Abstract
Background: Carpometacarpal (CMC) osteoarthritis (OA) of the thumb is a painful condition that affects over 15% of individuals above the age of 30 and up to 30% of post-menopausal women. Dry needling (DN) has been found to reduce pain and disability in a variety of neuromusculoskeletal conditions; however, DN in the management of CMC OA has not been well studied. Methods: Consecutive patients with clinical and radiographic evidence of CMC OA were treated with DN. The primary outcome measure was pain using the Numerical Pain Rating Scale (NPRS) at 12 weeks. Secondary outcome measures were the Upper Extremity Functional Index (UEFI-20) and the Global Rating of Change (GROC) scale. Outcome measures were collected at baseline, 4 weeks, 8 weeks, and 12 weeks. Results: Nine patients were treated for six sessions of periosteal DN over 3 weeks. Compared to baseline, statistically significant and clinically meaningful improvements were observed in thumb pain (NPRS mean difference: 2.6; p = 0.029) and function (UEFI-20 mean difference: 21.3; p = 0.012) at 12 weeks. Conclusion: Statistically significant and clinically meaningful within-group improvements in thumb pain and function were observed at 12 weeks following six sessions of periosteal DN treatment. DN may be a useful intervention in the management of patients with CMC OA of the thumb.
1. Introduction
Background
Carpometacarpal (CMC) osteoarthritis (OA) of the thumb is a debilitating hand-wrist joint condition that affects 15% of individuals over the age of 30 and up to 30% of post-menopausal women [1,2]. CMC OA thumb pain limits gripping, grasping, and dexterity activities, making several common functional activities difficult to perform, such as holding a cup, combing hair, carrying objects, or gripping a steering wheel.
A primary cause of CMC OA is thought to be associated with radial subluxation from the deterioration of the anterior oblique and dorsoradial ligaments of the thumb, leading to joint incongruence, radial subluxation, erosion of the articular cartilage, inflammation, pain, and joint stiffness [3]. Compensatory hyperextension of the metacarpophalangeal (MCP) joint is often seen as the condition progresses [4].
The diagnosis of CMC OA is based on radiographic findings and a clinical examination [1,2,5]. The clinical signs of CMC OA include a positive “hump” sign (a large lump over the CMC joint), a positive grind test, joint crepitation, swelling, point tenderness, weakness, and the inability to abduct the thumb. Carpal tunnel syndrome, De Quervain’s tenosynovitis, lateral epicondylitis, trigger thumb, and radial nerve entrapment are common co-existing conditions often seen with CMC OA. Current conservative treatments include bracing, activity modification, cortisone injection, and occupational and/or physical therapy [1].
Several studies seem to associate the etiology of musculoskeletal pain with poor circulation [6,7]. DN uses the insertion of solid filiform needles into the areas of neuromuscular tissue dysfunction to elicit acute tissue damage and, in turn, create a healing response by increasing blood flow to an area [8]. Notably, DN has been found to be effective in reducing pain, stiffness and/or disability associated with OA in the knee and upper extremity by improving circulation to the joint and surrounding tissues (muscle, nerve, bone, cartilage) [9,10,11,12,13,14,15,16,17,18,19].
Clinically meaningful improvements in pain and disability have been reported in a number of trials where periosteal needling—i.e., moving the needle close to the bone, joint line, or cartilage, or tapping the needle repeatedly onto the bone—was utilized in patients with hip or knee OA [20,21,22,23]. Notably, electroacupuncture appears to stimulate cartilage repair in individuals with knee OA. More specifically, following 20-min sessions over 4 weeks of 7-point, low-frequency electroacupuncture, Zhang et al. [24] reported significantly lower T2 values on magnetic resonance imaging at the anteromedial and anterolateral tibial subregions in 100 knees of 50 patients with knee OA.
In addition, interleukin-6 mRNA expression in bone marrow has been found to diminish following acupuncture (i.e., needling without injectate), thereby limiting inflammation and inhibiting the myelogenic osteoclast activity driving osteoarthritic degeneration [25]. Moreover, acupuncture may improve joint lubrication through changes in the hyaluronic acid within the synovial fluid [26]. Notably, acupuncture (i.e., periarticular needling without injectate) has also been found to increase microcirculation to the knee joint [10,11]. In addition, electroacupuncture has been found to block the release of local inflammatory cytokines in the synovia of osteoarthritic joints [27] and block the release of systemic inflammatory factors in the periaqueductal gray of the brain stem [28].
