A Machine-Learning-Based Method to Detect Degradation of Motor Control Stability with Implications to Diagnosis of Presymptomatic Parkinson’s Disease: A Simulation Study
Abstract
:1. Introduction
2. Methods
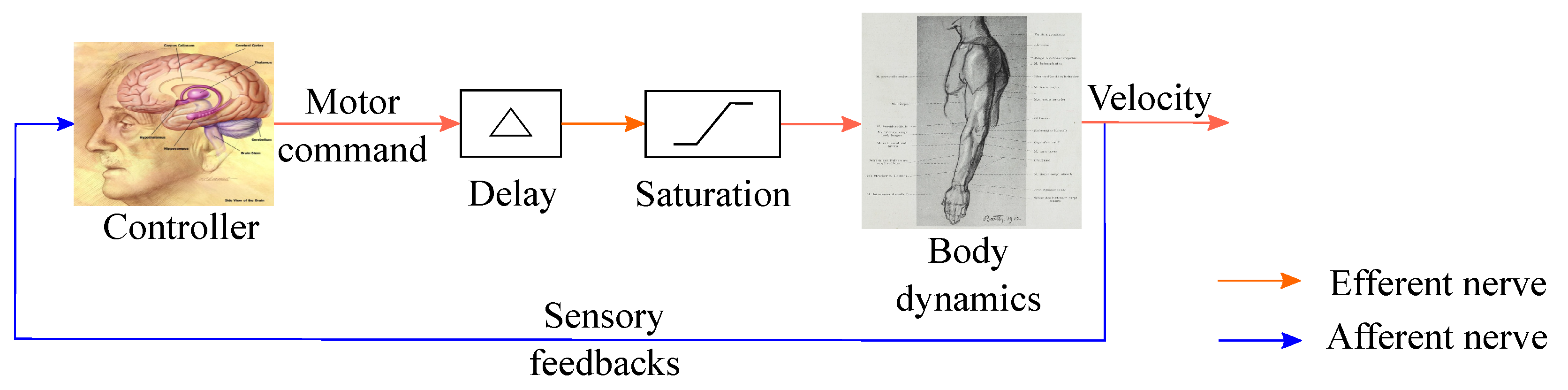
2.1. Sensorimotor Loop Representation
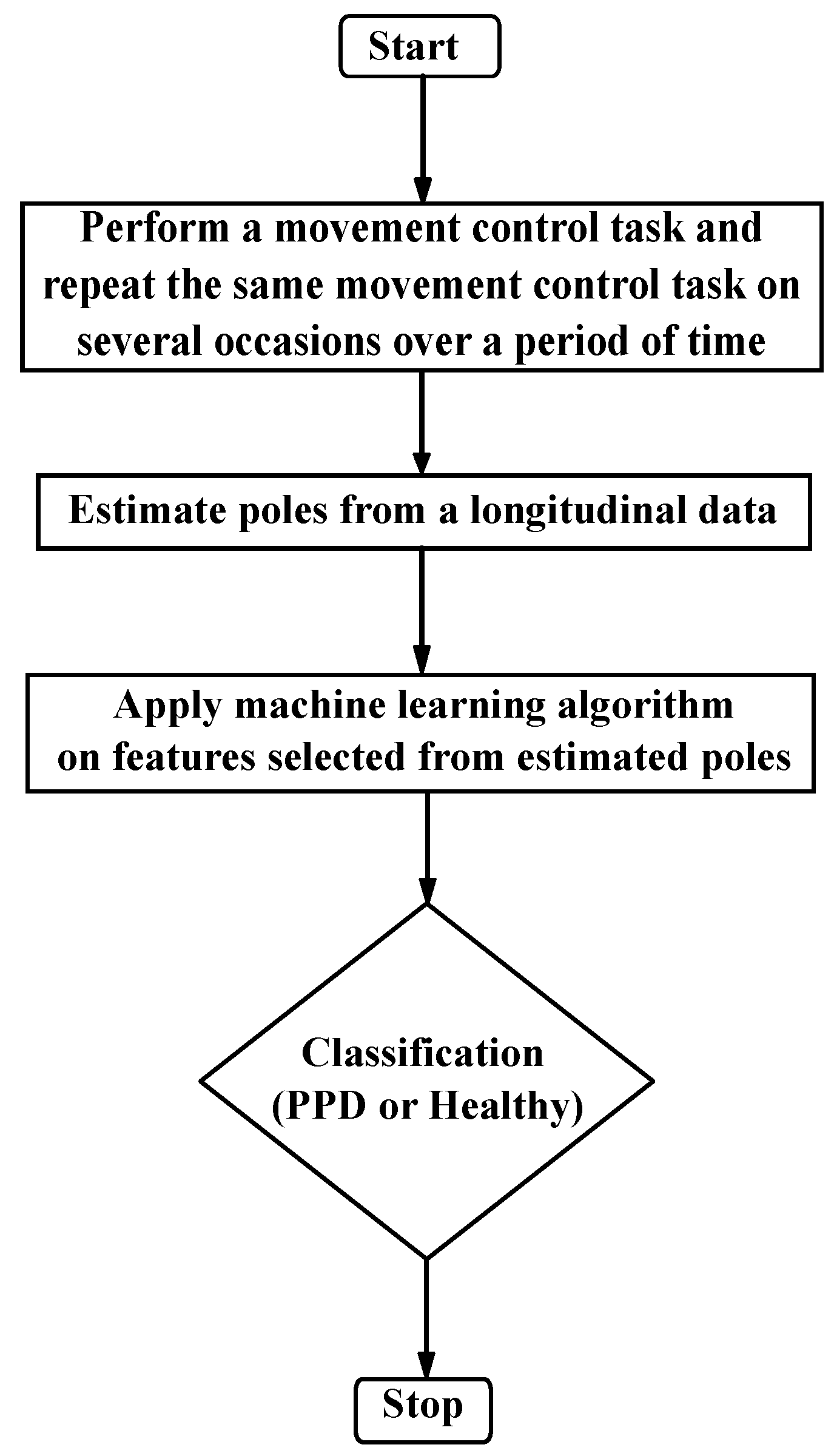
2.2. Proposed Approach for Detecting Presymptomatic Parkinson’s Disease (PPD)
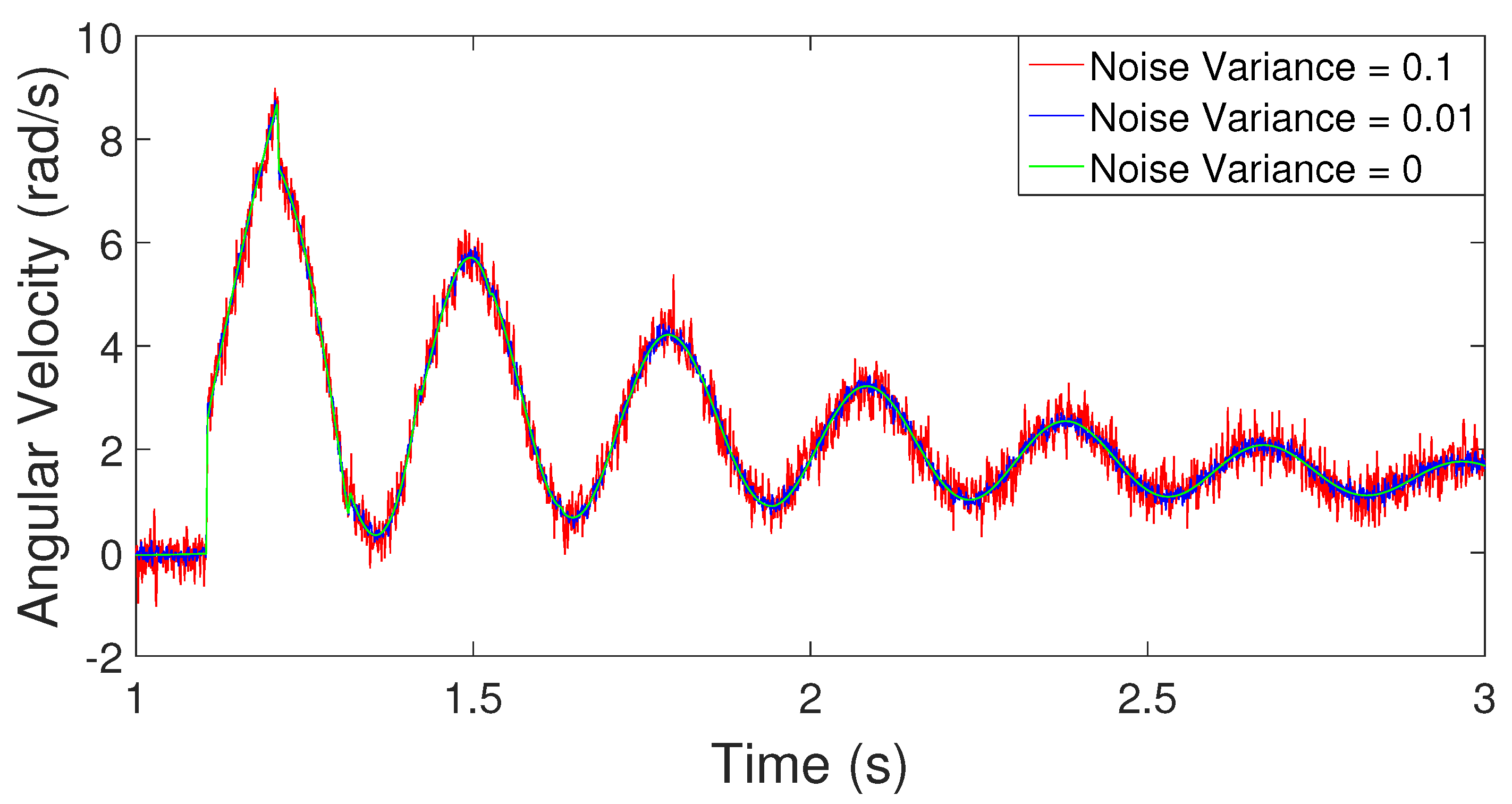
2.3. Simulation Example
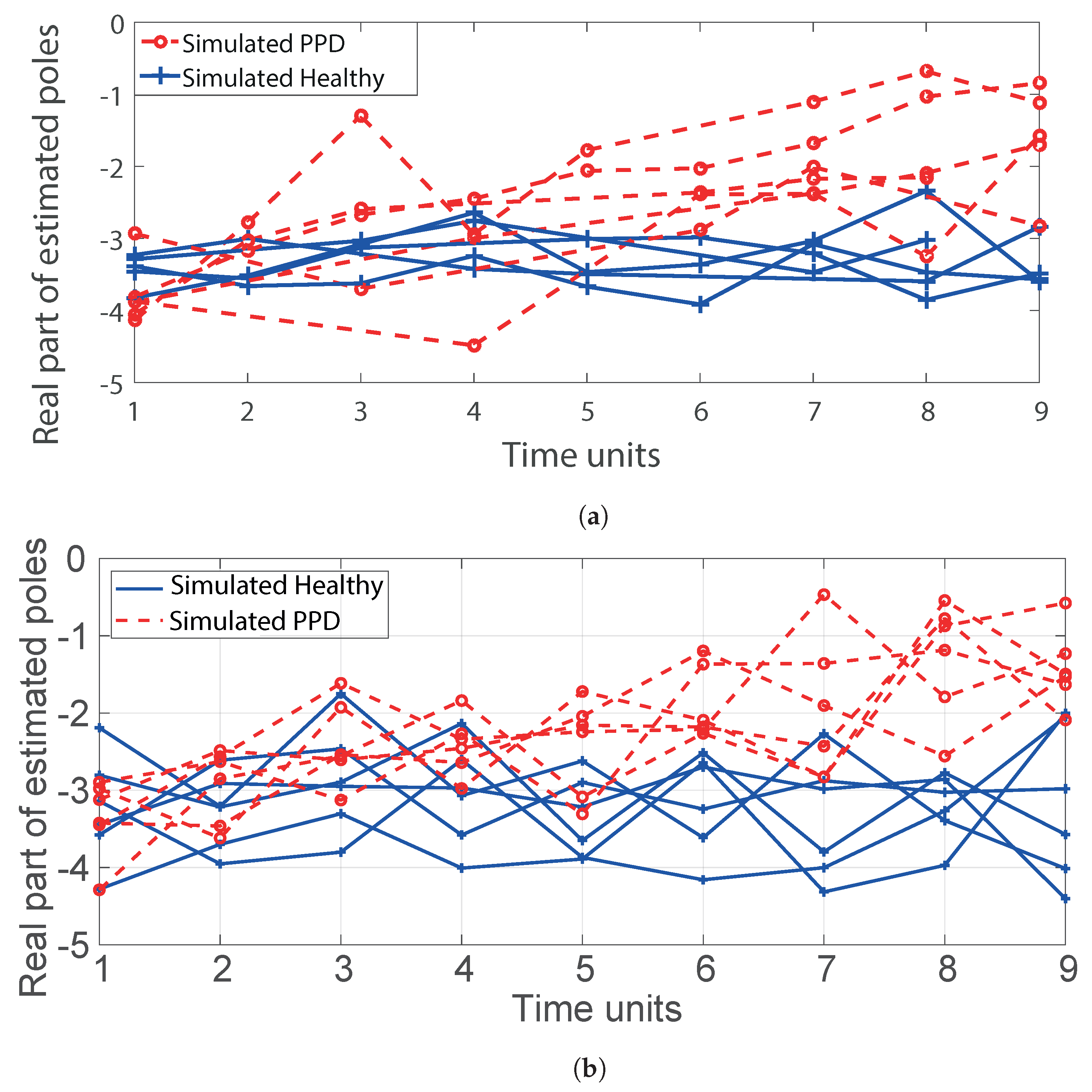
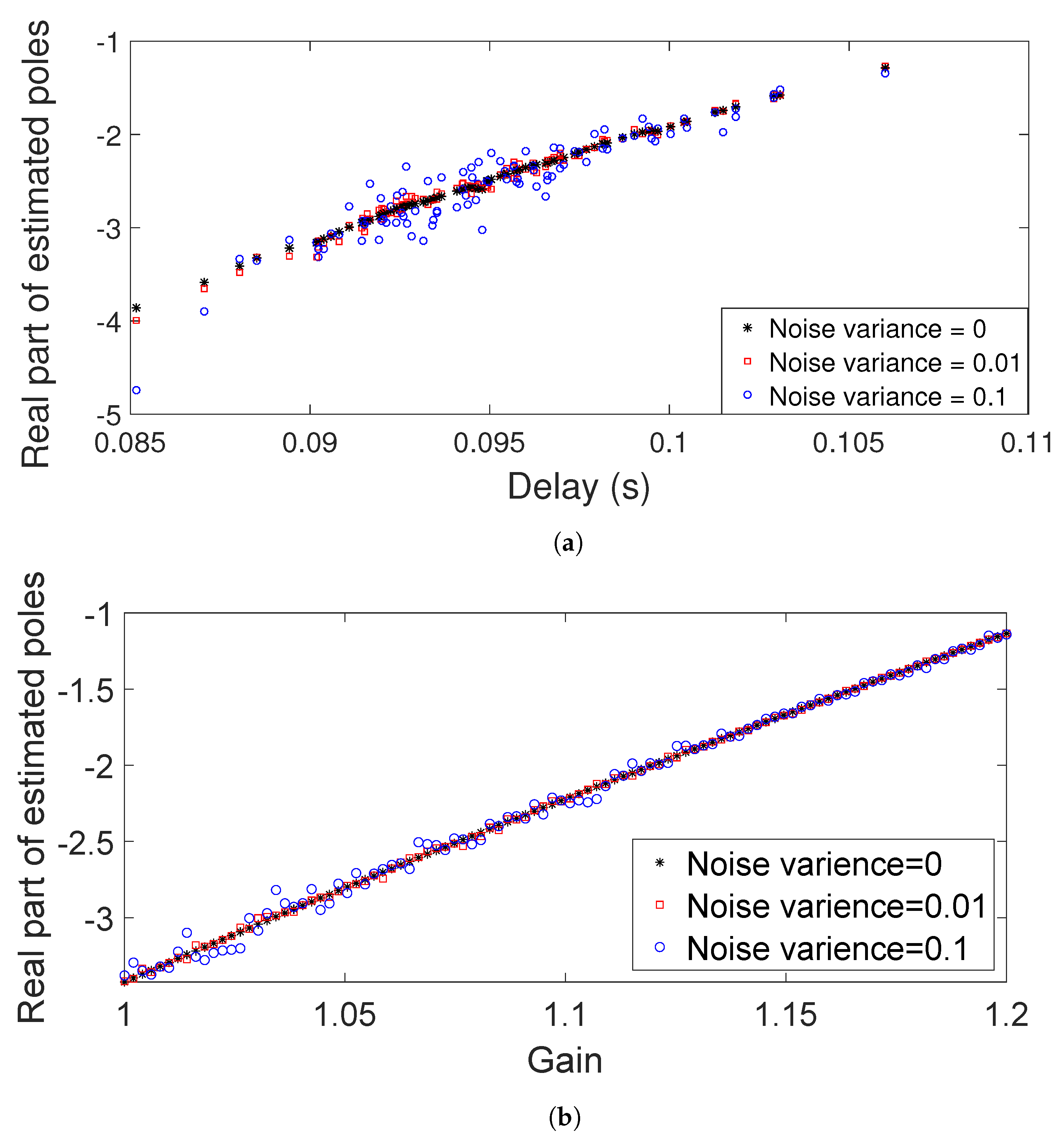
2.3.1. Estimation of Poles from Data
2.3.2. Machine Learning Algorithm for Classification
- Support Vector Machine: Support Vector Machine (SVM) [60] is a supervised machine learning algorithm, that given the training data with their features and class labels identified a priori, determines the maximum margin hyperplane in feature space. Maximum margin hyperplane is a plane in feature space from which the distance to the nearest data points of both classes is maximized. Once the maximum margin hyperplane is determined, depending on the position of the new data set in the feature space, that is, whether new data are lying below the hyperplane or above the hyperplane, each new data set is classified as either Class 1 or Class 2. In the case of outliers in data or data that are not linearly separable, a variant of SVM is used that finds a non-linear boundary to separate both classes of data. For further