Clinical Link between the BARD Score at Diagnosis and Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Clinical Data
2.3. ANCA Measurement
2.4. Formula of the BARD Score
2.5. All-Cause Mortality
2.6. Statistical Analyses
3. Results
3.1. Characteristics of AAV Patients
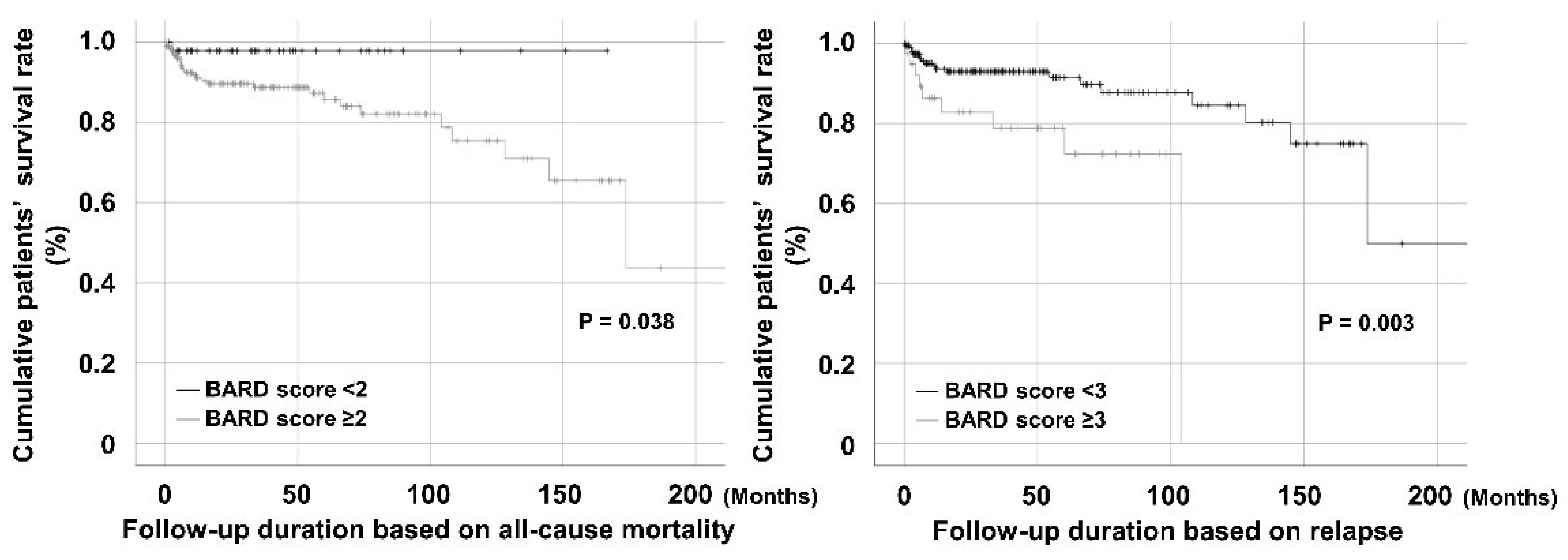
3.2. Comparison of the Cumulative Survival Rates
3.3. Cox Hazards Model Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cui, H.; Li, N.; Wei, Y.; Lai, S.; Yang, Y.; Yin, X.; Chen, D.F. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: A meta-analysis study. Hepatol. Res. 2016, 46, 862–870. [Google Scholar] [CrossRef]
- Raszeja-Wyszomirska, J.; Szymanik, B.; Ławniczak, M.; Kajor, M.; Chwist, A.; Milkiewicz, P.; Hartleb, M. Validation of the BARD scoring system in Polish patients with nonalcoholic fatty liver disease (NAFLD). BMC Gastroenterol. 2010, 10, 67. [Google Scholar] [CrossRef]
- De Carli, M.A.; de Carli, L.A.; Correa, M.B.; Junqueira, G., Jr.; Tovo, C.V.; Coral, G.P. Performance of noninvasive scores for the diagnosis of advanced liver fibrosis in morbidly obese with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2020, 32, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Celiński, K.; Prozorow-Król, B.; Swatek, J.; Słomka, M.; Lach, T. The BARD score and the NAFLD fibrosis score in the assessment of advanced liver fibrosis in nonalcoholic fatty liver disease. Med. Sci. Monit. 2012, 18, CR735–CR740. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.D.; Almond, M.K.; Burns, A.; Ellis, P.; Gaskin, G.; Neild, G.H.; Plaisance, M.; Pusey, C.; Jayne, D.R.; Pan-Thames Renal Research Group. Outcome of ANCA-associated renal vasculitis: A 5-year retrospective study. Am. J. Kidney Dis. 2003, 41, 776–784. [Google Scholar] [CrossRef]
- Park, P.G.; Pyo, J.Y.; Ahn, S.S.; Choi, H.J.; Song, J.J.; Park, Y.B.; Huh, J.H.; Lee, S.W. Fatty Liver Index Independently Predicts All-Cause Mortality in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis but No Substantial Liver Disease. Front. Cardiovasc. Med. 2022, 9, 848121. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Apekey, T.A.; Seddoh, D.; Walley, J. Liver enzymes and risk of all-cause mortality in general populations: A systematic review and meta-analysis. Int. J. Epidemiol. 2014, 43, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Choo, E.; Lee, S. Study of hospitalization and mortality in Korean diabetic patients using the diabetes complications severity index. BMC Endocr. Disord. 2020, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.T.; Hunder, G.G.; Lie, J.T.; Michel, B.A.; Bloch, D.A.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990, 33, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Toumelin, P.L.; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011, 90, 19–27. [Google Scholar] [CrossRef] [PubMed]
