Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements
Abstract
:1. Introduction
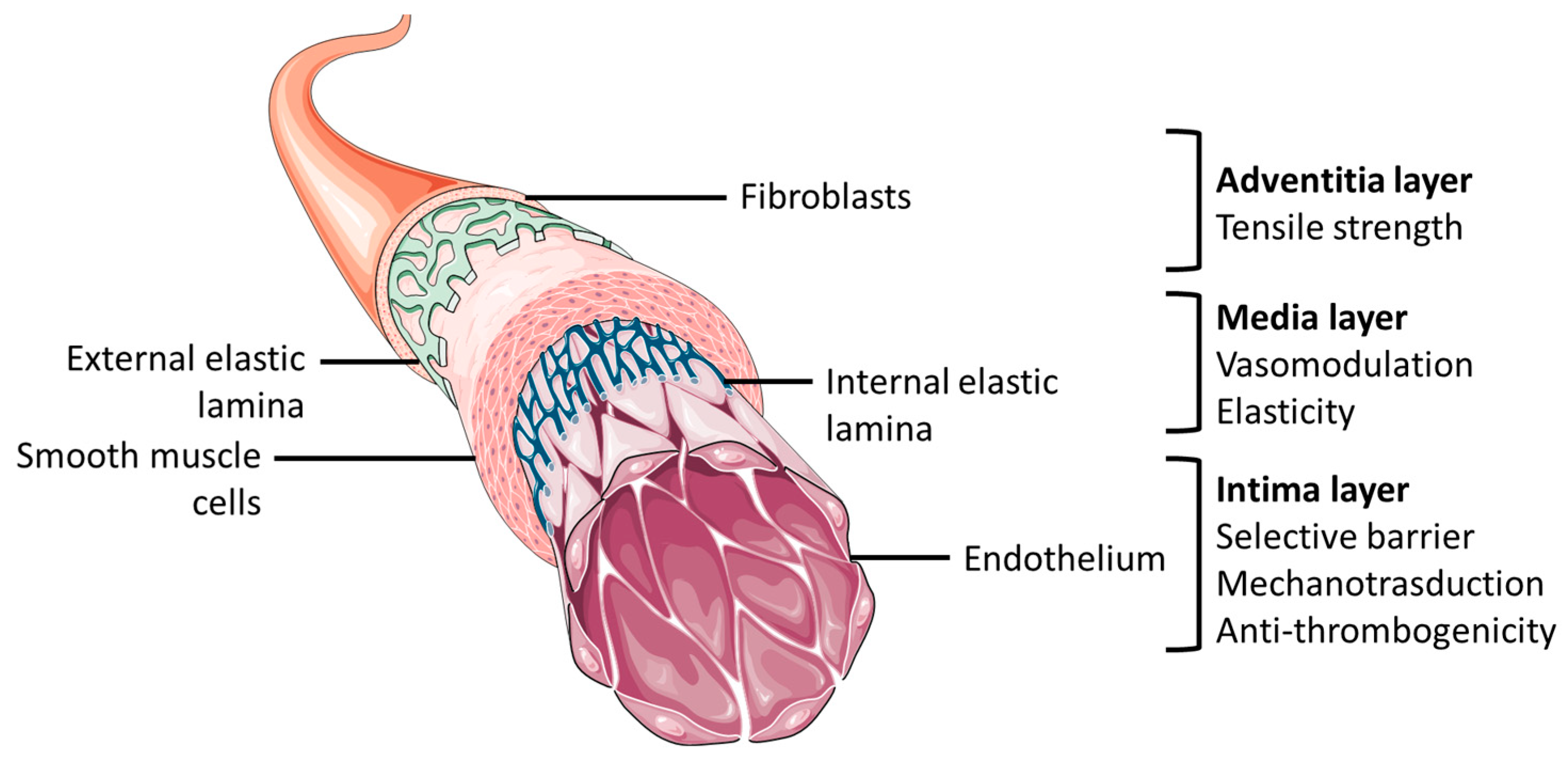
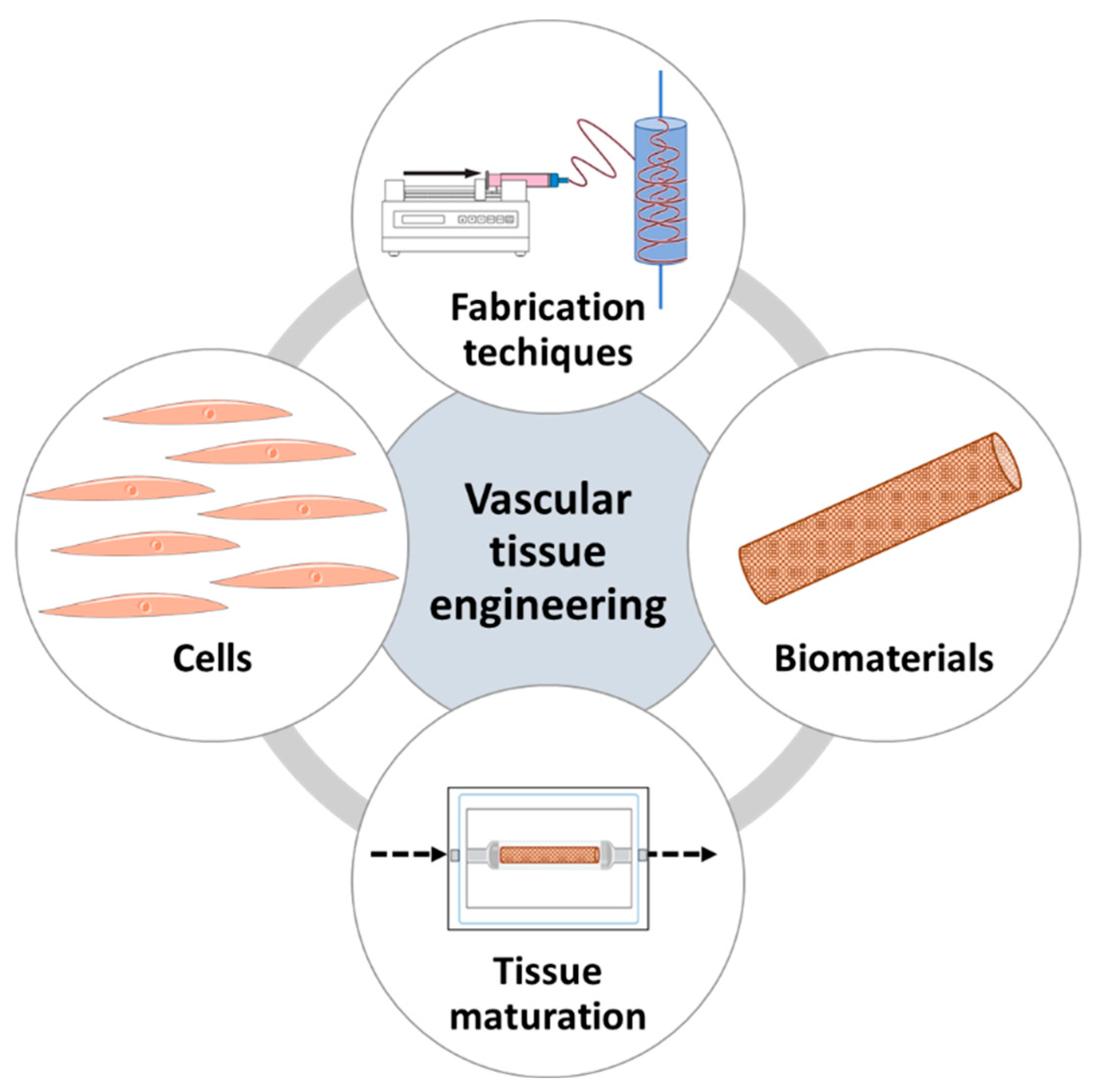
2. Vascular Tissue Engineering Requirements and Fundamentals
2.1. Cell Sources in Vascular Tissue Engineering
2.2. Biomaterials in Vascular Tissue Engineering
2.3. Fabrication Techniques
2.3.1. Cell Sheet Engineering
2.3.2. Decellularized Vessels
2.3.3. Molding
2.3.4. Electrospinning
2.3.5. 3D Printing
2.4. Tissue Maturation
3. Natural Biomaterials for Vascular Tissue Engineering
3.1. Collagen
3.2. Gelatin
3.3. Fibrin
3.4. Elastin
3.5. Silk
3.6. Chitosan
3.7. Decellularized Extracellular Matrix
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.C. Epidemiology of Cardiovascular Disease and Its Risk Factors in Korea. Glob. Health Med. 2021, 3, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS): Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal, Upper and Lower Extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Tan, R.P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering Artificial Blood Vessels from Natural Materials. Trends Biotechnol. 2022, 40, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Farkouh, M.E.; Domanski, M.; Sleeper, L.A.; Siami, F.S.; Dangas, G.; Mack, M.; Yang, M.; Cohen, D.J.; Rosenberg, Y.; Solomon, S.D.; et al. Strategies for Multivessel Revascularization in Patients with Diabetes. N. Engl. J. Med. 2012, 367, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Brilakis, E.S. Saphenous Vein Graft Failure: Seeing the Bigger Picture. J. Thorac. Dis. 2019, 11, S1441–S1444. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.R.; Simons, K.H.; Jukema, J.W.; Braun, J.; Quax, P.H.A. Vein Graft Failure: From Pathophysiology to Clinical Outcomes. Nat. Rev. Cardiol. 2016, 13, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Motwani, J.G.; Topol, E.J. Aortocoronary Saphenous Vein Graft Disease: Pathogenesis, Predisposition, and Prevention. Circulation 1998, 97, 916–931. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; de Winter, R.J.; Alexander, J.H. Saphenous Vein Graft Failure After Coronary Artery Bypass Surgery: Pathophysiology, Management, and Future Directions. Ann. Surg. 2013, 257, 824–833. [Google Scholar] [CrossRef]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef]
- Stöwe, I.; Pissarek, J.; Moosmann, P.; Pröhl, A.; Pantermehl, S.; Bielenstein, J.; Radenkovic, M.; Jung, O.; Najman, S.; Alkildani, S.; et al. Ex Vivo and In Vivo Analysis of a Novel Porcine Aortic Patch for Vascular Reconstruction. Int. J. Mol. Sci. 2021, 22, 7623. [Google Scholar] [CrossRef]
- van de Laar, B.C.; van Heusden, H.C.; Pasker-de Jong, P.C.; van Weel, V. Omniflow II Biosynthetic Grafts versus Expanded Polytetrafluoroethylene Grafts for Infrainguinal Bypass Surgery. A Single-Center Retrospective Analysis. Vascular 2022, 30, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J.-H. In Vivo Performance of Decellularized Vascular Grafts: A Review Article. Int. J. Mol. Sci. 2018, 19, 2101. [Google Scholar] [CrossRef] [PubMed]
- Stieglmeier, F.; Grab, M.; König, F.; Büch, J.; Hagl, C.; Thierfelder, N. Mapping of Bovine Pericardium to Enable a Standardized Acquirement of Material for Medical Implants. J. Mech. Behav. Biomed. Mater. 2021, 118, 104432. [Google Scholar] [CrossRef] [PubMed]
- Obiweluozor, F.O.; Emechebe, G.A.; Kim, D.-W.; Cho, H.-J.; Park, C.H.; Kim, C.S.; Jeong, I.S. Considerations in the Development of Small-Diameter Vascular Graft as an Alternative for Bypass and Reconstructive Surgeries: A Review. Cardiovasc. Eng. Technol. 2020, 11, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-X.; Wei, Q.-Q.; Mo, H.-L.; Ren, Y.; Zhang, W.; Lu, H.-J.; Joung, Y.K. Challenges and Advances in Materials and Fabrication Technologies of Small-Diameter Vascular Grafts. Biomater. Res. 2023, 27, 58. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Soto, M.A.; Polanía-Sandoval, C.A.; Aragón-Rivera, A.M.; Buitrago, D.; Ayala-Velásquez, M.; Velandia-Sánchez, A.; Peralta Peluffo, G.; Cruz, J.C.; Muñoz Camargo, C.; Camacho-Mackenzie, J.; et al. Small Diameter Cell-Free Tissue-Engineered Vascular Grafts: Biomaterials and Manufacture Techniques to Reach Suitable Mechanical Properties. Polymers 2022, 14, 3440. [Google Scholar] [CrossRef] [PubMed]
- Skovrind, I.; Harvald, E.B.; Juul Belling, H.; Jørgensen, C.D.; Lindholt, J.S.; Andersen, D.C. Concise Review: Patency of Small-Diameter Tissue-Engineered Vascular Grafts: A Meta-Analysis of Preclinical Trials. Stem Cells Transl. Med. 2019, 8, 671–680. [Google Scholar] [CrossRef]
- Gupta, P.; Mandal, B.B. Silk Biomaterials for Vascular Tissue Engineering Applications. Acta Biomater. 2021, 134, 79–106. [Google Scholar] [CrossRef]
- Chen, S.-G.; Ugwu, F.; Li, W.-C.; Caplice, N.M.; Petcu, E.; Yip, S.P.; Huang, C.-L. Vascular Tissue Engineering: Advanced Techniques and Gene Editing in Stem Cells for Graft Generation. Tissue Eng. Part B Rev. 2021, 27, 14–28. [Google Scholar] [CrossRef]
- Weinberg, C.B.; Bell, E. A Blood Vessel Model Constructed from Collagen and Cultured Vascular Cells. Science 1986, 231, 397–400. [Google Scholar] [CrossRef]
- Iwaki, R.; Shoji, T.; Matsuzaki, Y.; Ulziibayar, A.; Shinoka, T. Current Status of Developing Tissue Engineering Vascular Technologies. Expert Opin. Biol. Ther. 2022, 22, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Soto, M.A.; Suarez Vargas, N.; Riveros, A.; Camargo, C.M.; Cruz, J.C.; Sandoval, N.; Briceño, J.C. Failure Analysis of TEVG’s I: Overcoming the Initial Stages of Blood Material Interaction and Stabilization of the Immune Response. Cells 2021, 10, 3140. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, F.; Guo, Z.; Zhao, Q. Tissue-Engineered Vascular Grafts and Regeneration Mechanisms. J. Mol. Cell. Cardiol. 2022, 165, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Thottappillil, N.; Nair, P.D. Scaffolds in Vascular Regeneration: Current Status. Vasc. Health Risk Manag. 2015, 11, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bosch-Rué, È.; Pérez, R.A.; Truskey, G.A. Biofabrication of Tissue Engineering Vascular Systems. APL Bioeng. 2021, 5, 021507. [Google Scholar] [CrossRef] [PubMed]