A 2018 multi-center randomized clinical trial of 242 patients with knee osteoarthritis found the inclusion of periosteal electrical dry needling to be more effective for their pain and disability reduction than manual therapy and exercise alone [29]. However, to date, there is conflicting and limited evidence for the use of DN in individuals with thumb pain associated with OA of the CMC joint. A recent randomized controlled trial of patients with basal thumb pain found no between-group difference in pain following six visits of verum acupuncture compared with sham acupuncture [30]; however, a recent case study found significant reductions in thumb pain following DN [31]. Notably, current systematic reviews do not include acupuncture or dry needling as a conservative treatment option for thumb pain [1,32,33]. The purpose of this case series was to explore the use of DN as an invasive but conservative treatment option in patients with CMC OA joint pain. This case report follows the CARE checklist [34].
2. Case Description
2.1. Patients
Consecutive patients who presented to the outpatient physical therapy clinic at Redington-Fairview General Hospital, Skowhegan, Maine, between August and December 2022 with a doctor’s prescription to treat thumb pain were screened for inclusion. Their ages ranged between 49 to 73 years, with a mean (SD) of 60.9 (7.7) years. The duration of symptoms ranged from 12 months to 60 months, with a mean (SD) of 29.3 (20.9) months. Baseline characteristics of the nine patients in this case series can be found in Table 1.

Table 1.
Baseline variables: demographics and outcome measures.
The inclusion criteria were as follows: (1) primary complaint of thumb pain lasting longer than 3 months, (2) pain with movement of the thumb, (3) limited hand function due to thumb pain, and (4) clinical evidence of CMC osteoarthritis (i.e., a positive grind test and point tenderness at the joint line). A positive grind test has been found as a valid and reliable test for the clinical diagnosis of symptomatic CMC OA [35], with a sensitivity of 0.42, specificity of 0.96, and an interrater reliability of 0.48 [36,37]
The exclusion criteria were as follows: (1) had received a steroid injection to the thumb within the past 3 months, (2) had prior surgery to the thumb, (3) had received physical or occupational therapy treatment for thumb pain within the previous 3 months, (4) had evidence of cervical radiculopathy or referred pain from the cervical spine, (5) had one or more contraindications to dry needling, including red flags (i.e., tumor, fracture, metabolic diseases), (6) a history of mastectomy or lymph node removal involving the ipsilateral side, (7) were currently pregnant, (8) had surgery involving the ipsilateral upper extremity within the past year, and (9) had pending legal action or a worker’s compensation claim regarding their thumb pain. All patients who met the criteria and agreed to participate underwent a formal informed-consent process by signing a consent form approved by the Institutional Review Board at Redington-Fairview General Hospital, Skowhegan, Maine, prior to the physical therapy evaluation.
2.2. Treating Clinician
The physical therapist who examined and treated all patients had 20 years of clinical experience and was a fellow in training within the American Academy of Manipulative Therapy Fellowship in Orthopaedic Manual Physical Therapy program. The clinician had 4 years of experience in dry needling, including a 30 h APTA-accredited course in dry needling and a 54 h dry needling certification training course that included content specific to the thumb.
2.3. Evaluation Procedure
For each patient, a thorough physical examination was conducted to verify the clinical signs of CMC osteoarthritis. This examination included a visual inspection for nodules, joint enlargement and swelling, palpation for tenderness at the joint and surrounding soft tissues, and compression/distraction special tests for joint pain and crepitus. Screening measures were performed, as indicated, to identify any co-existing injuries of the upper extremity or cervical spine. Measures of strength and the active range of motion were also recorded.
2.4. Outcome Measures
Patients completed the Numeric Pain Rating Scale (NPRS) and the Upper Extremity Functional Index-20 (UEFI-20) at the initial evaluation and 4, 8, and 12-week intervals following the baseline examination. The primary outcome measure was the NPRS at 12 weeks. The NPRS was used to measure the thumb pain intensity. Patients reported the average intensity of thumb pain over the past week using an 11-point scale ranging from 0 (no pain) to 10 (worst pain imaginable) at baseline, 4 weeks, 8 weeks, and 12 weeks following the initial treatment session [38]. The NPRS has been found to be a reliable and valid instrument for the assessment of pain intensity [39,40,41]. The MCID for the NPRS has been reported to be 1.74 in patients with chronic musculoskeletal pain conditions [41]; however, the MCID for thumb-related pain has not yet been established. Nevertheless, a change of 2 points or a 30% decrease in pain from baseline has previously been considered the MCID in patients with chronic musculoskeletal pain conditions [41,42].