details, refer to [60].Feature Selection: Feature selection is an important aspect of the success of machine learning algorithms. A good choice of features helps improve classification performance, lower computational complexity, build more generalizable models and decrease the required storage [61]. The aim of feature selection is to extract features from data that represent the characteristics of each of the classes or groups. Since we are interested in tracking the stability of the human sensorimotor system and only the real part of estimated poles in the complex plane governs the stability, we only use the real part of the estimated poles for the rest of our analysis. We explore two sets of choices (C1 and C2) for feature selection. These choices of features are then used as input to the SVM to classify whether a particular individual is healthy or has PPD.C1: Since we are interested in the trend of the real part of the estimated poles over a period of time, the percentage changes in the real parts of poles with respect to the baseline (the estimated poles from the simulated response of the first movement control test) and the percentage change in the successive difference in the real parts of poles between simulated responses over a period of time are the features worth considering. However, in reality, it is very likely that the clinical task is not performed at fixed intervals of time for all subjects. Hence, we consider the rate of percentage change (either per month or per year) for both of the above-mentioned quantities as features. Based on preliminary simulation results, we find that only three features are sufficient for robust classification, namely, minimum percentage change rate in the real part of the poles, maximum percentage change rate in the successive difference between tests, and real part from the first clinical movement control test.The calculation of the percentage change rate in the real part of the poles and percentage change rate in the successive difference between tests are given in Table 1. Here, N is the total number of clinical movement control tests that are conducted possibly at irregular time intervals. is the time duration between two trials, where . to are the real parts of estimated poles, with subscripts indicating the test number.C2: For a second choice of features, since we are interested in determining whether the real part of estimated poles has an increasing trend, we use hypothesis testing as a tool to detect a statistically significant increasing trend in the presence of noisy data. With this approach, we use the statistical value of slope and the constant of the real part of estimated poles as features for the classification algorithm. Further, we also take the real part of the estimated poles of the simulation response of the first movement control test as the third feature as an indicator of the baseline for each individual.