- Durán-Rey, D.; Crisóstomo, V.; Sánchez-Margallo, J.A.; Sánchez-Margallo, F.M. Systematic Review of Tissue-Engineered Vascular Grafts. Front. Bioeng. Biotechnol. 2021, 9, 771400. [Google Scholar] [CrossRef]
- Gupta, P.; Mandal, B.B. Tissue-Engineered Vascular Grafts: Emerging Trends and Technologies. Adv. Funct. Mater. 2021, 31, 2100027. [Google Scholar] [CrossRef]
- Chlupáč, J.; Filová, E.; Bačáková, L. Blood Vessel Replacement: 50 Years of Development and Tissue Engineering Paradigms in Vascular Surgery. Physiol. Res. 2009, 58 (Suppl. S2), S119–S140. [Google Scholar] [CrossRef]
- Blume, C.; Kraus, X.; Heene, S.; Loewner, S.; Stanislawski, N.; Cholewa, F.; Blume, H. Vascular Implants—New Aspects for in Situ Tissue Engineering. Eng. Life Sci. 2022, 22, 344–360. [Google Scholar] [CrossRef]
- Wang, Y.; Keshavarz, M.; Barhouse, P.; Smith, Q. Strategies for Regenerative Vascular Tissue Engineering. Adv. Biol. 2022, 7, e2200050. [Google Scholar] [CrossRef]
- Liu, C.; Niu, K.; Xiao, Q. Updated Perspectives on Vascular Cell Specification and Pluripotent Stem Cell-Derived Vascular Organoids for Studying Vasculopathies. Cardiovasc. Res. 2020, 118, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shi, X.; Lin, Y.; Yuan, Y.; Kural, M.H.; Wang, J.; Ellis, M.W.; Anderson, C.W.; Zhang, S.-M.; Riaz, M.; et al. Efficient Differentiation of Human Induced Pluripotent Stem Cells into Endothelial Cells under Xenogeneic-Free Conditions for Vascular Tissue Engineering. Acta Biomater. 2021, 119, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Jouda, H.; Larrea Murillo, L.; Wang, T. Current Progress in Vascular Engineering and Its Clinical Applications. Cells 2022, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Chaikof, E.L. Biomaterials for Vascular Tissue Engineering. Regen. Med. 2010, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Hielscher, D.; Kaebisch, C.; Braun, B.J.V.; Gray, K.; Tobiasch, E. Stem Cell Sources and Graft Material for Vascular Tissue Engineering. Stem Cell Rev. Rep. 2018, 14, 642–667. [Google Scholar] [CrossRef] [PubMed]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer- and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Mosca, C.; Ramella, M.; Carmagnola, I.; Chiono, V.; Ciardelli, G.; Cannas, M. Biological Evaluation of Materials for Cardiovascular Application: The Role of the Short-Term Inflammatory Response in Endothelial Regeneration. J. Biomed. Mater. Res. Part A 2013, 101, 3131–3140. [Google Scholar] [CrossRef]
- Stahl, A.; Hao, D.; Barrera, J.; Henn, D.; Lin, S.; Moeinzadeh, S.; Kim, S.; Maloney, W.; Gurtner, G.; Wang, A.; et al. A Bioactive Compliant Vascular Graft Modulates Macrophage Polarization and Maintains Patency with Robust Vascular Remodeling. Bioact. Mater. 2022, 19, 167–178. [Google Scholar] [CrossRef]
- Leal, B.B.J.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts. Front. Cardiovasc. Med. 2021, 7, 592361. [Google Scholar] [CrossRef]
- Viera Rey, D.F.; St-Pierre, J.-P. 6—Fabrication Techniques of Tissue Engineering Scaffolds. In Handbook of Tissue Engineering Scaffolds: Volume One; Mozafari, M., Sefat, F., Atala, A., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 109–125. ISBN 978-0-08-102563-5. [Google Scholar]
- Wang, Z.; Mithieux, S.M.; Weiss, A.S. Fabrication Techniques for Vascular and Vascularized Tissue Engineering. Adv. Healthc. Mater. 2019, 8, 1900742. [Google Scholar] [CrossRef]
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef] [PubMed]
- von Bornstädt, D.; Wang, H.; Paulsen, M.J.; Goldstone, A.B.; Eskandari, A.; Thakore, A.; Stapleton, L.; Steele, A.N.; Truong, V.N.; Jaatinen, K.; et al. Rapid Self-Assembly of Bioengineered Cardiovascular Bypass Grafts from Scaffold-Stabilized, Tubular Bi-Level Cell Sheets. Circulation 2018, 138, 2130–2144. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, R.; Ahsan, T.; Larouche, D.; Lévesque, P.; Dubé, J.; Auger, F.A.; Nerem, R.M.; Germain, L. A Novel Single-Step Self-Assembly Approach for the Fabrication of Tissue-Engineered Vascular Constructs. Tissue Eng. Part A 2010, 16, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Catto, V.; Farè, S.; Freddi, G.; Tanzi, M. Vascular Tissue Engineering: Recent Advances in Small Diameter Blood Vessel Regeneration. ISRN Vasc. Med. 2014, 2014, 27. [Google Scholar] [CrossRef]
- Goins, A.; Webb, A.R.; Allen, J.B. Multi-Layer Approaches to Scaffold-Based Small Diameter Vessel Engineering: A Review. Mater. Sci. Eng. C 2019, 97, 896–912. [Google Scholar] [CrossRef] [PubMed]
- L’heureux, N.; Pâquet, S.; Labbé, R.; Germain, L.; Auger, F.A. A Completely Biological Tissue-Engineered Human Blood Vessel. FASEB J. 1998, 12, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.; Gluais, M.; Da Silva, N.; Rey, S.; Grémare, A.; Magnan, L.; Kawecki, F.; L’Heureux, N. Cell-Assembled Extracellular Matrix (CAM) Sheet Production: Translation from Using Human to Large Animal Cells. J. Tissue Eng. 2021, 12, 2041731420978327. [Google Scholar] [CrossRef]
- Magnan, L.; Labrunie, G.; Fénelon, M.; Dusserre, N.; Foulc, M.-P.; Lafourcade, M.; Svahn, I.; Gontier, E.; Vélez V, J.H.; McAllister, T.N.; et al. Human Textiles: A Cell-Synthesized Yarn as a Truly “Bio” Material for Tissue Engineering Applications. Acta Biomater. 2020, 105, 111–120. [Google Scholar] [CrossRef]
- McAllister, T.N.; Maruszewski, M.; Garrido, S.A.; Wystrychowski, W.; Dusserre, N.; Marini, A.; Zagalski, K.; Fiorillo, A.; Avila, H.; Manglano, X.; et al. Effectiveness of Haemodialysis Access with an Autologous Tissue-Engineered Vascular Graft: A Multicentre Cohort Study. Lancet 2009, 373, 1440–1446. [Google Scholar] [CrossRef]
- Wystrychowski, W.; Garrido, S.A.; Marini, A.; Dusserre, N.; Radochonski, S.; Zagalski, K.; Antonelli, J.; Canalis, M.; Sammartino, A.; Darocha, Z.; et al. Long-Term Results of Autologous Scaffold-Free Tissue-Engineered Vascular Graft for Hemodialysis Access. J. Vasc. Access 2022, 11297298221095994. [Google Scholar] [CrossRef]