The secondary outcome measures included the Upper Extremity Functional Index (UEFI-20) and Global Rating of Change scale (GROC). The UEFI-20 was recorded at baseline, 4 weeks, 8 weeks, and 12 weeks. The UEFI-20 has been found to possess strong construct validity and high inter-examiner reliability [43,44]. Scores range from 0 to 80, with higher scores indicating higher function. Patients rated their self-perceived changes in function using a 15-point GROC questionnaire based on a scale described by Jaeschke et al. [45]. The MCID for the GROC has not been established; however, scores of +4 and +5 are considered indicative of moderate changes in patient status [45].
2.5. Intervention
All participants received DN 2 x per week for 3 weeks for a total of 6 treatments.
Previous needling studies have used one needle inserted into the dorsal mid thenar eminence into the belly of the adductor pollicis brevis, several acupoints around the CMC joint line, and into the extensor carpi radialis longus and along the radial nerve pathway but the needles were not placed into the key muscles in the thenar eminence (opponens pollicis, abductor pollicis brevis, flexor pollicis brevis).
For this study, a standardized dry needling protocol was used to ensure consistency (Figure 1, Table 2).
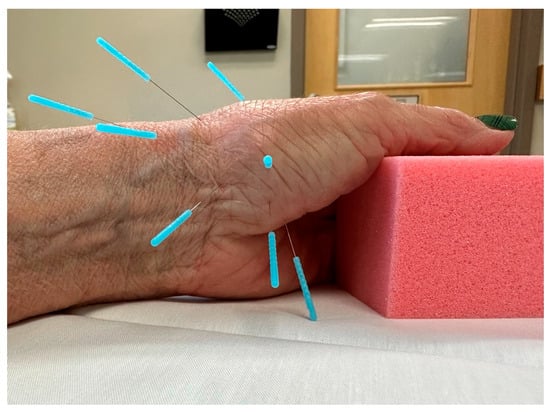
Figure 1.
Standardized dry needling protocol (8 needles) for CMC OA.

Table 2.
Standardized 8-point dry needling protocol for CMC OA.
The standardized dry needling protocol included eight points that targeted muscle, periarticular, and perineural tissue of the affected thumb. Needles were inserted to approximate the anatomical target, then rotated to achieve myofascial tenting and elicit a deqi response (i.e., a dull ache, heaviness, spreading, distention, or warmth) [46]. Unidirectional needle rotation is a recommended technique for eliciting myofascial release and a chemical healing cascade [47]. The needles were re-manipulated if necessary every 5 min and were left in situ for a total of 20 min. Two sizes of Seirin J type neeldes were utlizied (0.18 × 15 mm and 0.25 × 30 mm).
2.6. Treatment Side Effects
The patients were asked to report any adverse events they experienced during the study. An adverse event was defined as sequelae of a 1-week duration, perceived as distressing and unacceptable to the patient that required further attention [47]. Particular attention was given to ecchymosis and post-needling soreness. Aside from minor and temporary bruising and soreness, no adverse events were reported.
2.7. Statistical Analysis
Data analysis was performed using SPSS 28.0 (Chicago, IL, USA). Descriptive statistics, including frequency counts for the categorical variables and measures of central tendency and dispersion for continuous variables, were calculated to summarize the findings. A normal distribution for the NPRS and UEFI-20 was assessed using the Kolmogorov–Smirnov test; both outcomes were normally distributed (p > 0.05). A one-way analysis of variance (ANOVA) for repeated measures, with a Greenhouse–Geisser epsilon correction, was used to compare the within-group scores over time for each continuous variable (1 for the NPRS data and 1 for the UEFI-20 data). Post hoc pairwise comparisons were performed to examine the difference between the baseline and each of the follow-up periods using the Bonferroni correction at an α level of 0.05. The statistical analysis was conducted at a 95% confidence level. All nine participants completed the outcomes through the 12-week follow-up. To quantify the magnitude of the treatment effect, the within-group effect sizes were calculated using Cohen’s d coefficient. An effect size of greater than 0.8 was considered large, an approximating 0.5 was considered moderate, and less than 0.2 was considered small.
3. Results
A single physical therapist screened 11 consecutive patients with thumb pain for eligibility. Of the 11 patients screened, 1 was excluded due to symptoms consistent with carpal tunnel syndrome and possible cervical radiculopathy, and 1 was excluded because their thumb pain was deemed to primarily be associated with dysfunction at the MCP joint. Therefore, nine patients met the inclusion criteria and consented to participate in the study. Nine patients (mean age, 60.9 years; range, 49–73) were treated for six visits over a 3-week period (2 times per week). All patients completed all six treatment sessions.
