Apigenin Isolated from the Medicinal Plant Elsholtzia rugulosa Prevents β-Amyloid 25–35-Induces Toxicity in Rat Cerebral Microvascular Endothelial Cells
Abstract
:1. Introduction
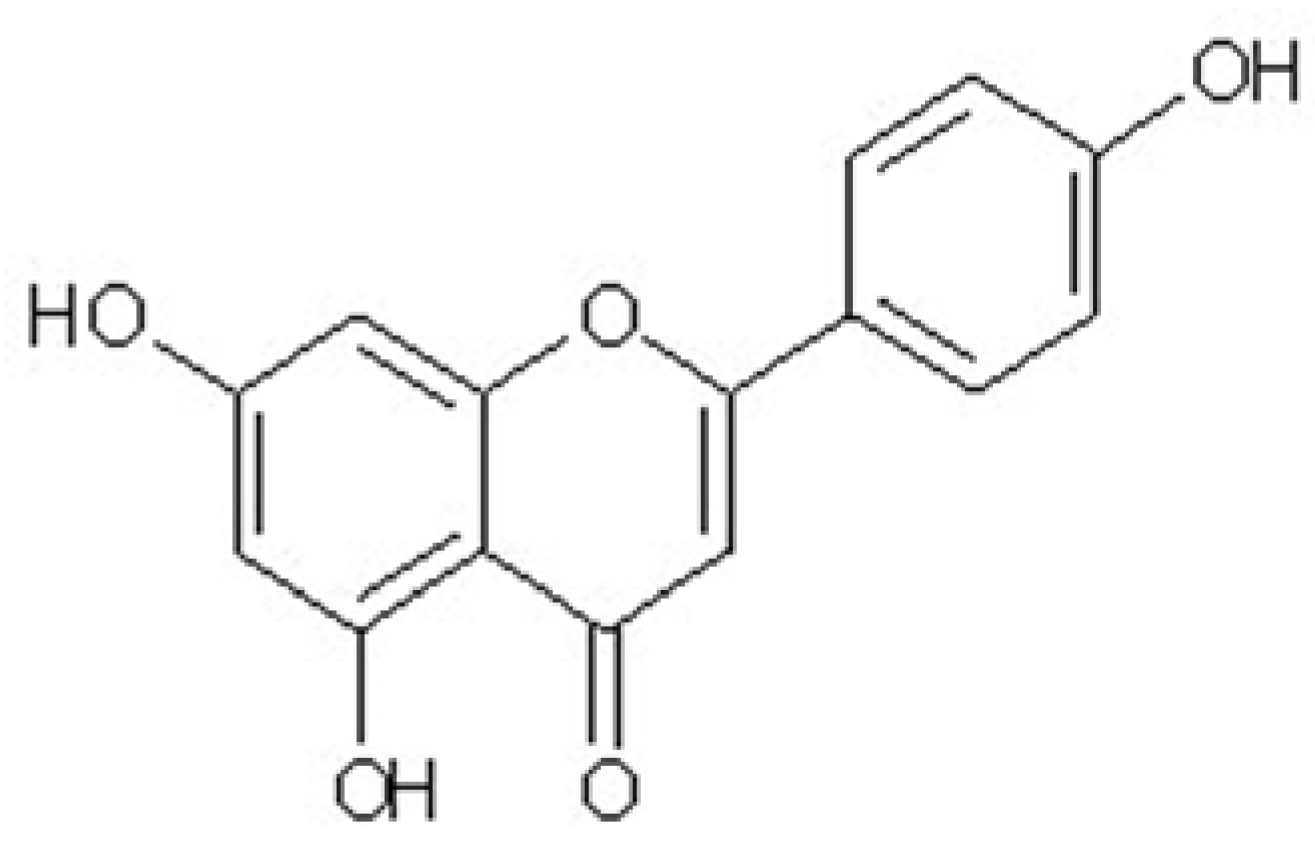
2. Results and Discussion
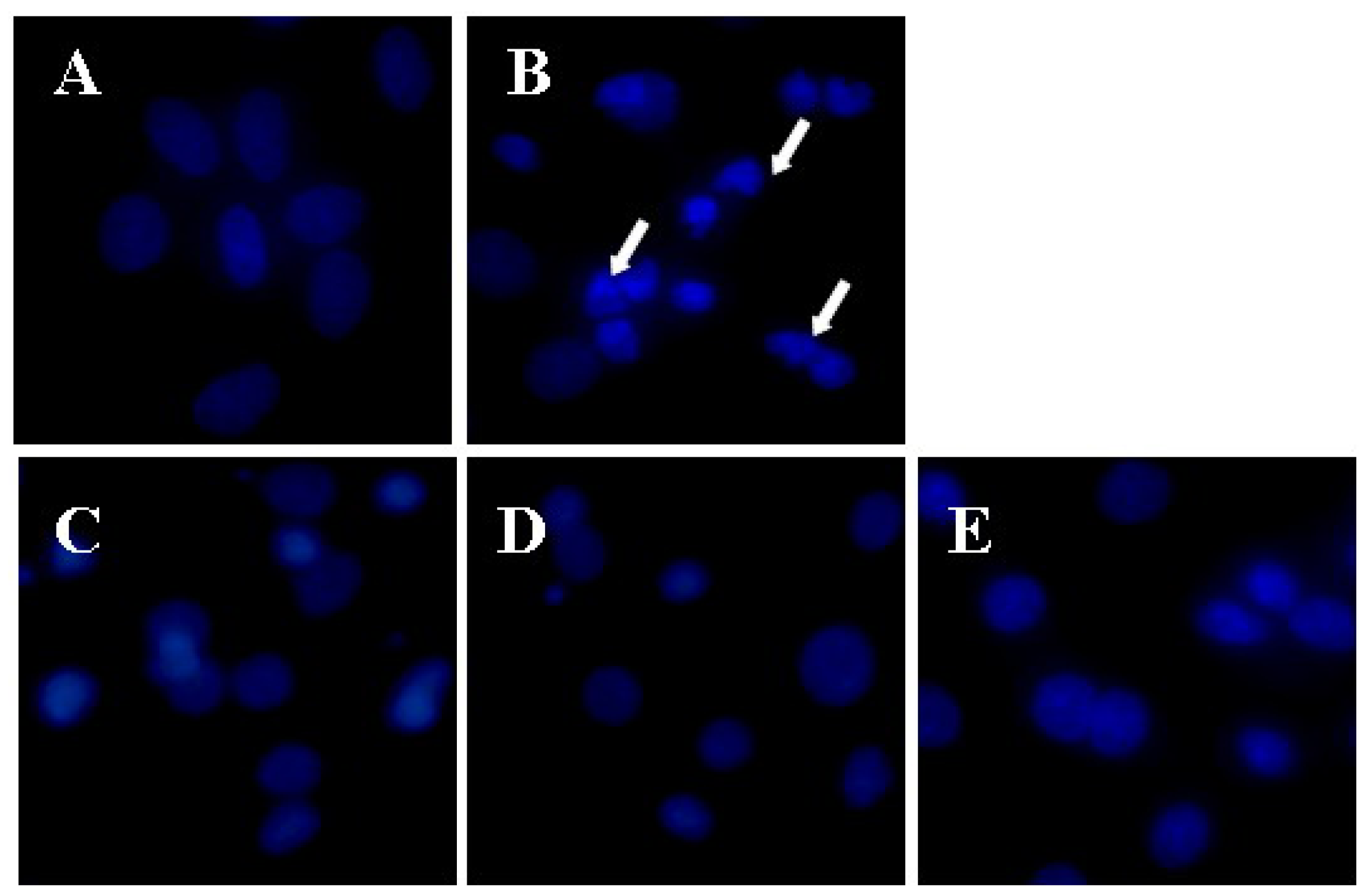
2.1. Apigenin Protected CMECs from Aβ25–35-Induced Cytotoxicity
| Group | Concentration/μmol∙L | Viability/OD490 | LDH/Fluorescence |
|---|---|---|---|
| Control | ─ | 1.00 ± 0.08 | 905.33 ± 60.75 |
| Aβ25–35 | 100 | 0.57 ± 0.08 *** | 1796.17 ± 152.29 *** |
| Aβ25+35 + Apigenin | 0.1 | 0.69 ± 0.03 ▲▲ | 1594.17 ± 83.39 ▲ |
| Aβ25+35 + Apigenin | 1.0 | 0.74 ± 0.03 ▲▲▲ | 1475.17 ± 111.82 ▲▲ |
| Aβ25+35 + Apigenin | 10.0 | 0.84 ± 0.03 ▲▲▲ | 1374.17 ± 131.10 ▲▲▲ |
2.2. Apigenin Regulated the Redox Imbalance of CMECs Against Aβ25–35-Induced Toxicity
| Group | Concentration/μmol∙L | Relative ROS content /Fluorescent intensity | SOD Inhibition rate/% |
|---|---|---|---|
| Control | ─ | 74.74 ± 10.53 | 33.96 ± 7.73 |
| Aβ25–35 | 100 | 211.32 ± 22.09 *** | 13.60 ± 4.47 ** |
| Aβ25+35 + Apigenin | 0.1 | 175.98 ± 17.10 ▲ | 20.56 ± 3.23 ▲ |
| Aβ25+35 + Apigenin | 1.0 | 168.72 ± 23.39 ▲ | 22.82± 3.51 ▲ |
| Aβ25+35 + Apigenin | 10.0 | 157.02 ± 14.54 ▲▲ | 26.74 ± 3.84 ▲▲ |
2.3. Apigenin Improved Barrier Function of CMECs against Aβ25–35-Induced Toxicity
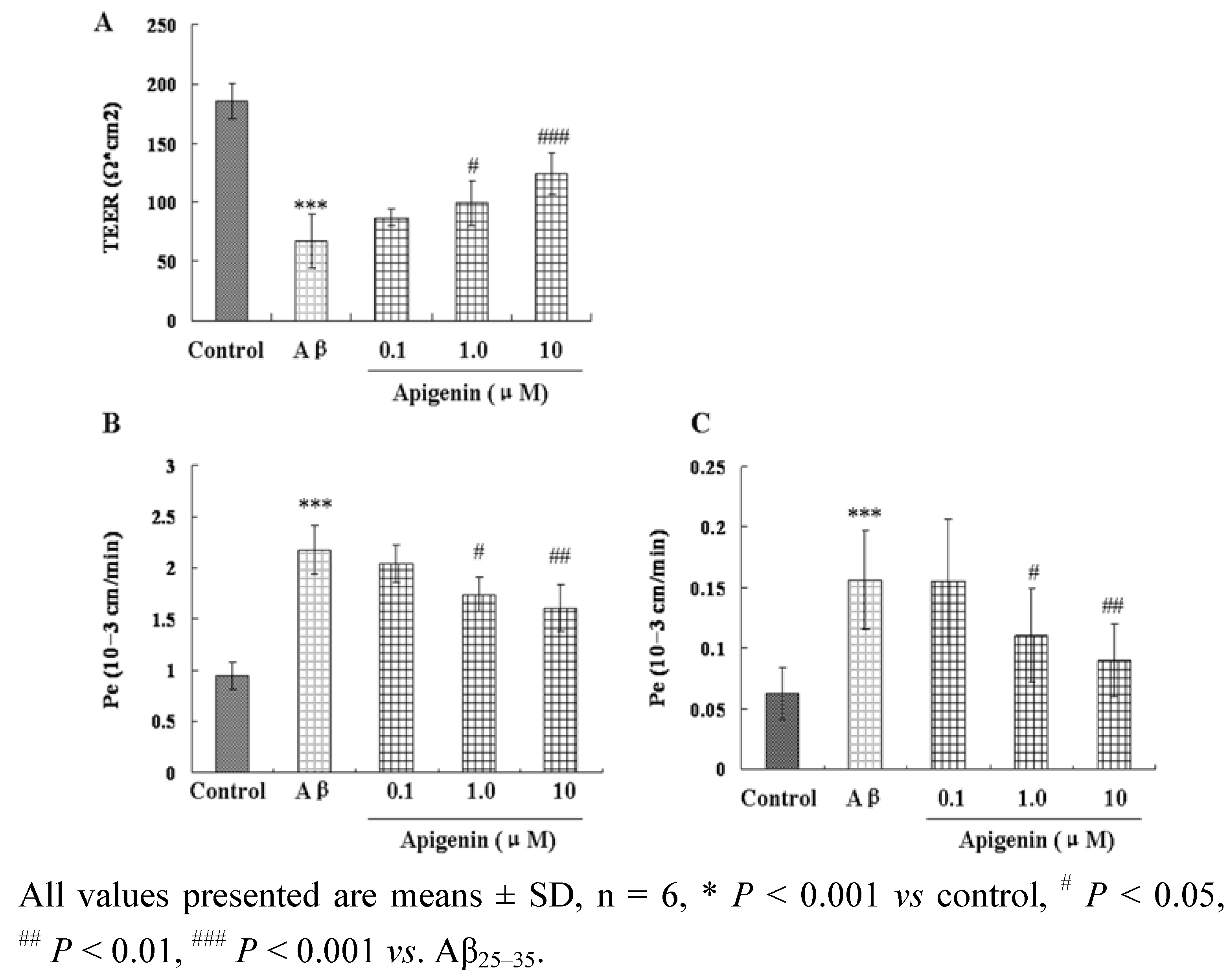
| Group | Concentration/μmol∙L | γ-GT/U.mg−1 protein | ALP/U.mg−1 protein |
|---|---|---|---|
| Control | ─ | 13.47 ± 1.14 | 13.75 ± 1.49 |
| Aβ25–35 | 100 | 6.08 ± 1.51 *** | 7.96 ± 1.75 ▲▲▲ |
| Aβ25+35 + Apigenin | 0.1 | 8.84 ± 0.94** | 10.88 ± 1.71 ▲▲ |
| Aβ25+35 + Apigenin | 1.0 | 10.25 ± 1.26 *** | 12.86 ± 2.01 ▲▲ |
| Aβ25+35 + Apigenin | 10.0 | 11.14 ± 0.46 *** | 13.73 ± 0.94 ▲▲▲ |
3. Experimental
3.1. Reagents
3.2. Plant Materials
3.3. Isolation of Apigenin
3.4. Animals
3.5. Cell Culture
3.6. Cell Toxicity Assays
3.7. Measurements of Intracellular ROS and SOD
3.8. Transendothelial Electrical Resistance (TEER)
3.9. Transendotheial Permeability for Sodium Fluorescein and FITC Labeled Albumin
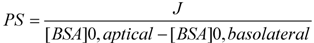