- Wang, X.; Chan, V.; Corridon, P.R. Decellularized Blood Vessel Development: Current State-of-the-Art and Future Directions. Front. Bioeng. Biotechnol. 2022, 10, 951644. [Google Scholar] [CrossRef]
- Conklin, B.S.; Richter, E.R.; Kreutziger, K.L.; Zhong, D.-S.; Chen, C. Development and Evaluation of a Novel Decellularized Vascular Xenograft. Med. Eng. Phys. 2002, 24, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, J.; Cai, Z.; Xing, Y.; Wang, C.; Guo, L.; Gu, Y. Decellularization of Porcine Carotid Arteries Using Low-Concentration Sodium Dodecyl Sulfate. Int. J. Artif. Organs 2021, 44, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Gou, K.; Hu, J.-J.; Baek, S. Mechanical Characterization of Human Umbilical Arteries by Thick-Walled Models: Enhanced Vascular Compliance by Removing an Abluminal Lining. J. Mech. Behav. Biomed. Mater. 2023, 142, 105811. [Google Scholar] [CrossRef]
- Fayon, A.; Menu, P.; El Omar, R. Cellularized Small-Caliber Tissue-Engineered Vascular Grafts: Looking for the Ultimate Gold Standard. npj Regen. Med. 2021, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, J.; Simsa, R.; Bogestål, Y.; Jenndahl, L.; Gustafsson-Hedberg, T.; Petronis, S.; Strehl, R.; Österberg, K. Individualized Tissue-Engineered Veins as Vascular Grafts: A Proof of Concept Study in Pig. J. Tissue Eng. Regen. Med. 2021, 15, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-Y.; Lin, Y.-W.; Wu, C.-C.; Lin, C.-Y.; Hsu, P.-S.; Periasamy, S.; Nagarajan, B.; Hsieh, D.-J.; Tsai, Y.-T.; Tsai, C.-S.; et al. Supercritical Carbon Dioxide-Decellularized Arteries Exhibit Physiologic-like Vessel Regeneration Following Xenotransplantation in Rats. Biomater. Sci. 2023, 11, 2566–2580. [Google Scholar] [CrossRef]
- Badylak, S.F.; Lantz, G.C.; Coffey, A.; Geddes, L.A. Small Intestinal Submucosa as a Large Diameter Vascular Graft in the Dog. J. Surg. Res. 1989, 47, 74–80. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Qiao, W.; Shi, J.; Qiu, X.; Dong, N. Application of Decellularized Vascular Matrix in Small-Diameter Vascular Grafts. Front. Bioeng. Biotechnol. 2023, 10, 1081233. [Google Scholar] [CrossRef]
- Mahara, A.; Somekawa, S.; Kobayashi, N.; Hirano, Y.; Kimura, Y.; Fujisato, T.; Yamaoka, T. Tissue-Engineered Acellular Small Diameter Long-Bypass Grafts with Neointima-Inducing Activity. Biomaterials 2015, 58, 54–62. [Google Scholar] [CrossRef]
- Schneider, K.H.; Rohringer, S.; Kapeller, B.; Grasl, C.; Kiss, H.; Heber, S.; Walter, I.; Teuschl, A.H.; Podesser, B.K.; Bergmeister, H. Riboflavin-Mediated Photooxidation to Improve the Characteristics of Decellularized Human Arterial Small Diameter Vascular Grafts. Acta Biomater. 2020, 116, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Tardalkar, K.; Marsale, T.; Bhamare, N.; Kshersagar, J.; Chaudhari, L.; Joshi, M.G. Heparin Immobilization of Tissue Engineered Xenogeneic Small Diameter Arterial Scaffold Improve Endothelialization. Tissue Eng. Regen. Med. 2022, 19, 505–523. [Google Scholar] [CrossRef]
- Caneparo, C.; Chabaud, S.; Bolduc, S. Reconstruction of Vascular and Urologic Tubular Grafts by Tissue Engineering. Processes 2021, 9, 513. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Iglesias-Mejuto, A.; García-González, C.A. Solvent-Free Approaches for the Processing of Scaffolds in Regenerative Medicine. Polymers 2020, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Rajan, N.; Habermehl, J.; Mantovani, D. Preparation and Characterization of a Scaffold for Vascular Tissue Engineering by Direct-Assembling of Collagen and Cells in a Cylindrical Geometry. Macromol. Biosci. 2007, 7, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Helms, F.; Lau, S.; Aper, T.; Zippusch, S.; Klingenberg, M.; Haverich, A.; Wilhelmi, M.; Böer, U. A 3-Layered Bioartificial Blood Vessel with Physiological Wall Architecture Generated by Mechanical Stimulation. Ann. Biomed. Eng. 2021, 49, 2066–2079. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, M.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun Nanofiber Scaffold for Vascular Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112373. [Google Scholar] [CrossRef]
- Ozdemir, S.; Yalcin-Enis, I.; Yalcinkaya, B.; Yalcinkaya, F. An Investigation of the Constructional Design Components Affecting the Mechanical Response and Cellular Activity of Electrospun Vascular Grafts. Membranes 2022, 12, 929. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, X.; Meng, X.; Guo, X.; Jiang, Y.; Xu, Y.; Li, Q.; Shen, C. Controllable Fiber Orientation and Nonlinear Elasticity of Electrospun Nanofibrous Small Diameter Tubular Scaffolds for Vascular Tissue Engineering. Biomed. Mater. 2019, 14, 035006. [Google Scholar] [CrossRef]
- Do, T.M.; Yang, Y.; Deng, A. Porous Bilayer Vascular Grafts Fabricated from Electrospinning of the Recombinant Human Collagen (RHC) Peptide-Based Blend. Polymers 2021, 13, 4042. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Y.; Shi, X.; Shen, H.; Ning, H.; Liu, H. 3D Printing of Tissue Engineering Scaffolds: A Focus on Vascular Regeneration. Bio-Design Manuf. 2021, 4, 344–378. [Google Scholar] [CrossRef] [PubMed]
- Fazal, F.; Raghav, S.; Callanan, A.; Koutsos, V.; Radacsi, N. Recent Advancements in the Bioprinting of Vascular Grafts. Biofabrication 2021, 13, 032003. [Google Scholar] [CrossRef] [PubMed]
- Weekes, A.; Bartnikowski, N.; Pinto, N.; Jenkins, J.; Meinert, C.; Klein, T.J. Biofabrication of Small Diameter Tissue-Engineered Vascular Grafts. Acta Biomater. 2022, 138, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.; Ramos, R.; Alexis Chando, P.; Zhou, L.; Reeser, K.; Jin, S.; Soman, P.; Ye, K. A Bioink Blend for Rotary 3D Bioprinting Tissue Engineered Small-Diameter Vascular Constructs. Acta Biomater. 2019, 95, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Nowicki, M.; Sun, H.; Hann, S.Y.; Cui, H.; Esworthy, T.; Lee, J.D.; Plesniak, M.; Zhang, L.G. 3D Bioprinting-Tunable Small-Diameter Blood Vessels with Biomimetic Biphasic Cell Layers. ACS Appl. Mater. Interfaces 2020, 12, 45904–45915. [Google Scholar] [CrossRef]