3.1. Thumb Pain (NPRS)
Using the Greenhouse–Geisser epsilon correction, a one-way repeated-measures ANOVA demonstrated a significant (F = 6.779; p = 0.024) decrease in thumb pain (NPRS) after six dry needling treatment sessions (Figure 2).
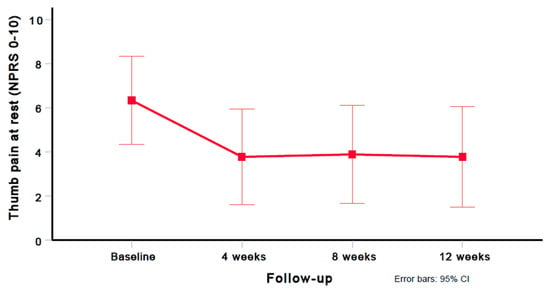
Figure 2.
Evolution of thumb pain intensity (NPRS 0–10). Values are mean and 95% confidence interval.
A significant improvement in thumb pain intensity (NPRS mean difference, 2.6) was observed between the baseline and 12 weeks (p = 0.029); however, no significant differences in thumb pain intensity (NPRS) were found between 4 and 8 weeks (p = 0.729), and 8 and 12 weeks (p = 0.594). Compared with the baseline, large (i.e., Cohen’s d ≥ 0.8) within-group effect sizes were observed for thumb pain intensity (NPRS) at 4 weeks (Cohen’s d = 0.875), 8 weeks (Cohen’s d = 0.939), and 12 weeks (Cohen’s d = 0.888). Table 3 provides the mean and standard deviation (SD) for the thumb pain intensity (NPRS) scores at all assessment periods (baseline, 4 weeks, 8 weeks, and 12 weeks). Table 4 provides the preintervention and postintervention scores for thumb pain intensity (NPRS) for each of the subjects at all time points.

Table 3.
Preintervention and postintervention scores for shoulder pain, disability, and GROC.

Table 4.
Preintervention and postintervention scores for thumb pain.
3.2. Upper Extremity Functional Index (UEFI-20)
Using the Greenhouse–Geisser epsilon correction, a one-way repeated measures ANOVA found a significant (F = 6.148; p = 0.010) decrease in disability (UEFI-20 score) after six sessions of DN (Figure 3).
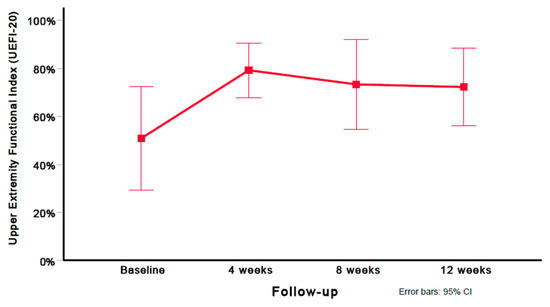
Figure 3.
Evolution of disability scores (UEFI-20). Values are mean and 95% confidence interval.
A significant improvement in disability (UEFI-20 mean difference: 21.33) was observed between the baseline and 12 weeks (p = 0.012); however, no significant differences in disability (UEFI-20) were found between 4 and 8 weeks (p = 0.511), and 8 and 12 weeks (p = 0.678). Compared with the baseline, large (i.e., Cohen’s d ≥ 0.8) within-group effect sizes were observed for disability (UEFI-20) at 4 weeks (Cohen’s d = 1.11), 8 weeks (Cohen’s d = 1.05), and 12 weeks (Cohen’s d = 1.08). Table 3 provides the mean and standard deviation (SD) for the disability (UEFI-20) scores at all assessment periods (baseline, 4 weeks, 8 weeks, and 12 weeks). Table 5 provides the preintervention and postintervention scores for UEFI-20 on each of the subjects at all time points.

Table 5.
Preintervention and postintervention scores for disability.
3.3. Global Rating of Change (GROC)
In nine patients with thumb pain due to CMC OA and after six sessions of DN, the mean (SD) GROC scores were +4.0 (2.1) at 4 weeks, +2.4 (3.0) at 8 weeks, and +2.2 (2.9) at 12 weeks follow-up, indicating “moderately better” and “slightly better” outcomes in the short and medium term, respectively.