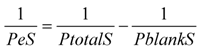
3.10. γ-Glutamyl Transpeptidase and Alkaline Phosphatase Activity Detection
3.11. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Kalaria, R.N. The role of cerebral ischemia in Alzheimer’s disease. Neurobiol. Aging 2000, 21, 321–330. [Google Scholar] [CrossRef]
- Han, B.H.; Zhou, M.L.; Abousaleh, F.; Brendza, R.P.; Dietrich, H.H.; Koenigsknecht-Talboo, J.; Cirrito, J.R.; Milner, E.; Holtzman, D.M.; Zipfel, G.J. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: Contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J. Neurosci. 2008, 28, 13542–13550. [Google Scholar]
- Yamada, M. Cerebral amyloid angiopathy: An overview. Neuropathology 2000, 20, 8–22. [Google Scholar] [CrossRef]
- Jellinger, K.A. Alzheimer disease and cerebrovascular pathology: An update. J. Neural. Transm. 2002, 109, 813–836. [Google Scholar] [CrossRef]
- Bell, R.D.; Zlokovic, B.V. Neurovascular mechanisms and bloodbrain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009, 118, 103–113. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Attems, J. Prevalence and pathogenic role of cerebrovascular lesions in Alzheimer disease. J. Neurol. Sci. 2005, 229, 37–41. [Google Scholar]
- Farkas, E.; Luiten, P.G. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog. Neurobiol. 2001, 64, 575–611. [Google Scholar] [CrossRef]
- Wisniewski, H.M; Vorbrodt, A.W.; Wegiel, J. Amyloid angiopathy and blood-brain barrier changes in Alzheimer’s disease. Ann. NY Acad. Sci. 1997, 826, 161–172. [Google Scholar]
- Suo, Z.; Tan, J.; Placzek, A.; Crawford, F.; Fang, C.; Mullan, M. Alzheimer’s beta-amyloid peptides induce inflammatory cascade in human vascular cells: The roles of cytokines and CD40. Brain Res. 1998, 80, 110–117. [Google Scholar]
- Giri, R.; Shen, Y.; Stins, M.; Du Yan, S.; Schmidt, A.M.; Stern, D.; Kim, K.S; Zlokovic, B.; Kalra, V.K. beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am. J. Physiol. Cell Physiol. 2000, 279, C1772–C1781. [Google Scholar]