- Mertsching, H.; Hansmann, J. Bioreactor Technology in Cardiovascular Tissue Engineering. In Bioreactor Systems for Tissue Engineering; Kasper, C., van Griensven, M., Pörtner, R., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 29–37. ISBN 978-3-540-69357-4. [Google Scholar]
- Mitchell, T.C.; Feng, N.L.; Lam, Y.T.; Michael, P.L.; Santos, M.; Wise, S.G. Engineering Vascular Bioreactor Systems to Closely Mimic Physiological Forces In Vitro. Tissue Eng. Part B Rev. 2023, 29, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Çelebi-Saltik, B.; Öteyaka, M.Ö.; Gökçinar-Yagci, B. Stem Cell-Based Small-Diameter Vascular Grafts in Dynamic Culture. Connect. Tissue Res. 2021, 62, 151–163. [Google Scholar] [CrossRef]
- Kobayashi, N.; Yasu, T.; Ueba, H.; Sata, M.; Hashimoto, S.; Kuroki, M.; Saito, M.; Kawakami, M. Mechanical Stress Promotes the Expression of Smooth Muscle-like Properties in Marrow Stromal Cells. Exp. Hematol. 2004, 32, 1238–1245. [Google Scholar] [CrossRef]
- Bono, N.; Meghezi, S.; Soncini, M.; Piola, M.; Mantovani, D.; Fiore, G.B. A Dual-Mode Bioreactor System for Tissue Engineered Vascular Models. Ann. Biomed. Eng. 2017, 45, 1496–1510. [Google Scholar] [CrossRef]
- Rizzi, S.; Mantero, S.; Boschetti, F.; Pesce, M. Luminal Endothelialization of Small Caliber Silk Tubular Graft for Vascular Constructs Engineering. Front. Cardiovasc. Med. 2022, 9, 1013183. [Google Scholar] [CrossRef] [PubMed]
- Pennings, I.; van Haaften, E.E.; Jungst, T.; Bulsink, J.A.; Rosenberg, A.J.W.P.; Groll, J.; Bouten, C.V.C.; Kurniawan, N.A.; Smits, A.I.P.M.; Gawlitta, D. Layer-Specific Cell Differentiation in Bi-Layered Vascular Grafts under Flow Perfusion. Biofabrication 2019, 12, 015009. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Qin, L.; Zhao, L.; Gui, L.; Ellis, M.W.; Huang, Y.; Kural, M.H.; Clark, J.A.; Ono, S.; Wang, J.; et al. Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human iPSCs. Cell Stem Cell 2020, 26, 251–261.e8. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, J.P.; Padalhin, A.; Lee, B.-T. Small-Diameter Decellularized Vascular Graft with Electrospun Polycaprolactone. Mater. Lett. 2021, 284, 128973. [Google Scholar] [CrossRef]
- Emechebe, G.A.; Obiweluozor, F.O.; Jeong, I.S.; Park, J.-K.; Park, C.H.; Kim, C.S. Merging 3D Printing with Electrospun Biodegradable Small-Caliber Vascular Grafts Immobilized with VEGF. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102306. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.-H.; Kim, T.-H.; Kim, T.-S.; Min, T.-J.; Lee, J.-H.; Yoo, S.-M.; Kim, J.-W.; Lee, J.-E.; Kim, C.-H.; Park, S.-H.; et al. Evaluation of 3D Templated Synthetic Vascular Graft Compared with Standard Graft in a Rat Model: Potential Use as an Artificial Vascular Graft in Cardiovascular Disease. Materials 2021, 14, 1239. [Google Scholar] [CrossRef] [PubMed]
- Lopera Higuita, M.; Lopera Giraldo, J.F.; Sarrafian, T.L.; Griffiths, L.G. Tissue Engineered Bovine Saphenous Vein Extracellular Matrix Scaffolds Produced via Antigen Removal Achieve High in Vivo Patency Rates. Acta Biomater. 2021, 134, 144–159. [Google Scholar] [CrossRef]
- Antunes, M.; Bonani, W.; Reis, R.L.; Migliaresi, C.; Ferreira, H.; Motta, A.; Neves, N.M. Development of Alginate-Based Hydrogels for Blood Vessel Engineering. Biomater. Adv. 2022, 134, 112588. [Google Scholar] [CrossRef]
- Cuenca, J.P.; Kang, H.-J.; Fahad, M.A.A.; Park, M.; Choi, M.; Lee, H.-Y.; Lee, B.-T. Physico-Mechanical and Biological Evaluation of Heparin/VEGF-Loaded Electrospun Polycaprolactone/Decellularized Rat Aorta Extracellular Matrix for Small-Diameter Vascular Grafts. J. Biomater. Sci. Polym. Ed. 2022, 33, 1664–1684. [Google Scholar] [CrossRef]
- Saunders, S.K.; Cole, S.Y.; Sierra, V.A.; Bracamonte, J.H.; Toldo, S.; Soares, J.S. Evaluation of Perfusion-Driven Cell Seeding of Small Diameter Engineered Tissue Vascular Grafts with a Custom-Designed Seed-and-Culture Bioreactor. PLoS ONE 2022, 17, e0269499. [Google Scholar] [CrossRef]
- Lu, X.; Zou, H.; Liao, X.; Xiong, Y.; Hu, X.; Cao, J.; Pan, J.; Li, C.; Zheng, Y. Construction of PCL-collagen@PCL@PCL-Gelatin Three-Layer Small Diameter Artificial Vascular Grafts by Electrospinning. Biomed. Mater. 2022, 18, 015008. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Shamloo, A.; Kalantarnia, F. Tubular TPU/SF Nanofibers Covered with Chitosan-Based Hydrogels as Small-Diameter Vascular Grafts with Enhanced Mechanical Properties. Sci. Rep. 2022, 12, 6179. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Jin, S.; Kim, S.; Son, D.; Shin, M. Tyramine-Functionalized Alginate-Collagen Hybrid Hydrogel Inks for 3D-Bioprinting. Polymers 2022, 14, 3173. [Google Scholar] [CrossRef] [PubMed]
- Caldiroli, A.; Pederzani, E.; Pezzotta, M.; Azzollini, N.; Fiori, S.; Tironi, M.; Rizzo, P.; Sangalli, F.; Figliuzzi, M.; Fiore, G.B.; et al. Hybrid Fibroin/Polyurethane Small-Diameter Vascular Grafts: From Fabrication Toin Vivopreliminary Assessment. Biomed. Mater. 2022, 17, 055015. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Karakaya, E.; Arslan, T.S.; Atila, D.; Erdogan, Y.K.; Arslan, Y.E.; Eskizengin, H.; Eylem, C.C.; Nemutlu, E.; Ercan, B.; et al. 3D Printing of Extracellular Matrix-Based Multicomponent, All-Natural, Highly Elastic, and Functional Materials toward Vascular Tissue Engineering. Adv. Healthc. Mater. 2023, 12, 2203044. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive Potential of Natural Biomaterials: Identification, Retention and Assessment of Biological Properties. Sig Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Brovold, M.; Almeida, J.I.; Pla-Palacín, I.; Sainz-Arnal, P.; Sánchez-Romero, N.; Rivas, J.J.; Almeida, H.; Dachary, P.R.; Serrano-Aulló, T.; Soker, S.; et al. Naturally-Derived Biomaterials for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1077, 421–449. [Google Scholar] [CrossRef]