4. Discussion
Nine patients with CMC OA were treated for six sessions of periosteal DN over 3 weeks. Statistically significant and clinically meaningful within-group improvements were observed in thumb pain (NPRS mean difference: 2.6; p = 0.029) and function (UEFI-20 mean difference: 21.3; p = 0.012) at 12 weeks. Notably, most improvement was observed at 4 weeks; however, this improvement appeared to persist following the completion of the treatment regimen at 3 weeks and through the final follow-up at 12 weeks. Four out of the nine subjects exceeded the MCID of a 2 or more points (NPRS 0–10) reduction in pain at 4, 8, and 12 weeks. For the disability, eight out of nine subjects exceeded the MCID of 10% improvement for the UEFI-20 at 4 weeks, and six out of nine subjects met the MCID of 10% improvement at 8 weeks and 12 weeks. In addition, six of the nine patients scored themselves at 4 or better at the 4-week interval, indicating that more than 60% of the patients reported a “moderate” improvement in their condition by the completion of the six sessions of periosteal dry needling to the CMC joint.
Barnard et al. found no difference between real and sham acupuncture for basal thumb pain [30]; in addition, Dickens and Lewith found no significant improvement in thumb pain following acupuncture in patients with CMC OA [48]. Nevertheless, unlike the current case series, periosteal needling targeting the bone, joint line, and periarticular connective tissue of the CMC joint was not utilized in the two prior studies [30,48]. Notably, a recent multi-center clinical trial of 242 patients with knee osteoarthritis found that the addition of periosteal electrical dry needling was more effective for pain and disability reduction than manual therapy and exercise alone [29]. Likewise, a 2021 case study reported significant reductions in thumb pain following DN [31].
The underlying mechanisms as to why the patients receiving DN around the CMC joint experienced a reduction in pain and an improvement in function remain to be elucidated. However, a number of studies have found periosteal needling—i.e., moving the needle close to the bone, cartilage, or joint line—leads to clinically meaningful improvements in pain and disability in patients with hip and/or knee OA [20,23,49]. More specifically, following 20-min sessions over 4 weeks of low-frequency electroacupuncture, Zhang et al. [24] reported significantly lower T2 values on MRIs at the anteromedial and anterolateral tibial subregions of 100 knees, suggesting electroacupuncture may play a role in the cartilage repair in individuals with knee OA. Notably, acupuncture has been found to diminish interleukin-6 mRNA expression in bone marrow, thereby reducing inflammation and inhibiting myelogenic osteoclast activity driving degeneration [25]. Additionally, increased levels of hyaluronic acid within the synovial fluid appear to be induced by acupuncture, thus enhancing joint lubrication [26]. Interestingly, local electroacupuncture has been found to enhance joint microcirculation at the knee [10,11]; furthermore, electroacupuncture has been found to block the release of local inflammatory cytokines (i.e., interleukin-1 β and tumor necrosis factor-α) in the synovia of osteoarthritic joints [27] and block the release of systemic inflammatory factors in the periaqueductal gray matter of the brain stem [28].
Limitations
Although a cause-and-effect relationship cannot be inferred from the results of a case series, clinically meaningful within-group changes in pain and function were observed; nevertheless, these changes may be due to natural history, changes in activity levels, the Hawthorn effect [50], and/or the therapeutic alliance [51]. Additionally, all treatment sessions were administered by a single physical therapist who was completing a postgraduate fellowship program in orthopedic manual physical therapy; therefore, the results may not be generalizable to all clinicians [52].
5. Conclusions
This case series suggests that periosteal DN may be a useful intervention for pain and disability reduction in patients with basal thumb pain associated with OA of the CMC joint. Randomized clinical trials are needed to determine the effectiveness and between-group effect sizes when compared to an active comparison or control group.
Author Contributions
A.S.W. and J.D. participated in the conception, design, data acquisition, statistical analyses, data interpretation, drafting, and revision of the manuscript. C.C., P.B. and F.M. were involved in the data interpretation, drafting, and revision of the manuscript. All data relevant to the study are included in the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board at Redington Fairview General Hospital, Skowhegan, Maine.
Informed Consent Statement
All patients provided informed consent before their participation in the study. All patients provided informed consent to publish the manuscript.
Data Availability Statement
Not applicable.
Acknowledgments
Redington Fairview General Hospital (RFGH) provided the facility and treatment supplies.
Conflicts of Interest
At the time of data collection, the lead author of this case series was a fellow-in-training within the American Academy of Manipulative Therapy (AAMT) Orthopaedic Manual Physical Therapy fellowship program. James Dunning is the Director of the AAMT Fellowship program in Orthopaedic Manual Physical Therapy. AAMT provides postgraduate training programs in diagnostic musculoskeletal ultrasonography, vestibular rehabilitation, spinal and extremity manipulation/mobilization, dry needling, and differential diagnosis to physical therapists, osteopaths, and medical physicians. James Dunning, Casey Charlebois, and Paul Bliton are instructors for AAMT. The other authors declare that they have no potential competing interests. None of the authors received any funding for this study.