- McAdoo, S.P.; Medjeral-Thomas, N.; Gopaluni, S.; Tanna, A.; Mansfield, N.; Galliford, J.; Griffith, M.; Levy, J.; Cairns, T.D.; Jayne, D.; et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol. Dial. Transplant. 2019, 34, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bezan, A.; Mrsic, E.; Krieger, D.; Stojakovic, T.; Pummer, K.; Zigeuner, R.; Hutterer, G.C.; Pichler, M. The Preoperative AST/ALT (De Ritis) Ratio Represents a Poor Prognostic Factor in a Cohort of Patients with Nonmetastatic Renal Cell Carcinoma. J. Urol. 2015, 194, 30–35. [Google Scholar] [CrossRef]
- Ewid, M.; Sherif, H.; Allihimy, A.S.; Alharbi, S.A.; Aldrewesh, D.A.; Alkuraydis, S.A.; Abazid, R. AST/ALT ratio predicts the functional severity of chronic heart failure with reduced left ventricular ejection fraction. BMC Res. Notes 2020, 13, 178. [Google Scholar] [CrossRef]
- Djakpo, D.K.; Wang, Z.Q.; Shrestha, M. The significance of transaminase ratio (AST/ALT) in acute myocardial infarction. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e279–e283. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Park, P.G.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.B.; Huh, J.H.; Lee, S.W. Effect of numbers of metabolic syndrome components on mortality in patients with antineutrophil cytoplasmic antibody-associated vasculitis with metabolic syndrome. Clin. Exp. Rheumatol. 2022, 40, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Dellavalle, R.; Danaei, G.; Ezzati, M.; Fahimi, A.; et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; Choi, G.H.; Han, W.K.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Choi, J.S.; Yang, S.C.; Choi, E.H.; Ahn, S.H.; et al. What are ‘true norma’ liver stiffness values using FibroScan?: A prospective study in healthy living liver and kidney donors in South Korea. Liver Int. 2010, 30, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sebode, M.; Weiler-Normann, C.; Liwinski, T.; Schramm, C. Autoantibodies in Autoimmune Liver Disease-Clinical and Diagnostic Relevance. Front. Immunol. 2018, 9, 609. [Google Scholar] [CrossRef]
| Variables | Values |
|---|---|
| At AAV diagnosis | |
| Demographic data | |
| Age (years) | 60.0 (48.3–69.0) |
| Male sex (N, (%)) | 82 (34.7) |
| Female sex (N, (%)) | 154 (65.3%) |
| BMI (kg/m2) | 22.4 (20.1–24.2) |
| Ex-smoker (N, (%)) | 13 (5.5) |
| AAV subtype (N, (%)) | |
| MPA | 124 (52.5) |
| GPA | 62 (26.3) |
| EGPA | 50 (21.2) |
| ANCA type and positivity (N, (%)) | |
| MPO-ANCA (or P-ANCA) positivity | 157 (66.5) |
| PR3-ANCA (or C-ANCA) positivity | 41 (17.4) |
| AAV-specific indices | |
| BVAS | 12.0 (7.0–18.0) |
| FFS | 1.0 (0–2.0) |
| Acute phase reactants | |
| ESR (mm/h) | 57.0 (21.3–96.0) |
| CRP (mg/L) | 13.5 (1.6–67.6) |
| Liver-related variables | |
| AST (IU/L) | 18.0 (15.0–24.0) |
| ALT (IU/L) | 16.0 (11.0–25.0) |
| AST/ALT ratio | 1.2 (0.9–1.6) |
| BARD score-related variables | |
| BMI ≥ 28 kg/m2 (N, (%)) | 7 (3.0) |