- Ullah, S.; Chen, X. Fabrication, Applications and Challenges of Natural Biomaterials in Tissue Engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ma, Z.; Yong, T.; Teo, W.E.; Ramakrishna, S. Fabrication of Collagen-Coated Biodegradable Polymer Nanofiber Mesh and Its Potential for Endothelial Cells Growth. Biomaterials 2005, 26, 7606–7615. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Fauzi, M.B. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Camasão, D.B.; Li, L.; Drouin, B.; Lau, C.; Reinhardt, D.P.; Mantovani, D. Physiologically Relevant Platform for an Advanced in Vitro Model of the Vascular Wall: Focus on in Situ Fabrication and Mechanical Maturation. Vitr. Model. 2022, 1, 179–195. [Google Scholar] [CrossRef]
- Bosch-Rué, È.; Díez-Tercero, L.; Delgado, L.M.; Pérez, R.A. Biofabrication of Collagen Tissue-Engineered Blood Vessels with Direct Co-Axial Extrusion. Int. J. Mol. Sci. 2022, 23, 5618. [Google Scholar] [CrossRef] [PubMed]
- Justin, A.W.; Cammarata, F.; Guy, A.A.; Estevez, S.R.; Burgess, S.; Davaapil, H.; Stavropoulou-Tatla, A.; Ong, J.; Jacob, A.G.; Saeb-Parsy, K.; et al. Densified Collagen Tubular Grafts for Human Tissue Replacement and Disease Modelling Applications. Biomater. Adv. 2023, 145, 213245. [Google Scholar] [CrossRef]
- Ma, W.; Wang, L.; Zhang, Q.; Dong, X.; Zhu, T.; Lu, S. Electrospun PCL/Collagen Hybrid Nanofibrous Tubular Graft Based on Post-Network Bond Processing for Vascular Substitute. Biomater. Adv. 2022, 139, 213031. [Google Scholar] [CrossRef]
- Jia, W.; Liu, L.; Li, M.; Zhou, Y.; Zhou, H.; Weng, H.; Gu, G.; Xiao, M.; Chen, Z. Construction of Enzyme-Laden Vascular Scaffolds Based on Hyaluronic Acid Oligosaccharides-Modified Collagen Nanofibers for Antithrombosis and in-Situ Endothelialization of Tissue-Engineered Blood Vessels. Acta Biomater. 2022, 153, 287–298. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef]
- Peng, K.; Liu, X.; Zhao, H.; Lu, H.; Lv, F.; Liu, L.; Huang, Y.; Wang, S.; Gu, Q. 3D Bioprinting of Reinforced Vessels by Dual-Cross-Linked Biocompatible Hydrogels. ACS Appl. Bio Mater. 2021, 4, 4549–4556. [Google Scholar] [CrossRef] [PubMed]
- Fazal, F.; Melchels, F.P.W.; McCormack, A.; Silva, A.F.; Callanan, A.; Koutsos, V.; Radacsi, N. A Vertical Additive-Lathe Printing System for the Fabrication of Tubular Constructs Using Gelatin Methacryloyl Hydrogel. J. Mech. Behav. Biomed. Mater. 2023, 139, 105665. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Pereira, J.; Aid-Launais, R.; Pavon-Djavid, G.; Ray, A.R.; Letourneur, D.; Meddahi-Pellé, A.; Gupta, B. Gelatin—Oxidized Carboxymethyl Cellulose Blend Based Tubular Electrospun Scaffold for Vascular Tissue Engineering. Int. J. Biol. Macromol. 2018, 107, 1922–1935. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, S.; Jiang, Y.; Shen, Q.; Li, L.; Shi, Y.; Xie, H.; Tian, J. A Preliminary Study on Polycaprolactone and Gelatin-Based Bilayered Tubular Scaffolds with Hierarchical Pore Size Constructed from Nano and Microfibers for Vascular Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2021, 32, 1791–1809. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, L.; Li, L.; Tang, Y.; Liu, Q.; Xie, H.; Tian, J.; Zhou, S.; Tang, G. Biomimetic Dual-Oriented/Bilayered Electrospun Scaffold for Vascular Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R. Fibrin Sealants in Surgical Practice: An Overview. Am. J. Surg. 2001, 182, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Ong, C.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of Vascular Graft Studies in Large Animal Models. Tissue Eng. Part B Rev. 2018, 24, 133–143. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Wu, Y.; Du, P.; Sun, L.; Yu, Z.; Song, S.; Yin, J.; Ma, X.; Jing, C.; et al. Preparation of PU/Fibrin Vascular Scaffold with Good Biomechanical Properties and Evaluation of Its Performance in Vitro and in Vivo. Int. J. Nanomed. 2020, 15, 8697–8715. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Wang, D.; Mu, S.; Lv, W.; Hao, Y.; Lu, X.; Zhang, G.; Nan, W.; Chen, H.; et al. Improved Mechanical Properties by Modifying Fibrin Scaffold with PCL and Its Biocompatibility Evaluation. J. Biomater. Sci. Polym. Ed. 2020, 31, 658–678. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Yang, L.; Sun, L.; Mu, S.; Zong, H.; Li, Q.; Wang, F.; Song, S.; Yang, C.; et al. Evaluation of Remodeling and Regeneration of Electrospun PCL/Fibrin Vascular Grafts in Vivo. Mater. Sci. Eng. C 2021, 118, 111441. [Google Scholar] [CrossRef]
- Elliott, M.B.; Matsushita, H.; Shen, J.; Yi, J.; Inoue, T.; Brady, T.; Santhanam, L.; Mao, H.-Q.; Hibino, N.; Gerecht, S. Off-the-Shelf, Heparinized Small Diameter Vascular Graft Limits Acute Thrombogenicity in a Porcine Model. Acta Biomater. 2022, 151, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Syedain, Z.H.; Meier, L.A.; Lahti, M.T.; Johnson, S.L.; Tranquillo, R.T. Implantation of Completely Biological Engineered Grafts Following Decellularization into the Sheep Femoral Artery. Tissue Eng. Part A 2014, 20, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Syedain, Z.H.; Graham, M.L.; Dunn, T.B.; O’Brien, T.; Johnson, S.L.; Schumacher, R.J.; Tranquillo, R.T. A Completely Biological “off-the-Shelf” Arteriovenous Graft That Recellularizes in Baboons. Sci. Transl. Med. 2017, 9, eaan4209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Mithieux, S.M.; Weiss, A.S. Fabricating Organized Elastin in Vascular Grafts. Trends Biotechnol. 2021, 39, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Torre, I.; Alonso, M.; Rodriguez-Cabello, J.-C. Elastin-Based Materials: Promising Candidates for Cardiac Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; de Kort, B.J.; Smits, A.I.P.M.; Weiss, A.S. Elastin in Vascular Grafts. In Tissue-Engineered Vascular Grafts; Walpoth, B., Bergmeister, H., Bowlin, G., Kong, D., Rotmans, J., Zilla, P., Eds.; Reference Series in Biomedical Engineering; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–32. ISBN 978-3-319-71530-8. [Google Scholar]
- Natsume, K.; Nakamura, J.; Sato, K.; Ohtsuki, C.; Sugawara-Narutaki, A. Biological Properties of Self-Assembled Nanofibers of Elastin-like Block Polypeptides for Tissue-Engineered Vascular Grafts: Platelet Inhibition, Endothelial Cell Activation and Smooth Muscle Cell Maintenance. Regen. Biomater. 2023, 10, rbac111. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Ryan, E.J.; Cameron, A.R.; O’Brien, F.J. Hierarchical Biofabrication of Biomimetic Collagen-Elastin Vascular Grafts with Controllable Properties via Lyophilisation. Acta Biomater. 2020, 112, 52–61. [Google Scholar] [CrossRef]