References
- Higgenbotham, C.; Boyd, A.; Busch, M.; Heaton, D.; Trumble, T. Optimal management of thumb basal joint arthritis: Challenges and solutions. Orthop. Res. Rev. 2017, 9, 93–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gillis, J.; Calder, K.; Williams, J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can. J. Plast. Surg. 2011, 19, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Riordan, E.; Robbins, S.; Deveza, L.; Duong, V.; Oo, W.; Wajon, A.; Bennell, K.; Eyles, J.; Jongs, R.; Linklater, J. Radial subluxation in relation to hand strength and radiographic severity in trapeziometacarpal osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; White, C.P.; Li, Y.K.; White, C.P. Five things to know about...carpometacarpal osteoarthritis of the thumb. Can. Med. Assoc. J. CMAJ 2013, 185, 149. [Google Scholar] [CrossRef]
- Van Heest, A.E.; Kallemeier, P. Thumb carpal metacarpal arthritis. JAAOS-J. Am. Acad. Orthop. Surg. 2008, 16, 140–151. [Google Scholar] [CrossRef]
- Hussain, S.M.; Wang, Y.; Shaw, J.E.; Magliano, D.J.; Wong, T.-Y.; Wluka, A.E.; Graves, S.; Tapp, R.J.; Cicuttini, F.M. Retinal arteriolar narrowing and incidence of knee replacement for osteoarthritis: A prospective cohort study. Osteoarthr. Cartil. 2015, 23, 589–593. [Google Scholar] [CrossRef]
- Findlay, D. Vascular pathology and osteoarthritis. Rheumatology 2007, 46, 1763–1768. [Google Scholar] [CrossRef]
- Kietrys, D.M.; Palombaro, K.M.; Azzaretto, E.; Hubler, R.; Schaller, B.; Schlussel, J.M.; Tucker, M. Effectiveness of dry needling for upper-quarter myofascial pain: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 620–634. [Google Scholar] [CrossRef]
- Fink, M.; Wipperman, B.; Gehrke, A. Non-specific effects of traditional Chinese acupuncture in osteoarthritis of the hip. Complement. Ther. Med. 2001, 9, 82–89. [Google Scholar] [CrossRef]
- Loaiza, L.A.; Yamaguchi, S.; Ito, M.; Ohshima, N. Electro-acupuncture stimulation to muscle afferents in anesthetized rats modulates the blood flow to the knee joint through autonomic reflexes and nitric oxide. Auton. Neurosci. 2002, 97, 103–109. [Google Scholar] [CrossRef]
- Ahsin, S.; Saleem, S.; Bhatti, A.M.; Iles, R.K.; Aslam, M. Clinical and endocrinological changes after electro-acupuncture treatment in patients with osteoarthritis of the knee. PAIN® 2009, 147, 60–66. [Google Scholar] [CrossRef]
- Haslam, R. A comparison of acupuncture with advice and exercises on the symptomatic treatment of osteoarthritis of the hip—A randomised controlled trial. Acupunct. Med. 2001, 19, 19–26. [Google Scholar] [CrossRef]
- Cagnie, B.; Barbe, T.; De Ridder, E.; Van Oosterwijck, J.; Cools, A.; Danneels, L. The influence of dry needling of the trapezius muscle on muscle blood flow and oxygenation. J. Manip. Physiol. Ther. 2012, 35, 685–691. [Google Scholar] [CrossRef]
- Takeda, W.; Wessel, J. Acupuncture for the treatment of pain of osteoarthritic knees. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1994, 7, 118–122. [Google Scholar] [CrossRef]
- Christensen, B.V.; Iuhl, I.; Vilbek, H.; Bülow, H.H.; Dreijer, N.; Rasmussen, H. Acupuncture treatment of severe knee osteoarthrosis: A long-term study. Acta Anaesthesiol. Scand. 1992, 36, 519–525. [Google Scholar] [CrossRef]
- Williamson, L.; Wyatt, M.; Yein, K.; Melton, J. Severe knee osteoarthritis: A randomized controlled trial of acupuncture, physiotherapy (supervised exercise) and standard management for patients awaiting knee replacement. Rheumatology 2007, 46, 1445–1449. [Google Scholar] [CrossRef]
- Trinh, K.; Zhou, F.; Belski, N.; Deng, J.; Wong, C.Y. The Effect of Acupuncture on Hand and Wrist Pain Intensity, Functional Status, and Quality of Life in Adults: A Systematic Review. Med. Acupunct. 2022, 34, 34–48. [Google Scholar] [CrossRef]