- Blanc, E.M.; Toborek, M.; Mark, R.J.; Hennig, B.; Mattson, M.P. Amyloid beta-peptide induces cell monolayer albumin permeability, impairs glucose transport, and induces apoptosis in vascular endothelial cells. J. Neurochem. 1997, 68, 1870–1881. [Google Scholar]
- Strazielle, N.; Ghersi-Egea, J.F.; Ghiso, J.; Dehouck, M.P.; Frangione, B.; Patlak, C.; Fenstermacher, J.; Gorevic, P. In vitro evidence that beta-amyloid peptide 1–40 diffuses across the blood-brain barrier and affects its permeability. J. Neuropathol. Exp. Neurol. 2000, 59, 29–38. [Google Scholar]
- Liu, R.; Lan, X.; Ying, J.; Du, G.H. Protective effects of luteolin against amyloid β25-35-induced toxicity on rat cerebral microvascular endothelial cells. Chin. J. Nat. Med. 2010, 8, 223–227. [Google Scholar]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Jaeger, L.B.; Dohgu, S.; Hwang, M.C.; Farr, S.A.; Murphy, M.P.; Fleegal Demotta, M.A.; Lynch, J.A.; Robinson, S.M.; Niehoff, M.L.; Johnson, S.N.; et al. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP1 antisense reduces blood brain barrier clearance and increases brain levels of amyloid-β protein and impairs cognition. J. Alzheimers Dis. 2009, 17, 553–570. [Google Scholar]
- Wu, C.Y. Flora of China; Science Press: Beijing, China, 1988; Volume 66, p. 308. [Google Scholar]
- Jiangshu New College of Medicine. The Dictionary of Chinese Medicine; Shanghai Press of Science and Technology: Shanghai, China, 1985; p. 2132. [Google Scholar]
- McVean, M.; Weinberg, W.C.; Pelling, J.C. A p21(waf1)-independent pathway for inhibitory phosphorylation of cyclin-dependent kinase p34(cdc2) and concomitant G(2)/M arrest by the chemopreventive flavonoid apigenin. Mol. Carcinog. 2002, 33, 36–43. [Google Scholar] [CrossRef]
- Wang, I.K.; Lin-Shiau, S.Y.; Lin, J.K. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur. J. Cancer 1999, 35, 1517–1525. [Google Scholar] [CrossRef]
- Vargo, M.A.; Voss, O.H.; Poustka, F.; Cardounel, A.J.; Grotewold, E.; Doseff, A.I. Apigenin-induced-apoptosis is mediated by the activation of PKCδ and caspases in leukemia cells. Biochem. Pharmacol. 2006, 72, 681–692. [Google Scholar] [CrossRef]