- Camasão, D.B.; González-Pérez, M.; Palladino, S.; Alonso, M.; Rodríguez-Cabello, J.C.; Mantovani, D. Elastin-like Recombinamers in Collagen-Based Tubular Gels Improve Cell-Mediated Remodeling and Viscoelastic Properties. Biomater. Sci. 2020, 8, 3536–3548. [Google Scholar] [CrossRef]
- Tanaka, T.; Abe, Y.; Cheng, C.-J.; Tanaka, R.; Naito, A.; Asakura, T. Development of Small-Diameter Elastin-Silk Fibroin Vascular Grafts. Front. Bioeng. Biotechnol. 2021, 8, 622220. [Google Scholar] [CrossRef]
- Wang, Z.; Mithieux, S.M.; Vindin, H.; Wang, Y.; Zhang, M.; Liu, L.; Zbinden, J.; Blum, K.M.; Yi, T.; Matsuzaki, Y.; et al. Rapid Regeneration of a Neoartery with Elastic Lamellae. Adv. Mater. 2022, 34, 2205614. [Google Scholar] [CrossRef]
- Settembrini, A.; Buongiovanni, G.; Settembrini, P.; Alessandrino, A.; Freddi, G.; Vettor, G.; Martelli, E. In-Vivo Evaluation of Silk Fibroin Small-Diameter Vascular Grafts: State of Art of Preclinical Studies and Animal Models. Front. Surg. 2023, 10, 1090565. [Google Scholar] [CrossRef] [PubMed]
- Janani, G.; Kumar, M.; Chouhan, D.; Moses, J.C.; Gangrade, A.; Bhattacharjee, S.; Mandal, B.B. Insight into Silk-Based Biomaterials: From Physicochemical Attributes to Recent Biomedical Applications. ACS Appl. Bio Mater. 2019, 2, 5460–5491. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Kluge, J.A.; Smoot, D.; Kluge, M.A.; Schmidt, D.F.; Paetsch, C.R.; Kim, P.S.; Kaplan, D.L. Fabricating Mechanically Improved Silk-Based Vascular Grafts by Solution Control of the Gel-Spinning Process. Biomaterials 2020, 230, 119567. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Fukuda, D.; Higashikuni, Y.; Hirata, Y.; Komuro, I.; Saotome, T.; Yamashita, Y.; Asakura, T.; Sata, M. Biodegradable Extremely-Small-Diameter Vascular Graft Made of Silk Fibroin Can Be Implanted in Mice. J. Atheroscler. Thromb. 2020, 27, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Durán-Rey, D.; Brito-Pereira, R.; Ribeiro, C.; Ribeiro, S.; Sánchez-Margallo, J.A.; Crisóstomo, V.; Irastorza, I.; Silván, U.; Lanceros-Méndez, S.; Sánchez-Margallo, F.M. Development of Silk Fibroin Scaffolds for Vascular Repair. Biomacromolecules 2023, 24, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Shen, W.; Yue, W. Biocompatibility of Silk Methacrylate/Gelatin-Methacryloyl Composite Hydrogel and Its Feasibility as a Vascular Tissue Engineering Scaffold. Biochem. Biophys. Res. Commun. 2023, 650, 62–72. [Google Scholar] [CrossRef]
- Szulc, M.; Lewandowska, K. Biomaterials Based on Chitosan and Its Derivatives and Their Potential in Tissue Engineering and Other Biomedical Applications—A Review. Molecules 2022, 28, 247. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Feng, Y. Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications. Gels 2023, 9, 373. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Feng, Y.; Yang, Y.; Wei, Z.; Zhao, W.; Zhao, C. A Chitosan Modified Asymmetric Small-Diameter Vascular Graft with Anti-Thrombotic and Anti-Bacterial Functions for Vascular Tissue Engineering. J. Mater. Chem. B 2020, 8, 568–577. [Google Scholar] [CrossRef]
- Fiqrianti, I.A.; Widiyanti, P.; Manaf, M.A.; Savira, C.Y.; Cahyani, N.R.; Bella, F.R. Poly-L-Lactic Acid (PLLA)-Chitosan-Collagen Electrospun Tube for Vascular Graft Application. J. Funct. Biomater. 2018, 9, 32. [Google Scholar] [CrossRef]
- Yin, A.; Zhuang, W.; Liu, G.; Lan, X.; Tang, Z.; Deng, Y.; Wang, Y. Performance of PEGylated Chitosan and Poly (L-Lactic Acid-Co-ε-Caprolactone) Bilayer Vascular Grafts in a Canine Femoral Artery Model. Colloids Surf. B Biointerfaces 2020, 188, 110806. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized Extracellular Matrix: New Promising and Challenging Biomaterials for Regenerative Medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized Extracellular Matrix-Based Bioinks for Engineering Tissue- and Organ-Specific Microenvironments. Chem. Rev. 2020, 120, 10608–10661. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xu, B.; Zhang, R.; Fan, Y.; Xie, H.; Li, X. Applications of Decellularized Materials in Tissue Engineering: Advantages, Drawbacks and Current Improvements, and Future Perspectives. J. Mater. Chem. B 2020, 8, 10023–10049. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, M.; Giri, P.S.; Mahapatra, S.; Pati, F.; Rath, S.N. Bioengineering Strategies for 3D Bioprinting of Tubular Construct Using Tissue-Specific Decellularized Extracellular Matrix. Int. J. Biol. Macromol. 2022, 223, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.-S.; Kim, B.S.; Choi, Y.-J.; Jang, W.B.; Hong, Y.J.; Kwon, S.-M.; et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv. Funct. Mater. 2017, 27, 1700798. [Google Scholar] [CrossRef]
- Shi, J.; Teng, Y.; Li, D.; He, J.; Midgley, A.C.; Guo, X.; Wang, X.; Yang, X.; Wang, S.; Feng, Y.; et al. Biomimetic Tri-Layered Small-Diameter Vascular Grafts with Decellularized Extracellular Matrix Promoting Vascular Regeneration and Inhibiting Thrombosis with the Salidroside. Mater. Today Bio 2023, 21, 100709. [Google Scholar] [CrossRef]
- Gao, H.; Hu, P.; Sun, G.; Wang, L.; Tian, Y.; Mo, H.; Liu, C.; Zhang, J.; Shen, J. Decellularized Scaffold-Based Poly(Ethylene Glycol) Biomimetic Vascular Patches Modified with Polyelectrolyte Multilayer of Heparin and Chitosan: Preparation and Vascular Tissue Engineering Applications in a Porcine Model. J. Mater. Chem. B 2022, 10, 1077–1084. [Google Scholar] [CrossRef]
- Gutowski, P.; Gage, S.M.; Guziewicz, M.; Ilzecki, M.; Kazimierczak, A.; Kirkton, R.D.; Niklason, L.E.; Pilgrim, A.; Prichard, H.L.; Przywara, S.; et al. Arterial Reconstruction with Human Bioengineered Acellular Blood Vessels in Patients with Peripheral Arterial Disease. J. Vasc. Surg. 2020, 72, 1247–1258. [Google Scholar] [CrossRef]