| T2DM (N, (%)) | 50 (21.2) |
| AST/ALT ratio ≥ 0.8 (N, (%)) | 187 (79.2) |
| BARD score | 2.0 (2.0–2.0) |
| BARD score ≥ 2 (N, (%)) | 189 (80.1) |
| BARD score ≥ 3 (N, (%)) | 40 (16.9) |
| During the follow-up duration | |
| Typical poor outcomes of AAV | |
| All-cause mortality (N, (%) | 28 (11.9) |
| Follow-up duration based on all-cause mortality (months) | 33.8 (9.9–68.4) |
| Medications (N, (%)) | |
| Glucocorticoids | 221 (93.6) |
| Cyclophosphamide | 130 (55.1) |
| Rituximab | 38 (16.1) |
| Mycophenolate mofetil | 33 (14.0) |
| Azathioprine | 126 (53.4) |
| Tacrolimus | 20 (8.5) |
| Methotrexate | 24 (10.2) |
| Variables | Univariable | Multivariable (BARD Score ≥ 2) | Multivariable (BARD Score ≥ 3) | ||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age (years) | 1.086 | 1.044–1.130 | <0.001 | 1.060 | 1.015–1.108 | 0.009 | 1.061 | 1.015–1.109 | 0.009 |
| Male sex | 2.094 | 0.989–4.431 | 0.053 | 2.698 | 1.181–6.160 | 0.019 | 2.327 | 1.035–5.233 | 0.041 |
| Female sex | 0.478 | 0.226–1.011 | 0.053 | ||||||
| Ex-smoker | 2.045 | 0.615–6.797 | 0.243 | ||||||
| MPO-ANCA (or P-ANCA) positivity | 1.776 | 0.750–4.207 | 0.191 | ||||||
| PR3-ANCA (or C-ANCA) positivity | 0.357 | 0.085–1.511 | 0.162 | ||||||
| BVAS | 1.117 | 1.064–1.174 | <0.001 | 1.087 | 1.025–1.154 | 0.006 | 1.100 | 1.035–1.168 | 0.002 |
| FFS | 2.208 | 1.543–3.160 | <0.001 | 1.282 | 0.829–1.981 | 0.264 | 1.383 | 0.880–2.173 | 0.160 |
| ESR | 1.009 | 1.000–1.018 | 0.058 | 0.990 | 0.976–1.004 | 0.162 | 0.989 | 0.976–1.003 | 0.135 |
| CRP | 1.008 | 1.003–1.014 | 0.004 | 1.007 | 0.998–1.015 | 0.131 | 1.006 | 0.998–1.014 | 0.170 |
| BARD score ≥ 2 | 6.307 | 0.855–46.514 | 0.071 | 6.432 | 0.796–51.974 | 0.081 | |||
| BARD score ≥ 3 | 3.267 | 1.421–7.512 | 0.005 | 2.866 | 1.175–6.991 | 0.021 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-G.; Park, P.-G.; Park, Y.-B.; Huh, J.-H.; Lee, S.-W. Clinical Link between the BARD Score at Diagnosis and Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. J. Clin. Med. 2023, 12, 5679. https://doi.org/10.3390/jcm12175679
Lee T-G, Park P-G, Park Y-B, Huh J-H, Lee S-W. Clinical Link between the BARD Score at Diagnosis and Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Journal of Clinical Medicine. 2023; 12(17):5679. https://doi.org/10.3390/jcm12175679
Chicago/Turabian StyleLee, Tae-Geom, Pil-Gyu Park, Yong-Beom Park, Ji-Hye Huh, and Sang-Won Lee. 2023. "Clinical Link between the BARD Score at Diagnosis and Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis" Journal of Clinical Medicine 12, no. 17: 5679. https://doi.org/10.3390/jcm12175679
APA StyleLee, T.-G., Park, P.-G., Park, Y.-B., Huh, J.-H., & Lee, S.-W. (2023). Clinical Link between the BARD Score at Diagnosis and Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Journal of Clinical Medicine, 12(17), 5679. https://doi.org/10.3390/jcm12175679






