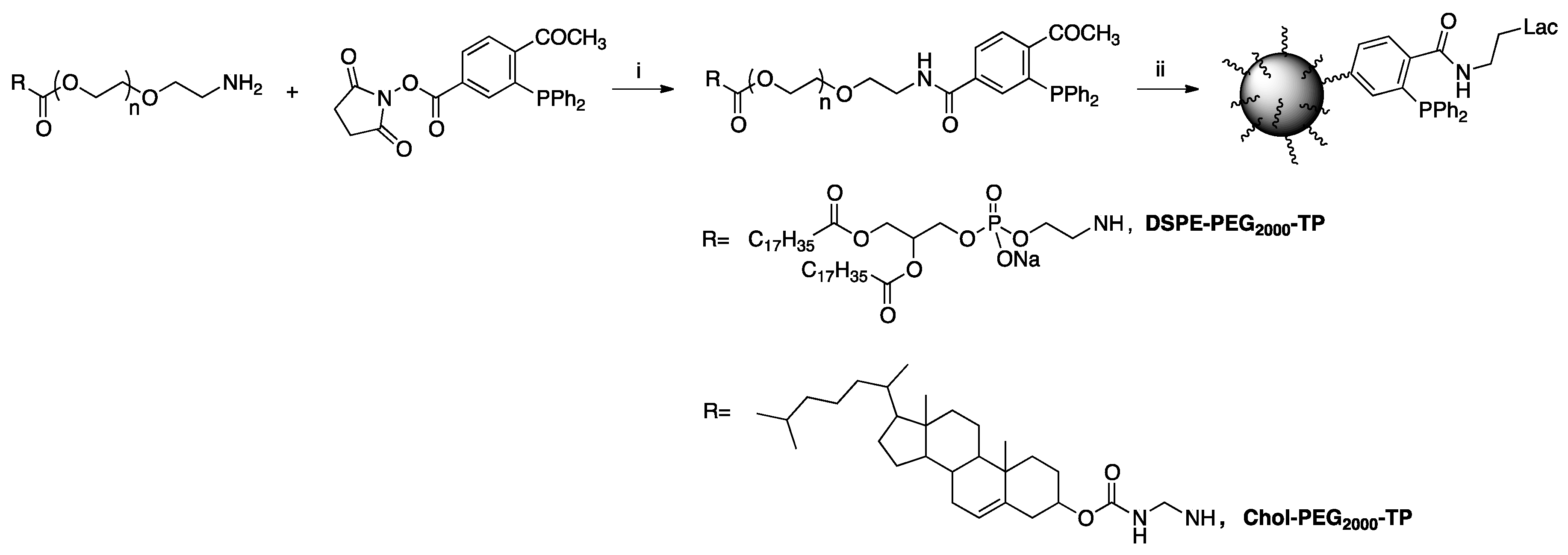

 | [23] | CuAAC click-chemistry |
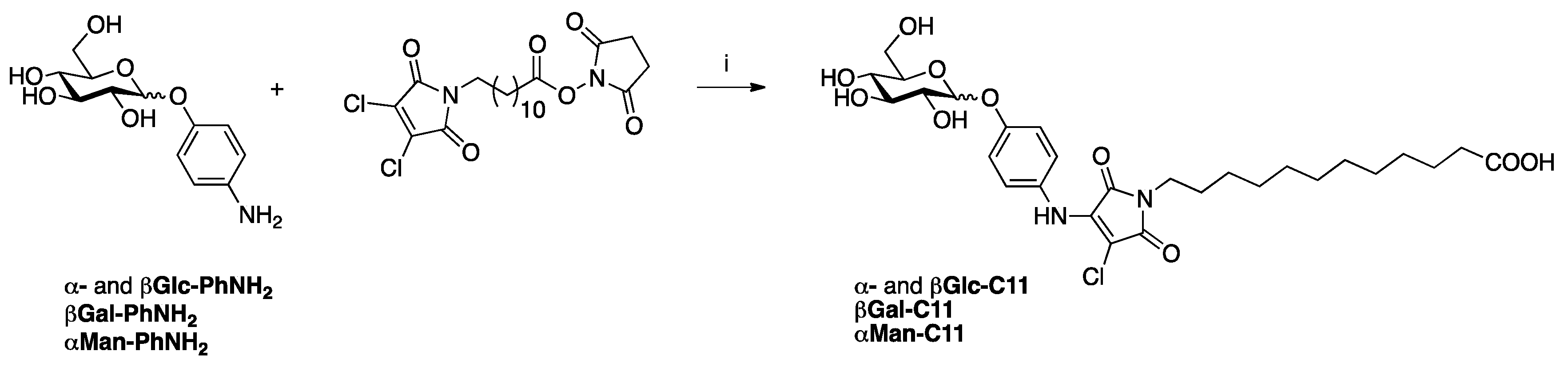
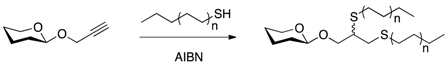 | [24] | Thiol-yne click chemistry |
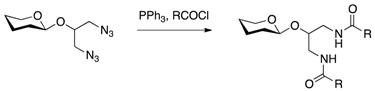 | [25] | Staudinger reaction + activated carbonyl |
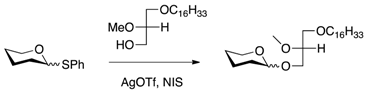 | [26] | Thioglycosidation method |
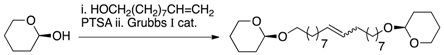 | [27] | Fisher glycosidation + metathesis |
 |
 |
| Staudinger reduction |
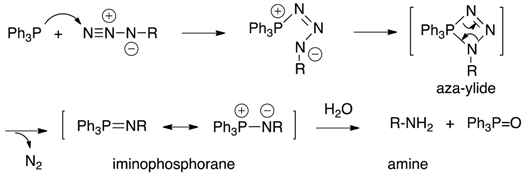 |
| Staudinger ligation |
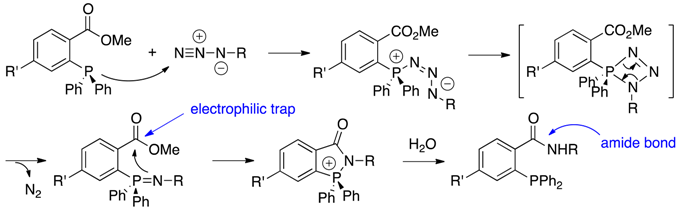 |
| Solvent | Concentration % (w/w) | Morphological Structure | G’ (Pa) | Thixotropic Behaviour | Tgelsol (°C) | Applications | Ref. |
|---|---|---|---|---|---|---|---|
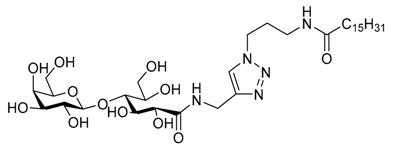 | |||||||
| Water | 2 | Wormlike micelles | ≈500 | Yes | 29 | Reduced biocompatibility | [109] |