- Lawson, J.H.; Glickman, M.H.; Ilzecki, M.; Jakimowicz, T.; Jaroszynski, A.; Peden, E.K.; Pilgrim, A.J.; Prichard, H.L.; Guziewicz, M.; Przywara, S.; et al. Bioengineered Human Acellular Vessels for Dialysis Access in Patients with End-Stage Renal Disease: Two Phase 2 Single-Arm Trials. Lancet 2016, 387, 2026–2034. [Google Scholar] [CrossRef]
- Drews, J.D.; Pepper, V.K.; Best, C.A.; Szafron, J.M.; Cheatham, J.P.; Yates, A.R.; Hor, K.N.; Zbinden, J.C.; Chang, Y.-C.; Mirhaidari, G.J.M.; et al. Spontaneous Reversal of Stenosis in Tissue-Engineered Vascular Grafts. Sci. Transl. Med. 2020, 12, eaax6919. [Google Scholar] [CrossRef]
| Biomaterial | Cell Type | Fabrication Technique | Tissue Maturation | Highlights | Year | Refs. |
|---|---|---|---|---|---|---|
| PCL | Human endothelial colony forming cells and multipotent mesenchymal stromal cells | Electrospinning and melt electrowriting | Perfusion bioreactor combining static maturation on outside layer and luminal shear stress dynamic stimulation | The bilayered TEVG showed a physiological-like cell organization and phenotype, due to the bioreactors design which allows the achievement of vascular layer-specific characteristics. | 2019 | [84] |
| Gelatin coated PGA | vSMCs derived from hiPSCs | Cell seeding on premade biodegradable scaffolds | Peristaltic pump bioreactor for incremental pulsatile stretching dynamic culturing | The hiPSCs-derived vSMCs seeded on the biodegradable scaffold produced cellularized collagenous TEVGs with physiological-like mechanical properties, which were maintained, along with patency, following in vivo implantation. | 2020 | [85] |
| ECM and PCL | Acellular | Decellularization and electrospinning | None | Small-diameter TEVG made by electrospinning PCL for reinforcing a decellularized vessel. The graft showed good integration between the materials, biocompatibility, and hemocompatibility. | 2020 | [86] |
| Polydioxanone and PCL | Acellular | Electrospinning and 3D printing | None | This bilayered TEVG, enriched with immobilized VEGF, proved to be a good conduit for vascular tissue regeneration, allowing for improved cellularization in vivo and in vitro. Moreover, it was able to maintain mechanical properties after in vivo implantation, due to the 3D-printed PCL reinforcement. | 2020 | [87] |
| Polyurethane | Acellular | Dip-coating on 3D-printed vascular templates | None | The synthetic graft showed excellent physiological-like mechanical properties, surpassing those of commercially available grafts. Furthermore, the TEVG proved to reduce thrombogenesis in vivo, with improved endothelialization of the graft. | 2021 | [88] |
| ECM | Acellular | Decellularization | None | A new decellularization method was developed to ensure antigen removal in the TEVG, with retention of ECM basement membrane. This allowed the achievement of a TEVG for small-diameter grafts with high patency rates after in vivo implantation. | 2021 | [89] |
| Alginate and collagen | Acellular | Molding | None | Natural-based TEVGs with tunable macro-architecture properties were produced. The cross-linking method developed proved to improve stability and mechanical properties while maintaining bioactivity. | 2022 | [90] |
| PCL and ECM | Acellular | Electrospinning | None | The TEVG, with heparin and VEGF added, showed excellent hemocompatibility and cell infiltration. Moreover, in vivo studies demonstrated the TEVGs‘ integration with a decreased thrombus risk. | 2022 | [91] |
| PCL | Murine vSMCs | Electrospinning | Perfusion-based bioreactor for seeding and culturing cells under dynamic conditions | The use of a low-cost and simple dynamic cell seeding and culturing bioreactor proved to produce a TEVG with more evenly distributed and viable cells compared to static conditions. | 2022 | [92] |
| PCL, collagen, and gelatin | Acellular | Electrospinning | None | An electrospun trilayered TEVG made with an inner PCL/collagen layer to improve endothelialization, a medial PCL layer, and an outer PCL/gelatin layer. The construct showed physiological-like ultrastructure of electrospun fibers and mechanical properties exceeding those of native vessels. | 2022 | [93] |
| Polyurethane, silk fibroin, gelatin, and chitosan | Acellular | Electrospinning and freeze-drying | None | Heparinized multicomponent TEVGs showed increased mechanical properties, cell integration, and ability to release heparin over time, producing antithrombotic characteristics. | 2022 | [94] |
| Alginate and collagen | Murine fibroblasts | 3D printing | None | The addition of collagen to the bioink proved to ameliorate the mechanical properties of the construct and increase cell adhesion and viability. | 2022 | [95] |
| Silk fibroin and polyurethane | Acellular | Electrospinning | None | Hybrid TEVGs, with physiological-like structure characteristics, were obtained. The small-calibre TEVGs showed good compliance, with adequate application up to 3 months after in vivo implantation. | 2022 | [96] |
| Alginate, hyaluronic acid, and ECM | Acellular | 3D printing | None | The approach produced a multi-component bioink that could be printed into a vascular graft with appropriate mechanical properties. Moreover, the TEVG also showed excellent angiogenic and anti-inflammatory activity in vitro. | 2023 | [97] |
| Biomaterial | Study | Outline | Year | Refs. |
|---|---|---|---|---|
| Collagen | In vitro | A trilayered cellularized physiological-like TEVG produced by molding and dynamic maturation, showing native vessel-like mechanical properties. | 2022 | [107] |
| In vitro | Bilayered and cellularized TEVGs made using coaxial extrusion, with high collagen concentrations for increased mechanical properties. | 2022 | [108] | |
| In vitro | A highly tailorable densified collagen construct with enhanced stability and mechanical properties and possibility of cellularization. | 2023 | [109] | |
| In vitro | Electrospun PCL/collagen/heparin TEVGs with ameliorated flexibility and bursting strength compared to native vessels. | 2022 | [110] | |
| In vitro/in vivo | Enzyme-laden hyaluronic acid/collagen/PCL electrospun scaffold favoring endothelialization and antithrombogenicity. | 2022 | [111] | |