- Support Vector Machine for Longitudinal Analysis (LSVM) A recently developed method, called Longitudinal Support Vector Machine (LSVM) [62], is a method specifically developed for longitudinal data. Here, each data point takes the form of a single time series. LSVM is shown to have higher accuracy compared to SVM, linear discriminant analysis (LDA), and functional linear discriminant analysis (FLDA). Note that once we have real parts of estimated poles over a period of time, this method is formulated such that an additional feature selection is not required.
3. Results
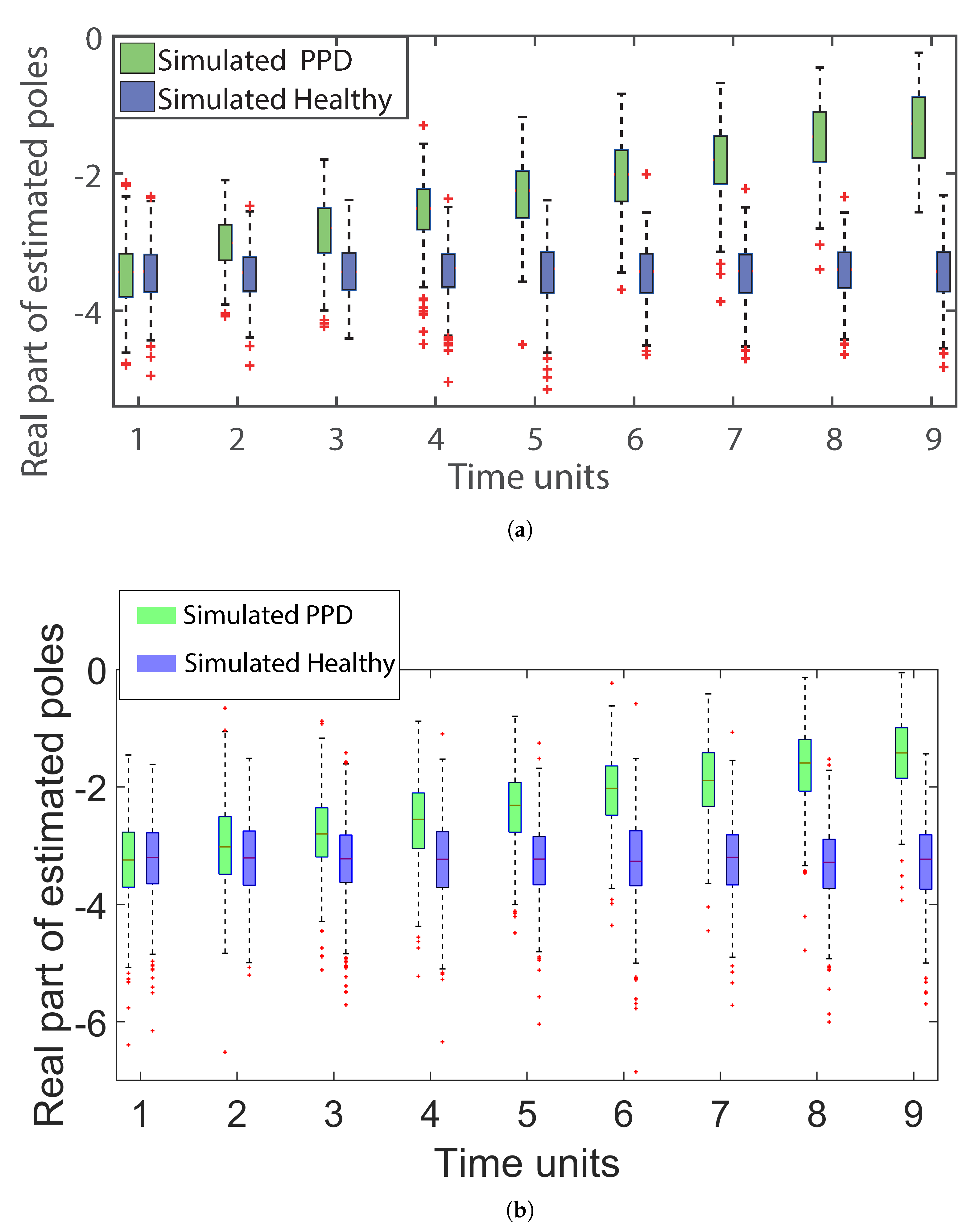
3.1. Classification Results
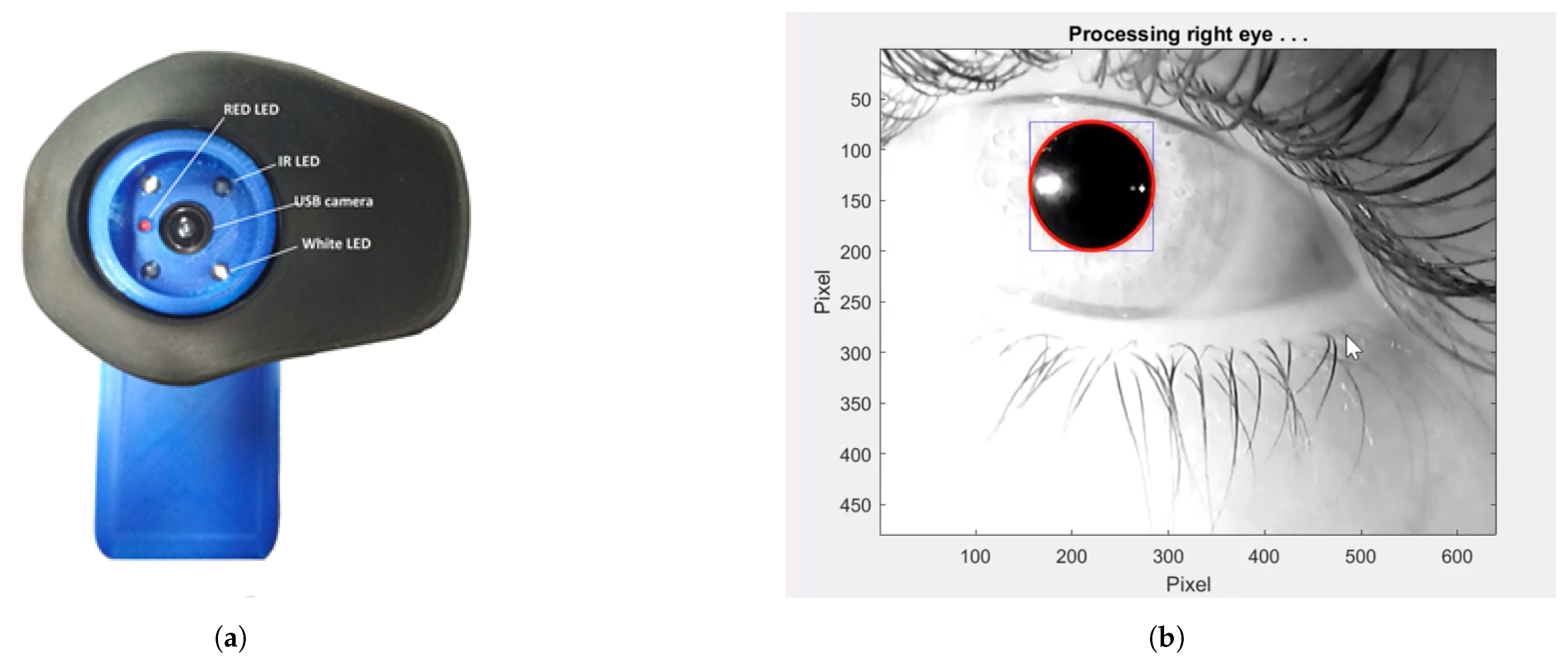
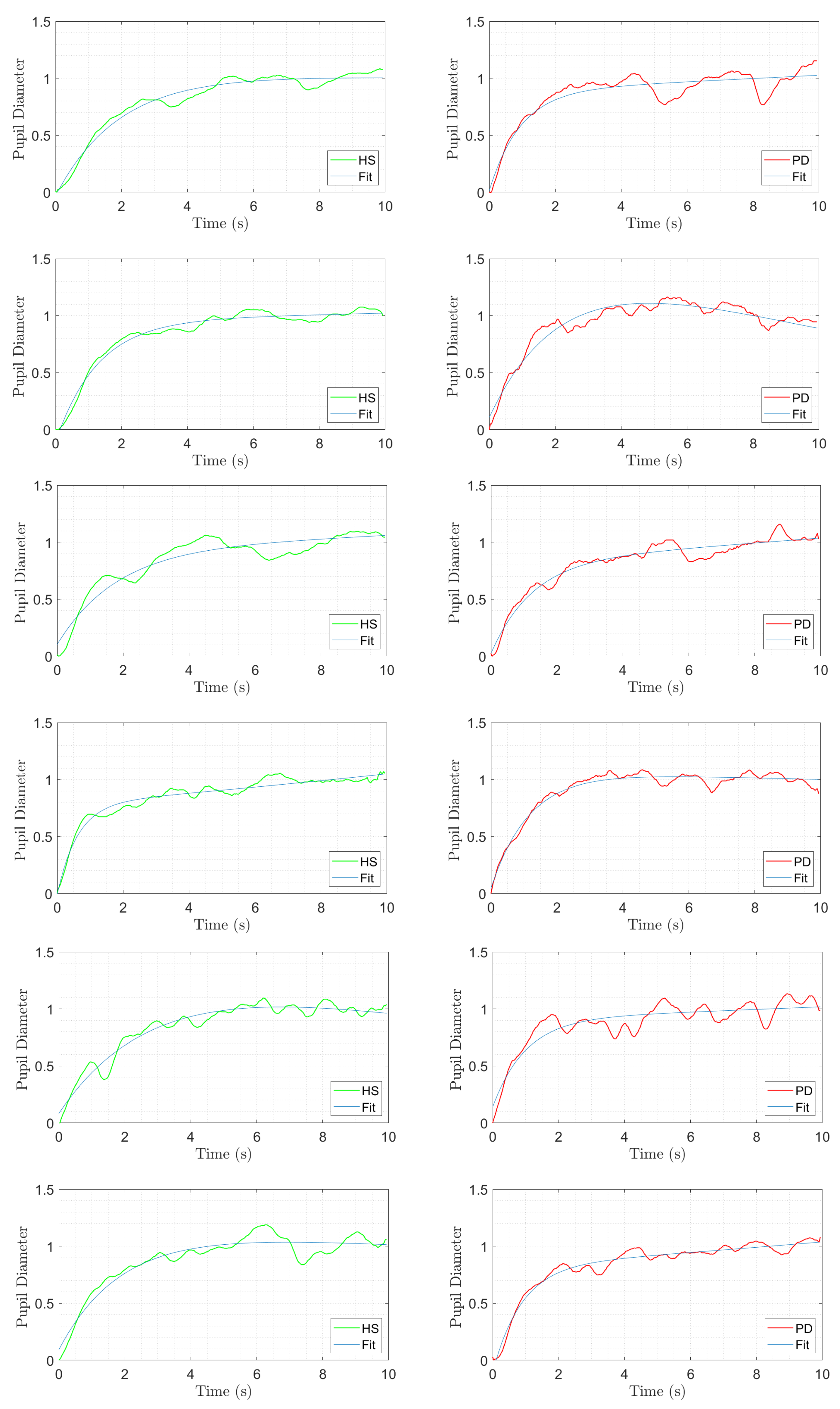
3.2. Example Task and Physiological Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Numerical Simulation of Data Set Using Simulation Example
Appendix A.2. Matrix Pencil Method (MPM) [58]
References
- Sharma, S.; Moon, C.S.; Khogali, A.; Haidous, A.; Chabenne, A.; Ojo, C.; Jelebinkov, M.; Kurdi, Y.; Ebadi, M. Biomarkers in Parkinson’s disease (recent update). Neurochem. Int. 2013, 63, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Fisch, L.; Srinivasan, S.; D’Amico, F.; Okada, T.; Eaton, C.; Robbins, C. Does this patient have Parkinson disease? JAMA 2003, 289, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Seppi, K.; Poewe, W. The concept of prodromal Parkinson’s disease. J. Park. Dis. 2015, 5, 681–697. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Greffard, S.; Verny, M.; Bonnet, A.M.; Beinis, J.Y.; Gallinari, C.; Meaume, S.; Piette, F.; Hauw, J.J.; Duyckaerts, C. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body—Associated neuronal loss in the substantia nigra. Arch. Neurol. 2006, 63, 584–588. [Google Scholar] [CrossRef]
- Sommer, U.; Hummel, T.; Cormann, K.; Mueller, A.; Frasnelli, J.; Kropp, J.; Reichmann, H. Detection of presymptomatic Parkinson’s disease: Combining smell tests, transcranial sonography, and SPECT. Mov. Disord. 2004, 19, 1196–1202. [Google Scholar] [CrossRef]
- Postuma, R.; Montplaisir, J. Predicting Parkinson’s disease—Why, when, and how? Park. Relat. Disord. 2009, 15, S105–S109. [Google Scholar] [CrossRef]
- Wu, Y.; Le, W.; Jankovic, J. Preclinical biomarkers of Parkinson disease. Arch. Neurol. 2011, 68, 22–30. [Google Scholar] [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. Biomarkers of Parkinson’s disease: Present and future. Metabolism 2015, 64, S40–S46. [Google Scholar] [CrossRef]
- Sherer, T.B. Biomarkers for Parkinson’s disease. Sci. Transl. Med. 2011, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Svetaa, S.; Pavithra, S.; Sakthivelan, R. Early Stage Parkinson’s Disease Diagnosis and Detection Using K-Nearest Neighbors Algorithm. In IoT Based Control Networks and Intelligent Systems; Springer: Singapore, 2023; pp. 15–25. [Google Scholar]
- Villagrana-Bañuelos, R.; Villagrana-Bañuelos, K.E.; Murillo, M.A.S.; Galván-Tejada, C.E.; Celaya-Padilla, J.M.; Galván-Tejada, J.I. Comparison of Three Supervised Machine Learning Classification Methods for the Diagnosis of PD. In Proceedings of the International Conference on Ubiquitous Computing and Ambient Intelligence, Riviera Maya, Mexico, 28–30 November 2023; pp. 314–319. [Google Scholar]
