Comparative Analysis of the Prevalence of Bovine Viral Diarrhea Virus in Cattle Populations Based on Detection Methods: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Data Extraction and Quality Assessment
2.3. Qualitative Data Selection and Analysis
2.4. Statistical and Meta-Analysis
3. Results
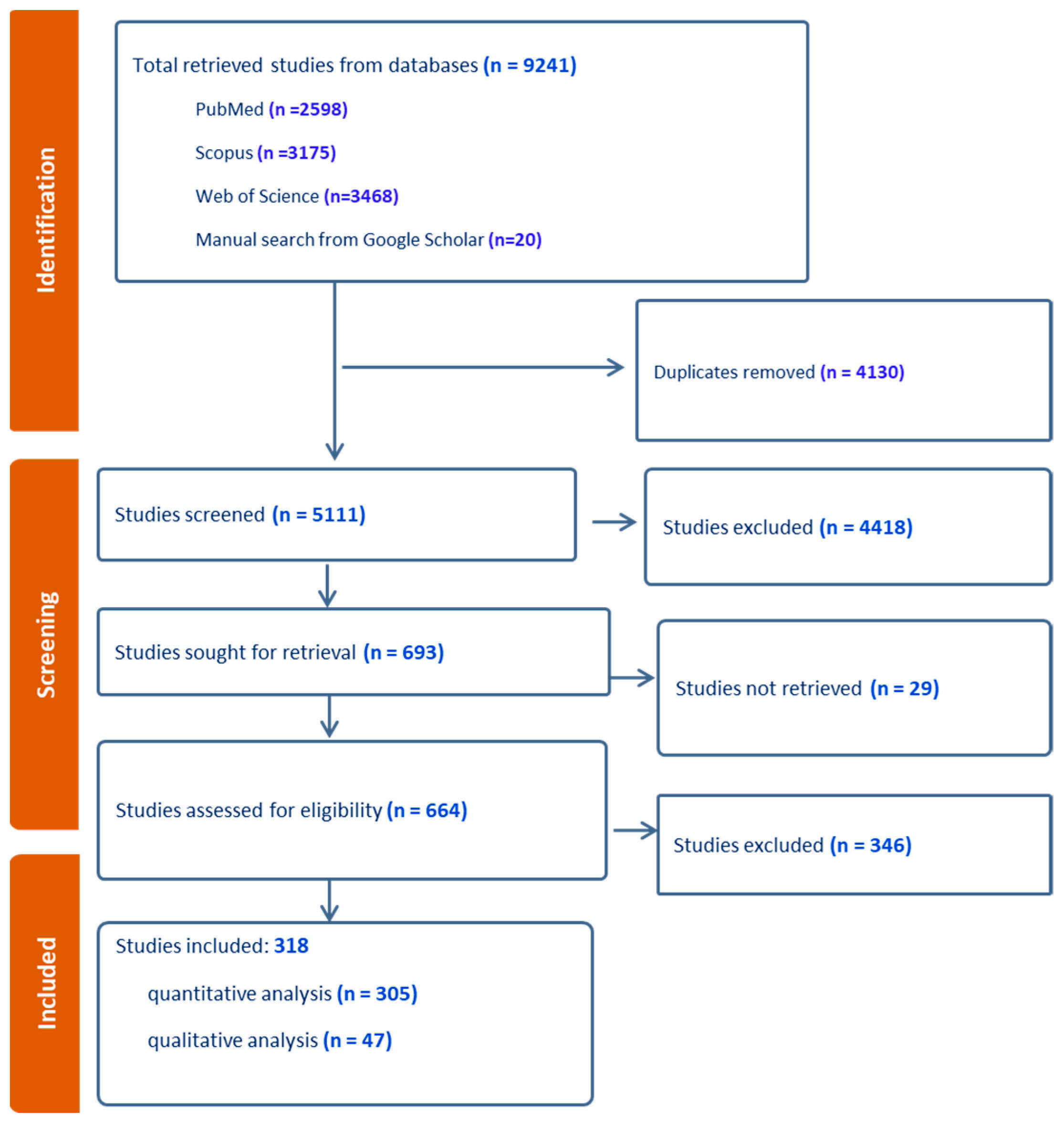
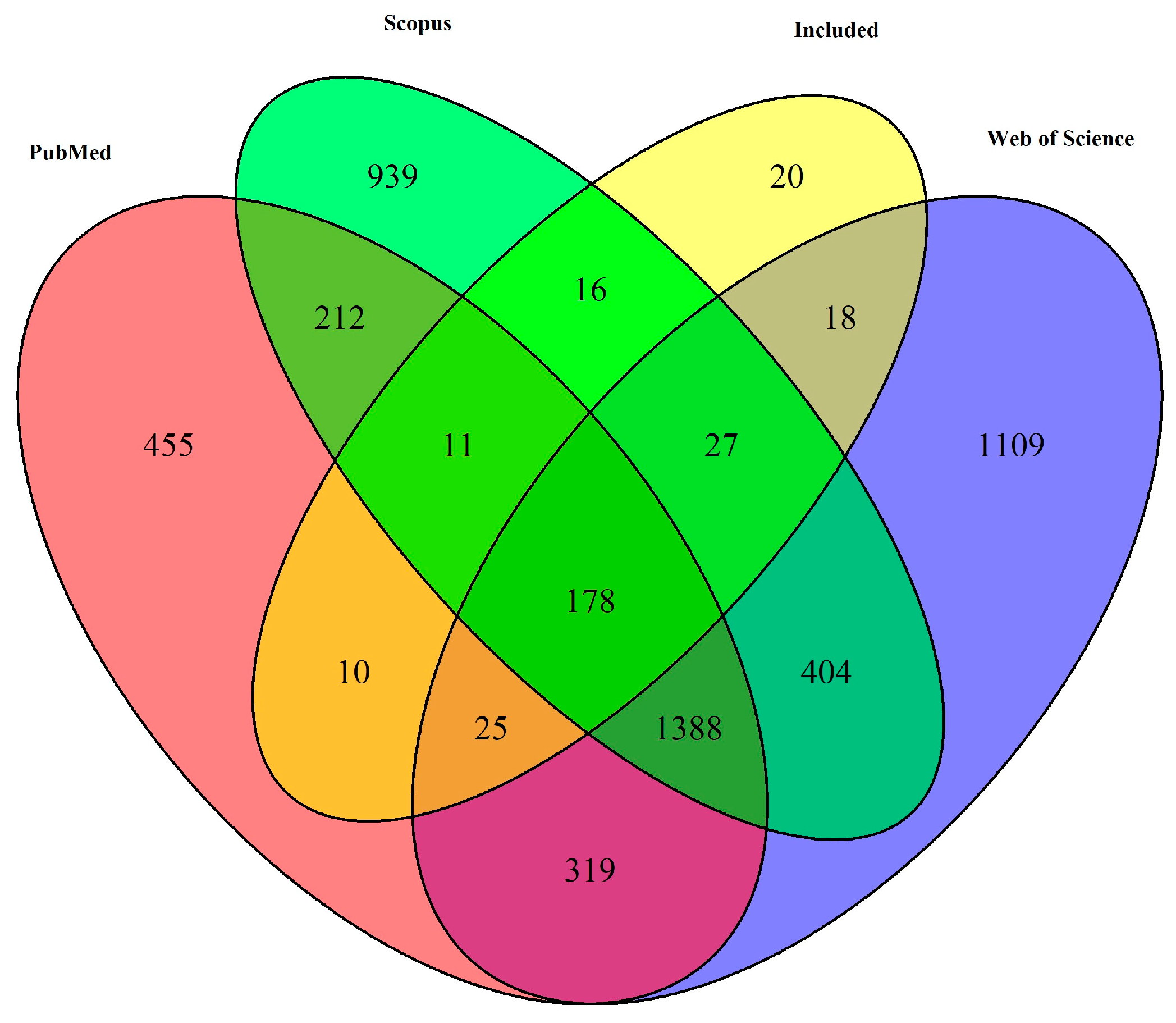
3.1. Study Selection and Characteristics
3.2. Prevalence of Bovine Viral Diarrhea Using Antibody Detection Methods