- Fotsis, T.; Pepper, M.S.; Aktas, E.; Breit, S.; Rasku, S.; Adlercreutz, H.; Wähälä, K.; Montesano, R.; Schweigerer, L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997, 57, 2916–2921. [Google Scholar]
- Yin, F.; Giuliano, A.E.; Law, R.E.; Van Herle, A.J. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001, 21, 413–420. [Google Scholar]
- Chen, D.; Daniel, K.G.; Chen, M.S.; Kuhn, D.J.; Landis-Piwowar, K.R.; Dou, Q.P. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem. Pharmacol. 2005, 69, 1421–1432. [Google Scholar] [CrossRef]
- Woodman, O.L.; Chan, E. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol. 2004, 31, 786–790. [Google Scholar] [CrossRef]
- Olszanecki, R.; Gebska, A.; Kozlovski, V.I.; Gryglewski, R.J. Flavonoids and nitric oxide synthase. J. Physiol. Pharmacol. 2002, 53, 571–584. [Google Scholar]
- Zhang, Y.H.; Park, Y.S.; Kim, T.J.; Fang, L.H.; Ahn, H.Y.; Hong, J.T.; Kim, Y.; Lee, C.K.; Yun, Y.P. Endothelium-dependent vasorelaxant and antiproliferative effects of apigenin. Gen. Pharmacol. 2000, 35, 341–347. [Google Scholar] [CrossRef]
- Guerrero, J.A.; Lozano, M.L.; Castillo, J.; Benavente-Garcia, O.; Vicente, V.; Rivera, J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J. Thromb. Haemost. 2005, 3, 369–376. [Google Scholar] [CrossRef]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Preston, J.E.; Hipkiss, A.R.; Himsworth, D.T.; Romero, I.A.; Abbott, N.J. Toxic effects of β-amyloid(25-35) on immortalized rat brain endothelial cell: Protection by carnosine, homocarnosine and β-alanine. Neurosci. Lett. 1998, 242, 105–108. [Google Scholar] [CrossRef]
- Folin, M.; Baiguera, S.; Tommasini, M.; Guidolin, D.; Conconi, M.T.; DeCarlo, E.; Nussdorfer, G.G.; Parnigotto, P.P. Effects of β-amyloid on rat neuromicrovascular endothelial cells cultured in vitro. Int. J. Mol. Med. 2005, 15, 929–935. [Google Scholar]
- Shukla, A.; Shukla, R.; Dikshit, M.; Srimal, R.C. Alterations in free radical scavenging mechanisms following blood-brain barrier disruption. Free Radic. Biol. Med. 1993, 15, 97–100. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005, 28, 202–208. [Google Scholar]
- Kneisel, U.; Wolburg, H. Tight junctions of the blood-brain barrier. Cell. Mol. Neurobiol. 2000, 20, 57–76. [Google Scholar] [CrossRef]