- Mei, J.; Desrosiers, C.; Frasnelli, J. Machine learning for the diagnosis of Parkinson’s disease: A review of literature. Front. Aging Neurosci. 2021, 13, 633752. [Google Scholar] [CrossRef] [PubMed]
- Naseer, A.; Rani, M.; Naz, S.; Razzak, M.I.; Imran, M.; Xu, G. Refining Parkinson’s neurological disorder identification through deep transfer learning. Neural Comput. Appl. 2020, 32, 839–854. [Google Scholar] [CrossRef]
- Rovini, E.; Maremmani, C.; Moschetti, A.; Esposito, D.; Cavallo, F. Comparative motor pre-clinical assessment in Parkinson’s disease using supervised machine learning approaches. Ann. Biomed. Eng. 2018, 46, 2057–2068. [Google Scholar] [CrossRef]
- Dezsi, L.; Vecsei, L. Monoamine oxidase B inhibitors in Parkinson’s disease. CNS Neurol. Disord. Drug Targets (Form. Curr. Drug Targets-CNS Neurol. Disord.) 2017, 16, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Rivest, J.; Barclay, C.L.; Suchowersky, O. COMT inhibitors in Parkinson’s disease. Can. J. Neurol. Sci. 1999, 26, S34–S38. [Google Scholar] [CrossRef]
- Rascol, O.; Negre-Pages, L.; Damier, P.; Delval, A.; Derkinderen, P.; Destée, A.; Fabbri, M.; Meissner, W.G.; Rachdi, A.; Tison, F.; et al. Utilization patterns of amantadine in Parkinson’s disease patients enrolled in the French COPARK study. Drugs Aging 2020, 37, 215–223. [Google Scholar] [CrossRef]
- Farley, B.G.; Koshland, G.F. Training BIG to move faster: The application of the speed–amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Exp. Brain Res. 2005, 167, 462–467. [Google Scholar] [CrossRef]
- Irwin-Carruthers, S. An approach to physiotherapy for Parkinson’s disease. S. Afr. J. Physiother. 1971, 25, 5. [Google Scholar] [CrossRef]
- Radder, D.L.; Lígia Silva de Lima, A.; Domingos, J.; Keus, S.H.; van Nimwegen, M.; Bloem, B.R.; de Vries, N.M. Physiotherapy in Parkinson’s disease: A meta-analysis of present treatment modalities. Neurorehabilit. Neural Repair 2020, 34, 871–880. [Google Scholar] [CrossRef]
- Murman, D.L. Early treatment of Parkinson’s disease: Opportunities for managed care. Am. J. Manag. Care 2012, 18, S183–S188. [Google Scholar] [PubMed]
- Konjevod, M.; Sáiz, J.; Barbas, C.; Bergareche, A.; Ardanaz, E.; Huerta, J.M.; Vinagre-Aragón, A.; Erro, M.E.; Chirlaque, M.D.; Abilleira, E.; et al. A Set of Reliable Samples for the Study of Biomarkers for the Early Diagnosis of Parkinson’s Disease. Front. Neurol. 2021, 13, 844841. [Google Scholar] [CrossRef] [PubMed]
- McInerney-Leo, A.; Hadley, D.W.; Gwinn-Hardy, K.; Hardy, J. Genetic testing in Parkinson’s disease. Mov. Disord. 2005, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Jankovic, J. Genetic testing in Parkinson disease: Promises and pitfalls. Arch. Neurol. 2006, 63, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.R.; Stewart, T.H.; Zhang, J. Premotor biomarkers for Parkinson’s disease—A promising direction of research. Transl. Neurodegener. 2012, 1, 11. [Google Scholar] [CrossRef]
- Ross, G.W.; Petrovitch, H.; Abbott, R.D.; Tanner, C.M.; Popper, J.; Masaki, K.; Launer, L.; White, L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 2008, 63, 167–173. [Google Scholar] [CrossRef]