| Gelatin | In vitro | 3D-printed GelMa constructs stabilized by dual cross-linking showing enhanced mechanical properties and endothelialization. | 2021 | [113] |
| None | A novel additive lathe printing method to achieve highly tunable GelMA tubular structures for VTE. | 2023 | [114] | |
| In vitro/in vivo | Electrospun gelatin cross-linked with oxidized carboxymethyl cellulose showing excellent biocompatibility both in vitro and in vivo. | 2017 | [115] | |
| In vitro | Gelatin was electrospun with PCL and PGE to increase mechanical properties and tailor ultrastructure, achieving cell adhesion and migration in the scaffold and edothelialization. | 2017 | [116] | |
| In vitro | Electrospun PCL, PGLA, and gelatin with controlled fiber orientation showing increased guidance for cell orientation and appropriate mechanical properties. | 2020 | [117] | |
| Fibrin | In vitro/in vivo | Electrospun PU/fibrin small-caliber TEVGs showed optima biocompatibility and mechanical properties, with graft patency and thrombosis risk reduction achieved up to 3 months after implantation. | 2020 | [120] |
| In vitro/in vivo | Electrospun PCL/fibrin grafts with increased mechanical properties demonstrated good hemocompatibility and biocompatibility. | 2020 | [121] | |
| In vivo | Electrospun PCL/fibrin small-caliber grafts studied in vivo up to 9 months, showed ability to induce neoartery regeneration. | 2021 | [122] | |
| In vitro/in vivo | Fibrin graft embedded with heparin for decreased thrombogenicity and showed stability after up to 12 months of storage. | 2022 | [123] | |
| In vitro/in vivo | Fibrin-based decellularized TEVG from ovine fibroblasts showed graft recellularization and good patency up to 6 months after implantation in ovine model. | 2014 | [124] | |
| In vitro/in vivo | Fibrin-based decellularized TEVG from human fibroblasts demonstrated no immune reactions, graft recellularization, and stability up to 6 months after implantation in baboon model. | 2017 | [125] | |
| Elastin | In vitro | Self-assembling functionalized elastin scaffold able to limit platelet adhesion and activation, promote endhotelialization, and induce SMCs’ contractile phenotype. | 2023 | [129] |
| In vitro | A multilayered elastin/collagen graft with highly controlled ultrastructure, showing good SMC biocompatibility and low immunogenicity. | 2020 | [130] | |
| In vitro | Molded cellularized collagen grafts with functionalized ELR addition demonstrated improved elastic-mechanical properties and cell functionality. | 2020 | [131] | |
| In vitro/in vivo | The addition of elastin to the silk fibroin scaffolds improved mechanical properties and cell adhesion, maintaining patency and bioactivity after implantation. | 2021 | [132] | |
| In vitro/in vivo | Tropoelastin lamellae embedded in PSG electrospun scaffolds led to formation of neoartery 8 months after in vivo implantation. | 2022 | [133] | |
| Silk | In vivo | Tunable gel spun silk TEVGs with high porosities showed improved mechanical properties and good cellularization after in vivo implantation. | 2020 | [136] |
| In vivo | Small-diameter braided silk fibroin grafts were used to understand graft remodeling after implantation, showing excellent biocompatibility and long-term spotipatency. | 2020 | [137] | |
| In vitro | Physico-chemical characterization of 3 different silk biomaterials was performed, all showing good biocompatibility for VTE applications. | 2023 | [138] | |
| In vitro | Cellularized silk electrospun grafts with dynamic stimulation for physiological-like EC monolayer. | 2022 | [83] | |
| In vitro/in vivo | Methacrylated silk and GelMa hydrogels showing enhanced mechanical properties, biocompatibility, and angiogenic potential both in vitro and in vivo. | 2023 | [139] | |
| In vitro | Bilayered electrospun chitosan and PCL grafts with antithrombogenic and antibacterial properties; in addition, demonstrated rapid endothelialization. | 2019 | [142] | |
| Chitosan | In vitro | Chitosan-rich collagen/PLLA TEVGs showed improved hemocompatibility and biocompatibility. | 2018 | [143] |
| In vitro/in vivo | Evaluation of chitosan/PLCL vascular grafts in large animal model demonstrated stability and biocompatibility up to 24 weeks. | 2020 | [144] | |
| Decellularized extracellular matrix | In vitro | A bioink made of dECM and ECs derived from the same vein sample, supplemented with mesenchymal stem cells showing ability to induce cell differentiation. | 2022 | [148] |
| In vitro/in vivo | dECM and alginate bioink, cellularized with endothelial progenitor cells, showing bioactivity and therapeutic potential for ischemic disease. | 2017 | [149] | |
| In vitro/in vivo | Electrospun dECM and PLCL loaded with salidroside demonstrated bioactivity with good endothelialization and ECM deposition in vitro and in vivo. | 2023 | [150] | |
| In vitro/in vivo | dECM scaffold modified with PEG, heparin, and chitosan showed appropriate mechanical properties and long-term patency in large in vivo model. | 2022 | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Francesco, D.; Pigliafreddo, A.; Casarella, S.; Di Nunno, L.; Mantovani, D.; Boccafoschi, F. Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements. Biomolecules 2023, 13, 1389. https://doi.org/10.3390/biom13091389
Di Francesco D, Pigliafreddo A, Casarella S, Di Nunno L, Mantovani D, Boccafoschi F. Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements. Biomolecules. 2023; 13(9):1389. https://doi.org/10.3390/biom13091389
Chicago/Turabian StyleDi Francesco, Dalila, Alexa Pigliafreddo, Simona Casarella, Luca Di Nunno, Diego Mantovani, and Francesca Boccafoschi. 2023. "Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements" Biomolecules 13, no. 9: 1389. https://doi.org/10.3390/biom13091389
APA StyleDi Francesco, D., Pigliafreddo, A., Casarella, S., Di Nunno, L., Mantovani, D., & Boccafoschi, F. (2023). Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements. Biomolecules, 13(9), 1389. https://doi.org/10.3390/biom13091389