- Butt, A.M.; Jones, H.C.; Abbott, N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats: A developmental study. J. Physiol. 1990, 429, 47–62. [Google Scholar]
- Harhaj, N.S.; Antonetti, D.A. Regulation of tight junctions and loss of barrier function in pathophysiology. Int. J. Biochem. Cell Biol. 2004, 36, 1206–1237. [Google Scholar] [CrossRef]
- Deli, M.A.; Abraham, C.S.; Kataoka, Y.; Niwa, M. Permeability studies on in vitro blood-brain barrier models: Physiology, pathology, and pharmacology. Cell. Mol. Neurobiol. 2005, 25, 59–127. [Google Scholar] [CrossRef]
- Gumbleton, M.; Audus, K.L. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J. Pharm. Sci. 2001, 90, 1681–1698. [Google Scholar] [CrossRef]
- Lawrenson, J.G.; Reid, A.R.; Finn, T.M.; Orte, C.; Allt, G. Cerebral and pial microvessels: Differential expression of gamma-glutamyl transpeptidase and alkaline phosphatase. Anat. Embryol. (Berl) 1999, 199, 29–34. [Google Scholar]
- Meyer, J.; Mischeck, U.; Veyhl, M.; Henzel, K.; Galla, H.J. Blood-brain barrier characteristic enzymatic properties in cultured brain capillary endothelial cells. Brain Res. 1990, 514, 305–309. [Google Scholar] [CrossRef]
- Frey, A.; Meckelein, B.; Weiler-Guttler, H.; Mockel, B.; Flach, R.; Gassen, H.G. Pericytes of the brain microvasculature express gamma-glutamyl transpeptidase. Eur. J. Biochem. 1991, 202, 421–429. [Google Scholar] [CrossRef]
- Roux, F.; Durieu-Trautmann, O.; Chaverot, N.; Claire, M.; Mailly, P.; Bourre, J.M.; Strosberg, A.D.; Couraud, P.O. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J. Cell. Physiol. 1994, 159, 101–113. [Google Scholar] [CrossRef]
- Wolf, S.; Gassen, H.G. Gamma-glutamyl transpeptidase, a blood-brain barrier associated membrane protein. Splitting peptides to transport amino acids. Adv. Exp. Med. Biol. 1997, 421, 37–45. [Google Scholar]
- Singh, J.; Chander, J.; Singh, S.; Singh, G.; Atal, C.K. Gamma-glutamyl transpeptidase: A novel biochemical marker in inflammation. Biochem. Pharmacol. 1986, 35, 3753–3760. [Google Scholar] [CrossRef]