- Haehner, A.; Hummel, T.; Hummel, C.; Sommer, U.; Junghanns, S.; Reichmann, H. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov. Disord. 2007, 22, 839–842. [Google Scholar] [CrossRef]
- Postuma, R.B.; Lang, A.E.; Massicotte-Marquez, J.; Montplaisir, J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology 2006, 66, 845–851. [Google Scholar] [CrossRef]
- Hughes, K.C.; Gao, X.; Baker, J.M.; Stephen, C.; Kim, I.Y.; Valeri, L.; Schwarzschild, M.A.; Ascherio, A. Non-motor features of Parkinson’s disease in a nested case—Control study of US men. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1288–1295. [Google Scholar] [CrossRef]
- La Morgia, C.; Romagnoli, M.; Pizza, F.; Biscarini, F.; Filardi, M.; Donadio, V.; Carbonelli, M.; Amore, G.; Park, J.C.; Tinazzi, M.; et al. Chromatic Pupillometry in Isolated Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2022, 37, 205–210. [Google Scholar] [CrossRef]
- Solana-Lavalle, G.; Galán-Hernández, J.C.; Rosas-Romero, R. Automatic Parkinson disease detection at early stages as a pre-diagnosis tool by using classifiers and a small set of vocal features. Biocybern. Biomed. Eng. 2020, 40, 505–516. [Google Scholar] [CrossRef]
- Er, M.B.; Isik, E.; Isik, I. Parkinson’s detection based on combined CNN and LSTM using enhanced speech signals with variational mode decomposition. Biomed. Signal Process. Control 2021, 70, 103006. [Google Scholar] [CrossRef]
- Wetzel, P.; Baron, M.; Gitchel, G. Automated Analysis System for the Detection and Screening of Neurological Disorders and Deficits. WO Patent App. PCT/US2014/023,923, 12 March 2014. [Google Scholar]
- Hufschmidt, H.J. Proprioceptive origin of parkinsonian tremor. Nature 1963, 367–368. Available online: https://www.nature.com/articles/200367a0 (accessed on 18 April 2023). [CrossRef] [PubMed]
- Stiles, R.N.; Pozos, R.S. A mechanical-reflex oscillator hypothesis for parkinsonian hand tremor. J. Appl. Physiol. 1976, 40, 990–998. [Google Scholar] [CrossRef]
- Stein, R.; Oĝuztöreli, M. Tremor and other oscillations in neuromuscular systems. Biol. Cybern. 1976, 22, 147–157. [Google Scholar] [CrossRef]
- Rack, P.; Ross, H. The role of reflexes in the resting tremor of Parkinson’s disease. Brain 1986, 109, 115–141. [Google Scholar] [CrossRef]
- Schnider, S.; Kwong, R.; Lenz, F.; Kwan, H. Detection of feedback in the central nervous system using system identification techniques. Biol. Cybern. 1989, 60, 203–212. [Google Scholar] [CrossRef]
- Beuter, A.; Milton, J.; Labrie, C.; Glass, L.; Gauthier, S. Delayed visual feedback and movement control in Parkinson’s disease. Exp. Neurol. 1990, 110, 228–235. [Google Scholar] [CrossRef]
- Beuter, A.; Bélair, J.; Labrie, C. Feedback and delays in neurological diseases: A modeling study using dynamical systems. Bull. Math. Biol. 1993, 55, 525–541. [Google Scholar] [CrossRef]
- Deuschl, G.; Raethjen, J.; Baron, R.; Lindemann, M.; Wilms, H.; Krack, P. The pathophysiology of parkinsonian tremor: A review. J. Neurol. 2000, 247, V33–V48. [Google Scholar] [CrossRef]
- Rodriguez-Oroz, M.C.; Jahanshahi, M.; Krack, P.; Litvan, I.; Macias, R.; Bezard, E.; Obeso, J.A. Initial clinical manifestations of Parkinson’s disease: Features and pathophysiological mechanisms. Lancet Neurol. 2009, 8, 1128–1139. [Google Scholar] [CrossRef]