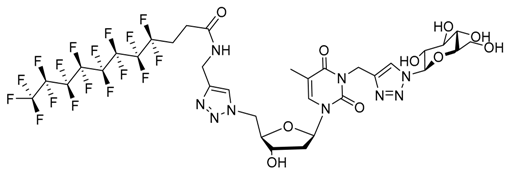 | |||||||
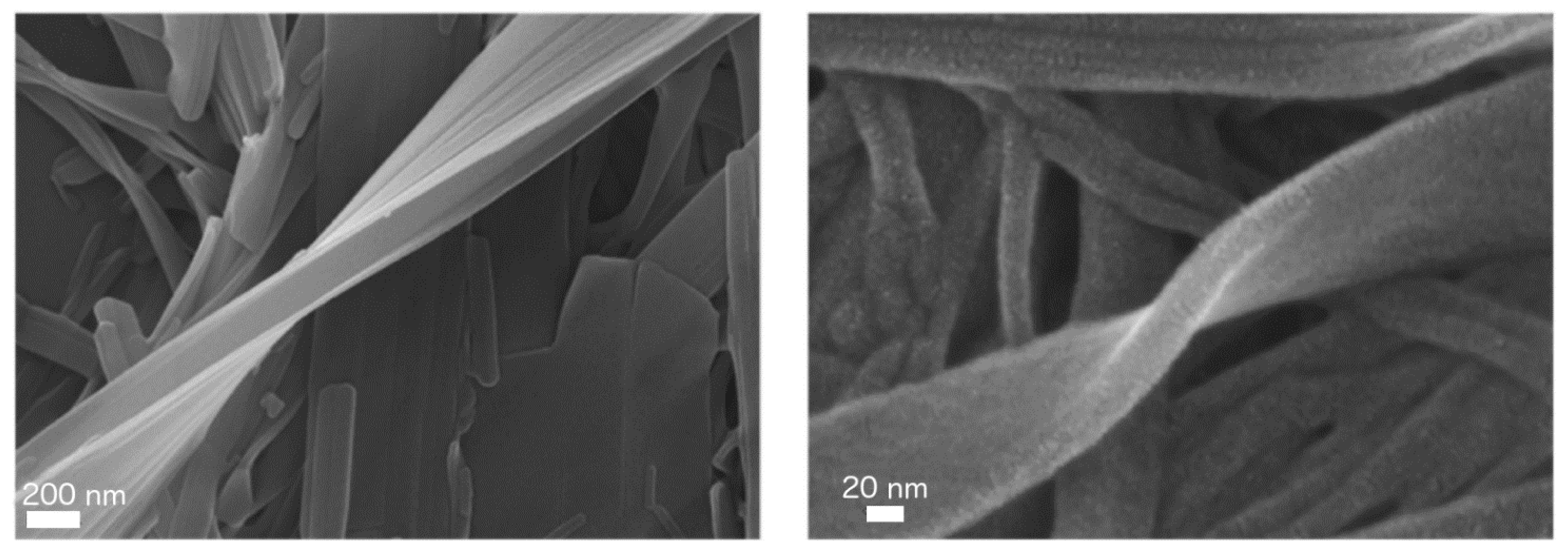
| Water | 1.5 | Nanofibers | 800 | Yes | 52 | Matrix for cell culture Biomaterial Decontamination | [101,102,110,111] |
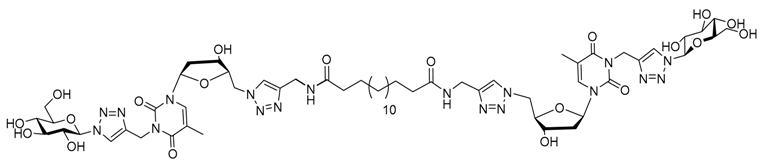 | |||||||
| Water | 11.55 mM | Nanofibers | 5 × 103 | Yes | 62 | Injection in mice Biomaterial | [106] |
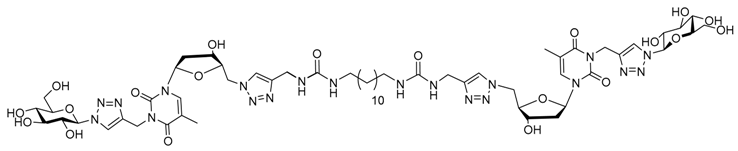 | |||||||
| Water | 11.55 mM | Nanofibers | 12 × 103 | Yes | 79 | Injection in mice No foreign body reaction | [106] |
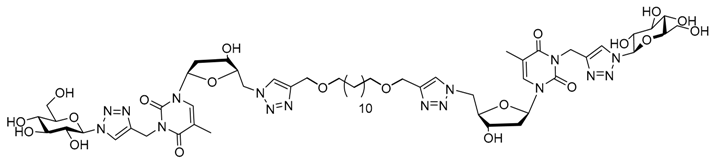 | |||||||
| Water | 2 | Nanofibers | 30 × 103 | Yes | 46 | Scaffold for stem cells | [103] |
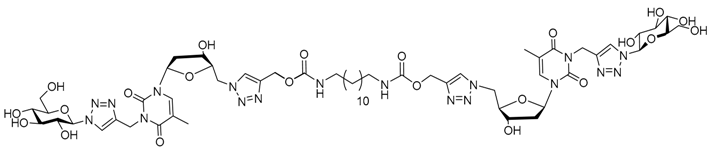 | |||||||
| Water | 4 | Nanofibers | 57 × 103 | Yes | 36 | No biomedical application for the moment | [105] |
 | |||||||
| Water | 5 | Nanofibers | 63 × 103 | - | - | Enzyme/cell responsive capsule | [108] |
 | |||||||
| Cyclohexane | 0.6 | Nanofibers | 100 × 103 | - | 49 | No application for the moment | [107] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latxague, L.; Gaubert, A.; Barthélémy, P. Recent Advances in the Chemistry of Glycoconjugate Amphiphiles. Molecules 2018, 23, 89. https://doi.org/10.3390/molecules23010089
Latxague L, Gaubert A, Barthélémy P. Recent Advances in the Chemistry of Glycoconjugate Amphiphiles. Molecules. 2018; 23(1):89. https://doi.org/10.3390/molecules23010089
Chicago/Turabian StyleLatxague, Laurent, Alexandra Gaubert, and Philippe Barthélémy. 2018. "Recent Advances in the Chemistry of Glycoconjugate Amphiphiles" Molecules 23, no. 1: 89. https://doi.org/10.3390/molecules23010089
APA StyleLatxague, L., Gaubert, A., & Barthélémy, P. (2018). Recent Advances in the Chemistry of Glycoconjugate Amphiphiles. Molecules, 23(1), 89. https://doi.org/10.3390/molecules23010089