- Kugelman, A.; Choy, H.A.; Liu, R.; Shi, M.M.; Gozal, E.; Forman, H.J. gamma-Glutamyl transpeptidase is increased by oxidative stress in rat alveolar L2 epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994, 11, 586–592. [Google Scholar]
- Yu, C.; Kastin, A.J.; Ding, Y.; Pan, W. Gamma glutamyl transpeptidase is a dynamic indicator of endothelial response to stroke. Exp. Neurol. 2007, 203, 116–122. [Google Scholar] [CrossRef]
- Plateel, M.; Dehouck, M.P.; Torpier, G.; Cecchelli, R.; Teissier, E. Hypoxia increases the susceptibility to oxidant stress and the permeability of the blood-brain barrier endothelial cell monolayer. J. Neurochem. 1995, 65, 2138–2145. [Google Scholar]
- Han, J.; Shuvaev, V.V.; Muzykantov, V.R. Catalase and SOD conjugated with PECAM antibody distinctly alleviate abnormal endothelial permeability caused by exogenous ROS and vascular endothelial growth factor. J. Pharmacol. Exp. Ther. 2011. Epub ahead of print. [Google Scholar]
- Boueiz, A.; Hassoun, P.M. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc. Res. 2009, 77, 26–34. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Yang, H.; Lan, X.; Ying, J.; Du, G. The flavonoid apigenin protects brain neurovascular coupling against amyloid-&beta25-35-induced toxicity in mice. J. Alzheimers Dis. 2011, 4, 85–100. [Google Scholar]
- Stamatovic, S.M.; Dimitrijevic, O.B.; Keep, R.F.; Andjelkovic, A.V. Protein kinase Calpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J. Biol. Chem. 2006, 281, 8379–8388. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, H.; Kojima, T.; Fujibe, M.; Soma, T.; Miyajima, H.; Nagasawa, K.; Wada, I.; Sawada, N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp. Cell. Res. 2003, 290, 275–288. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez Vizcaíno, F.; Utrilla, P.; Jiménez, J.; Tamargo, J.; Zarzuelo, A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen. Pharmacol. 1993, 24, 857–862. [Google Scholar]
- He, C.; Xiao, W.; Li, M.; Peng, Y.; Xu, L.; Gu, J.; Xiao, P. Chemical constituents from seeds of Paeonia suffruticosa. Zhongguo Zhong Yao Za Zhi 2010, 35, 1428–1431. [Google Scholar]