- Zhang, D.; Poignet, P.; Bo, A.P.; Ang, W.T. Exploring peripheral mechanism of tremor on neuromusculoskeletal model: A general simulation study. IEEE Trans. Biomed. Eng. 2009, 56, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Tremor: Pathophysiology. Park. Relat. Disord. 2014, 20, S118–S122. [Google Scholar] [CrossRef]
- Palanthandalam-Madapusi, H.J.; Goyal, S. Is Parkinsonian Tremor a limit cycle? J. Mech. Med. Biol. 2011, 11, 1017–1023. [Google Scholar] [CrossRef]
- Milton, J.G. Time delays and the control of biological systems: An overview. IFAC-PapersOnLine 2015, 48, 87–92. [Google Scholar] [CrossRef]
- Shah, V.V.; Goyal, S.; Palanthandalam-Madapusi, H.J. Clinical Facts Along with a Feedback Control Perspective Suggest That Increased Response Time Might Be the Cause of Parkinsonian Rest Tremor. J. Comput. Nonlinear Dyn. 2017, 12, 011007. [Google Scholar] [CrossRef]
- Chumacero, E.; Yang, J.; Chagdes, J.R. Effect of sensory-motor latencies and active muscular stiffness on stability for an ankle-hip model of balance on a balance board. J. Biomech. 2018, 75, 77–88. [Google Scholar] [CrossRef]
- Edwards, R.; Beuter, A.; Glass, L. Parkinsonian tremor and simplification in network dynamics. Bull. Math. Biol. 1999, 61, 157–177. [Google Scholar] [CrossRef]
- Elble, R.J. Central mechanisms of tremor. J. Clin. Neurophysiol. 1996, 13, 133–144. [Google Scholar] [CrossRef]
- Wilson, S.K. The Croonian Lectures on Some Disorders of Motility and of Muscle Tone, with Special Reference to the Corpus Striatum. Lancet 1925, 206, 1–10. [Google Scholar]
- Evarts, E.; Teräväinen, H.; Calne, D. Reaction Time in Parkinson’s Disease. Brain J. Neurol. 1981, 104, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Bloxham, C.; Dick, D.; Moore, M. Reaction Times and Attention in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1987, 50, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Heilman, K.M.; Bowers, D.; Watson, R.T.; Greer, M. Reaction Times in Parkinson Disease. Arch. Neurol. 1976, 33, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.V.; Goyal, S.; Palanthandalam-Madapusi, H.J. A Possible Explanation of How High-Frequency Deep Brain Stimulation Suppresses Low-Frequency Tremors in Parkinson’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 2498–2508. [Google Scholar] [CrossRef]
- Sarkar, T.K.; Pereira, O. Using the matrix pencil method to estimate the parameters of a sum of complex exponentials. Antennas Propag. Mag. IEEE 1995, 37, 48–55. [Google Scholar] [CrossRef]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.V.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Tang, J.; Alelyani, S.; Liu, H. Feature selection for classification: A review. Data Classif. Algorithms Appl. 2014, 37. [Google Scholar] [CrossRef]
- Kristiaan, P.; Hong-Li, Z. Support Vector Machines for Longitudinal Analysis. IEEE Trans. Pattern Anal. Mach. Intell. 2015. Available online: https://www.semanticscholar.org/paper/Support-Vector-Machines-for-Longitudinal-Analysis-Pelckmans-Zeng/6f42b35ccdd2876841d435070f47ac032c06838f#citing-papers (accessed on 18 April 2023).