- Manheimer, E.; Cheng, K.; Linde, K.; Lao, L.; Yoo, J.; Wieland, S.; van der Windt, D.A.; Berman, B.M.; Bouter, L.M. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst. Rev. 2010, 20, CD001977. [Google Scholar] [CrossRef]
- Lewit, K. The needle effect in the relief of myofascial pain. Pain 1979, 6, 83–90. [Google Scholar] [CrossRef]
- Weiner, D.K.; Moore, C.G.; Morone, N.E.; Lee, E.S.; Kwoh, C.K. Efficacy of periosteal stimulation for chronic pain associated with advanced knee osteoarthritis: A randomized, controlled clinical trial. Clin. Ther. 2013, 35, 1703–1720.e5. [Google Scholar] [CrossRef]
- Mavrommatis, C.I.; Argyra, E.; Vadalouka, A.; Vasilakos, D.G. Acupuncture as an adjunctive therapy to pharmacological treatment in patients with chronic pain due to osteoarthritis of the knee: A 3-armed, randomized, placebo-controlled trial. PAIN® 2012, 153, 1720–1726. [Google Scholar] [CrossRef]
- Ugreja, R.A.; Prem, V. Effectiveness of dry needling techniques in patients with knee osteoarthritis: A systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2021, 27, 328–338. [Google Scholar] [CrossRef]
- McIndoe, A.; Young, K.; Bone, M. A comparison of acupuncture with intra-articular steroid injection as analgesia for osteoarthritis of the hip. Acupunct. Med. 1995, 13, 67–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, F.; Wang, Y.; Wu, Z. Influence of acupuncture in treatment of knee osteoarthritis and cartilage repairing. Am. J. Transl. Res. 2016, 8, 3995–4002. [Google Scholar]
- Liu, X.; Shen, L.; Wu, M.; Wu, B.; Gao, L.; Hu, W.; Zhang, A. Effects of acupuncture on myelogenic osteoclastogenesis and IL-6 mRNA expression. J. Tradit. Chin. Med. Chung I Tsa Chih Ying Wen Pan 2004, 24, 144–148. [Google Scholar]
- Li, Z.-D.; Cao, L.-H.; Wang, S.-C. Effect of moxibustion in treating knee joint osteoarthritis and its relation with contents of hyaluronic acid in serum and synovial fluid. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi Chin. J. Integr. Tradit. West. Med. 2009, 29, 883–885. [Google Scholar]
- Huang, J.; Zhuo, L.-S.; Wang, Y.-Y.; Peng, Z.-L.; Huang, Y.-R.; Wang, Y.; Yang, L. Effects of electroacupuncture on synovia IL-1beta and TNF-alpha contents in the rabbit with knee osteoarthritis. Zhen Ci Yan Jiu Acupunct. Res. 2007, 32, 115–118. [Google Scholar]
- Cao, S.; Wang, C.; Ma, X.; Wang, X.; Huang, J.; Zhang, C. Imaging diagnosis for chronic lateral ankle ligament injury: A systemic review with meta-analysis. J. Orthop. Surg. Res. 2018, 13, 122. [Google Scholar] [CrossRef]
- Dunning, J.; Butts, R.; Young, I.; Mourad, F.; Galante, V.; Bliton, P.; Tanner, M.; Fernandez-de-Las-Penas, C. Periosteal Electrical Dry Needling as an Adjunct to Exercise and Manual Therapy for Knee Osteoarthritis: A Multicenter Randomized Clinical Trial. Clin. J. Pain. 2018, 34, 1149–1158. [Google Scholar] [CrossRef]
- Barnard, A.; Jansen, V.; Swindells, M.G.; Arundell, M.; Burke, F.D. A randomized controlled trial of real versus sham acupuncture for basal thumb joint arthritis. J. Hand Surg. Eur. Vol. 2020, 45, 488–494. [Google Scholar] [CrossRef]
- Voss, M.R.; Donnay, R.L.; Homa, J.K. The effects of dry needling on the thumb: A case report. Hong Kong J. Occup. Ther. 2021, 34, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, J.H.; Valdes, K.; Pedersini, P.; Berjano, P. Thumb carpometacarpal osteoarthritis: A musculoskeletal physiotherapy perspective. J. Bodyw. Mov. Ther. 2019, 23, 908–912. [Google Scholar] [CrossRef]
- Shridhar, V.; Williams, S. Basal thumb arthritis: Treatment strategies for managing pain. Aust. J. Gen. Pract. 2020, 49, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob. Adv. Health Med. 2013, 2, 38–43. [Google Scholar] [CrossRef]
- Merritt, M.M.; Roddey, T.S.; Costello, C.; Olson, S. Diagnostic value of clinical grind test for carpometacarpal osteoarthritis of the thumb. J. Hand Ther. 2010, 23, 261–268. [Google Scholar] [CrossRef]