- Liu, R.; Zhang, L.; Lan, X.; Li, L.; Zhang, T.T.; Sun, J.H.; Du, G.H. Protection by borneol on cortical neurons against oxygen-glucose deprivation/reperfusion: Involvement of anti-oxidation and anti-inflammation through nuclear transcription factor κappaB signaling pathway. Neuroscience 2011, 176, 408–419. [Google Scholar] [CrossRef]
- Liu, R.; Gao, M.; Yang, Z.H.; Du, G.H. Pinocembrin protects rat brain against oxidation and apoptosis induced by ischemia-reperfusion both in vivo and in vitro. Brain Res. 2008, 1216, 104–115. [Google Scholar]
- Veszelka, S.; Pasztoi, M.; Farkas, A.E.; Krizbai, I.; Dung, N.T.K.; Niwa, M.; Abraham, C.S.; Deli, M.A. Pentosan polysulfate protects brain endothelial cells against bacterial lipopolysaccharideinduced damages. Neurochem. Int. 2007, 50, 219–228. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, L.; Hou, L.; Sun, H.; Yan, X.; Sun, X.; Li, J.; Bian, Y.; Chu, Y.; Liu, Q. Apigenin Isolated from the Medicinal Plant Elsholtzia rugulosa Prevents β-Amyloid 25–35-Induces Toxicity in Rat Cerebral Microvascular Endothelial Cells. Molecules 2011, 16, 4005-4019. https://doi.org/10.3390/molecules16054005
Zhao L, Hou L, Sun H, Yan X, Sun X, Li J, Bian Y, Chu Y, Liu Q. Apigenin Isolated from the Medicinal Plant Elsholtzia rugulosa Prevents β-Amyloid 25–35-Induces Toxicity in Rat Cerebral Microvascular Endothelial Cells. Molecules. 2011; 16(5):4005-4019. https://doi.org/10.3390/molecules16054005
Chicago/Turabian StyleZhao, Le, Lin Hou, Huijun Sun, Xin Yan, Xifeng Sun, Jianguang Li, Yong Bian, Yu Chu, and Qingshan Liu. 2011. "Apigenin Isolated from the Medicinal Plant Elsholtzia rugulosa Prevents β-Amyloid 25–35-Induces Toxicity in Rat Cerebral Microvascular Endothelial Cells" Molecules 16, no. 5: 4005-4019. https://doi.org/10.3390/molecules16054005
APA StyleZhao, L., Hou, L., Sun, H., Yan, X., Sun, X., Li, J., Bian, Y., Chu, Y., & Liu, Q. (2011). Apigenin Isolated from the Medicinal Plant Elsholtzia rugulosa Prevents β-Amyloid 25–35-Induces Toxicity in Rat Cerebral Microvascular Endothelial Cells. Molecules, 16(5), 4005-4019. https://doi.org/10.3390/molecules16054005