- De Lima, E.R.; Andrade, A.O.; Pons, J.L.; Kyberd, P.; Nasuto, S.J. Empirical mode decomposition: A novel technique for the study of tremor time series. Med. Biol. Eng. Comput. 2006, 44, 569–582. [Google Scholar] [CrossRef]
- Gitchel, G.T.; Wetzel, P.A.; Baron, M.S. Pervasive ocular tremor in patients with Parkinson disease. Arch. Neurol. 2012, 69, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Flowers, K.; Downing, A. Predictive control of eye movements in Parkinson disease. Ann. Neurol. 1978, 4, 63–66. [Google Scholar] [CrossRef]
- Senders, J.W.; Cruzen, M. Tracking Performance on Combined Compensatory and Pursuit Tasks; Wright Air Development Center, Air Research and Development Command, United States Air Force: Dayton, OH, USA, 1952; Volume 52, p. 39. [Google Scholar]
- Giza, E.; Fotiou, D.; Bostantjopoulou, S.; Katsarou, Z.; Karlovasitou, A. Pupil light reflex in Parkinson’s disease: Evaluation with pupillometry. Int. J. Neurosci. 2011, 121, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Micieli, G.; Tassorelli, C.; Martignoni, E.; Pacchetti, C.; Bruggi, P.; Magri, M.; Nappi, G. Disordered pupil reactivity in Parkinson’s disease. Clin. Auton. Res. 1991, 1, 55–58. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Hong, J.H.; Yoo, J. Analysis of pupillometer results according to disease stage in patients with Parkinson’s disease. Sci. Rep. 2021, 11, 17880. [Google Scholar] [CrossRef] [PubMed]
- Heenan, M.; Scheidt, R.A.; Woo, D.; Beardsley, S.A. Intention tremor and deficits of sensory feedback control in multiple sclerosis: A pilot study. J. Neuroeng. Rehabil. 2014, 11, 170. [Google Scholar] [CrossRef]
- Thompson, J.D.; Franz, J.R. Do kinematic metrics of walking balance adapt to perturbed optical flow? Hum. Mov. Sci. 2017, 54, 34–40. [Google Scholar] [CrossRef]
- Franz, J.R.; Francis, C.A.; Allen, M.S.; O’Connor, S.M.; Thelen, D.G. Advanced age brings a greater reliance on visual feedback to maintain balance during walking. Hum. Mov. Sci. 2015, 40, 381–392. [Google Scholar] [CrossRef]
- Cona, F.; Pizza, F.; Provini, F.; Magosso, E. An improved algorithm for the automatic detection and characterization of slow eye movements. Med. Eng. Phys. 2014, 36, 954–961. [Google Scholar] [CrossRef]
- King, R.C.; Villeneuve, E.; White, R.J.; Sherratt, R.S.; Holderbaum, W.; Harwin, W.S. Application of data fusion techniques and technologies for wearable health monitoring. Med. Eng. Phys. 2017, 42, 1–12. [Google Scholar] [CrossRef]
- Meel-van den Abeelen, A.S.; van Beek, A.H.; Slump, C.H.; Panerai, R.B.; Claassen, J.A. Transfer function analysis for the assessment of cerebral autoregulation using spontaneous oscillations in blood pressure and cerebral blood flow. Med. Eng. Phys. 2014, 36, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Panerai, R.; Rennie, J.; Kelsall, A.; Evans, D. Frequency-domain analysis of cerebral autoregulation from spontaneous fluctuations in arterial blood pressure. Med. Biol. Eng. Comput. 1998, 36, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, J.; Harrou, F.; Sun, Y. Early detection of Parkinson’s disease using deep learning and machine learning. IEEE Access 2020, 8, 147635–147646. [Google Scholar] [CrossRef]
- Rehman, R.Z.U.; Del Din, S.; Guan, Y.; Yarnall, A.J.; Shi, J.Q.; Rochester, L. Selecting clinically relevant gait characteristics for classification of early Parkinson’s disease: A comprehensive machine learning approach. Sci. Rep. 2019, 9, 17269. [Google Scholar] [CrossRef]
- Senturk, Z.K. Early diagnosis of Parkinson’s disease using machine learning algorithms. Med. Hypotheses 2020, 138, 109603. [Google Scholar] [CrossRef] [PubMed]
| Features | Formula for Calculation | |
|---|---|---|
| C1: | 1. Percentage change rate in real part of pole | |
| 2. Percentage change rate in successive difference of real part of poles between tests | ||
| Methods | Training Synthetic Data Set | ||||
|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | False Positive (%) | False Negative (%) | Error in Classification (%) | |
| LVSM | 96.73 | 100 | 0 | 3.27 | 1.67 |
| SVM (C1) | 96.40 | 98.97 | 1.03 | 3.60 | 2.34 |
| SVM (C2) | 98.36 | 99.66 | 0.34 | 1.64 | 1 |
| Validation Synthetic Data Set | |||||
| LVSM | 97.42 | 100 | 0 | 2.58 | 1.25 |
| SVM (C1) | 95.36 | 99.51 | 0.49 | 4.64 | 2.5 |
| SVM (C2) | 98.96 | 99.02 | 0.98 | 1.04 | 1 |
| Methods | Training Synthetic Data Set | ||||
|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | False Positive (%) | False Negative (%) | Error in Classification (%) | |
| LVSM | 96.65 | 100 | 0 | 3.35 | 1.67 |
| SVM (C1) | 96.66 | 98.33 | 1.67 | 3.34 | 2.5 |
| SVM (C2) | 97.65 | 99.35 | 0.65 | 2.35 | 1.5 |
| Validation Synthetic Data Set | |||||
| LVSM | 96.51 | 100 | 0 | 3.49 | 1.75 |
| SVM (C1) | 96.02 | 97.99 | 2.01 | 3.98 | 3 |
| SVM (C2) | 98.01 | 97.49 | 2.51 | 1.99 | 2.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, V.V.; Jadav, S.; Goyal, S.; Palanthandalam-Madapusi, H.J. A Machine-Learning-Based Method to Detect Degradation of Motor Control Stability with Implications to Diagnosis of Presymptomatic Parkinson’s Disease: A Simulation Study. Appl. Sci. 2023, 13, 9502. https://doi.org/10.3390/app13179502
Shah VV, Jadav S, Goyal S, Palanthandalam-Madapusi HJ. A Machine-Learning-Based Method to Detect Degradation of Motor Control Stability with Implications to Diagnosis of Presymptomatic Parkinson’s Disease: A Simulation Study. Applied Sciences. 2023; 13(17):9502. https://doi.org/10.3390/app13179502
Chicago/Turabian StyleShah, Vrutangkumar V., Shail Jadav, Sachin Goyal, and Harish J. Palanthandalam-Madapusi. 2023. "A Machine-Learning-Based Method to Detect Degradation of Motor Control Stability with Implications to Diagnosis of Presymptomatic Parkinson’s Disease: A Simulation Study" Applied Sciences 13, no. 17: 9502. https://doi.org/10.3390/app13179502