- Jensen, G.M.; Hack, L.M.; Nordstrom, T.; Gwyer, J.; Mostrom, E. National Study of Excellence and Innovation in Physical Therapist Education: Part 2—A Call to Reform. Phys. Ther. 2017, 97, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Choa, R.; Parvizi, N.; Giele, H. A prospective case-control study to compare the sensitivity and specificity of the grind and traction-shift (subluxation-relocation) clinical tests in osteoarthritis of the thumb carpometacarpal joint. J. Hand Surg. Eur. Vol. 2014, 39, 282–285. [Google Scholar] [CrossRef]
- Jensen, M.P.; Karoly, P.; Braver, S. The measurement of clinical pain intensity: A comparison of six methods. Pain 1986, 27, 117–126. [Google Scholar] [CrossRef]
- Cleland, J.A.; Childs, J.D.; Whitman, J.M. Psychometric Properties of the Neck Disability Index and Numeric Pain Rating Scale in Patients with Mechanical Neck Pain. Arch. Phys. Med. Rehabil. 2008, 89, 69–74. [Google Scholar] [CrossRef]
- Young, I.A.; Cleland, J.A.; Michener, L.A.; Brown, C. Reliability, construct validity, and responsiveness of the neck disability index, patient-specific functional scale, and numeric pain rating scale in patients with cervical radiculopathy. Am. J. Phys. Med. Rehabil. 2010, 89, 831–839. [Google Scholar] [CrossRef]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Salaffi, F.; Stancati, A.; Silvestri, C.A.; Ciapetti, A.; Grassi, W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur. J. Pain 2004, 8, 283–291. [Google Scholar] [CrossRef]
- Chesworth, B.M.; Hamilton, C.B.; Walton, D.M.; Benoit, M.; Blake, T.A.; Bredy, H.; Burns, C.; Chan, L.; Frey, E.; Gillies, G. Reliability and validity of two versions of the upper extremity functional index. Physiother. Can. 2014, 66, 243–253. [Google Scholar] [CrossRef]
- Hamilton, C.B.; Chesworth, B.M. A Rasch-validated version of the upper extremity functional index for interval-level measurement of upper extremity function. Phys. Ther. 2013, 93, 1507–1519. [Google Scholar] [CrossRef]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Butts, R.; Mourad, F.; Young, I.; Flannagan, S.; Perreault, T. Dry needling: A literature review with implications for clinical practice guidelines. Phys. Ther. Rev. 2014, 19, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Carlesso, L.C. Standardization of adverse event terminology and reporting in orthopaedic physical therapy: Application to the cervical spine. J. Orthop. Sports Phys. Ther. 2010, 40, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Dickens, W.; Lewith, G.T. A single-blind, controlled and randomised clinical trial to evaluate the effect of acupuncture in the treatment of trapezio-metacarpal osteoarthirits. Complement. Med. Res. 1989, 3, 5–8. [Google Scholar]
- Weiner, D.K.; Rudy, T.E.; Morone, N.; Glick, R.; Kwoh, C.K. Efficacy of periosteal stimulation therapy for the treatment of osteoarthritis-associated chronic knee pain: An initial controlled clinical trial. J. Am. Geriatr. Soc. 2007, 55, 1541–1547. [Google Scholar] [CrossRef]
- McCarney, R.; Warner, J.; Iliffe, S.; van Haselen, R.; Griffin, M.; Fisher, P. The Hawthorne Effect: A randomised, controlled trial. BMC Med. Res. Methodol. 2007, 7, 30. [Google Scholar] [CrossRef]
- Kinney, M.; Seider, J.; Beaty, A.F.; Coughlin, K.; Dyal, M.; Clewley, D. The impact of therapeutic alliance in physical therapy for chronic musculoskeletal pain: A systematic review of the literature. Physiother. Theory Pract. 2020, 36, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Rodeghero, J.; Wang, Y.-C.; Flynn, T.; Cleland, J.A.; Wainner, R.S.; Whitman, J.M. The impact of physical therapy residency or fellowship education on clinical outcomes for patients with musculoskeletal conditions. J. Orthop. Sports Phys. Ther. 2015, 45, 86–96. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).