3.3. Prevalence of Bovine Viral Diarrhea Using Antigen Detection Methods
3.4. Prevalence of Bovine Viral Diarrhea Virus Using Nucleic Acid Detection Methods
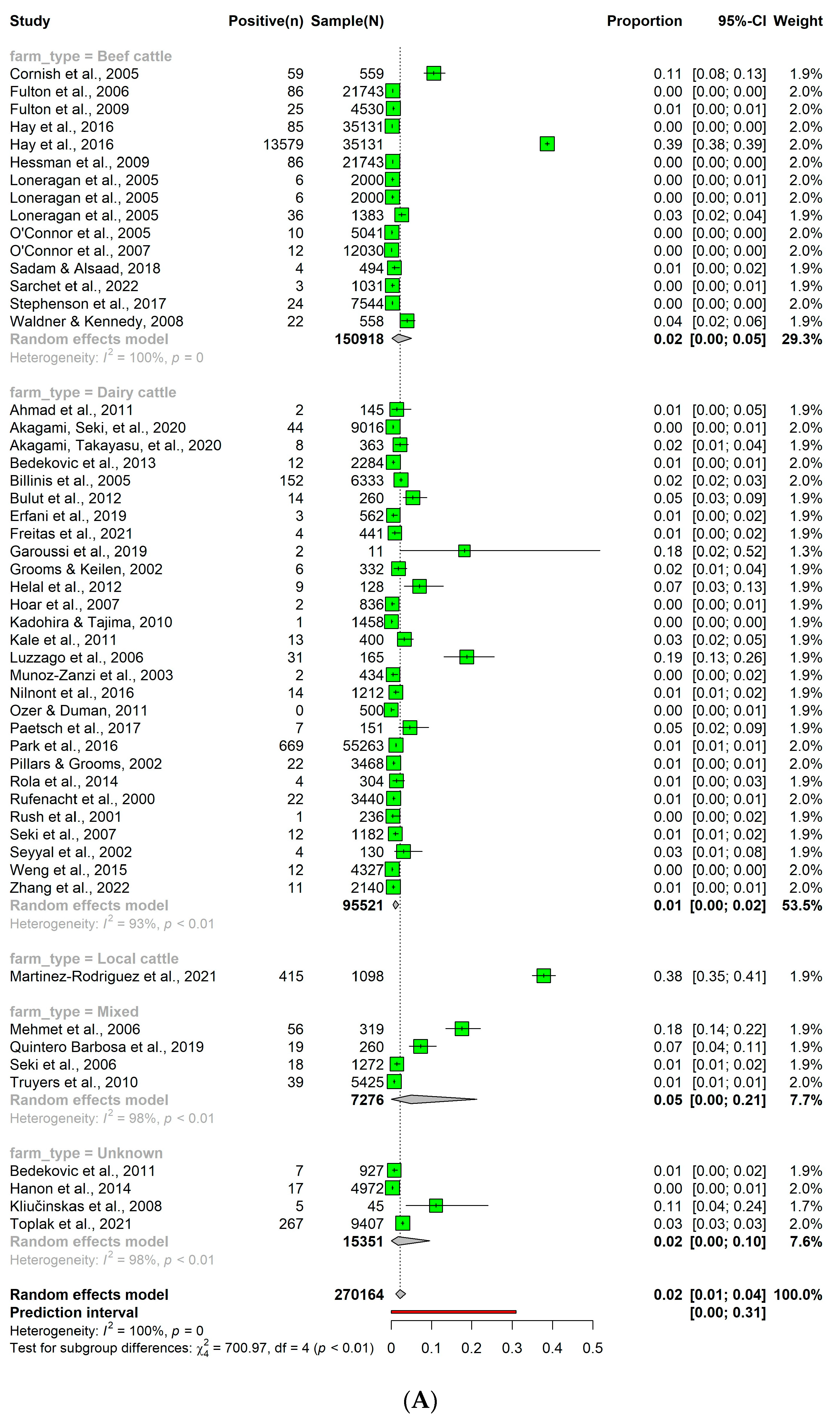
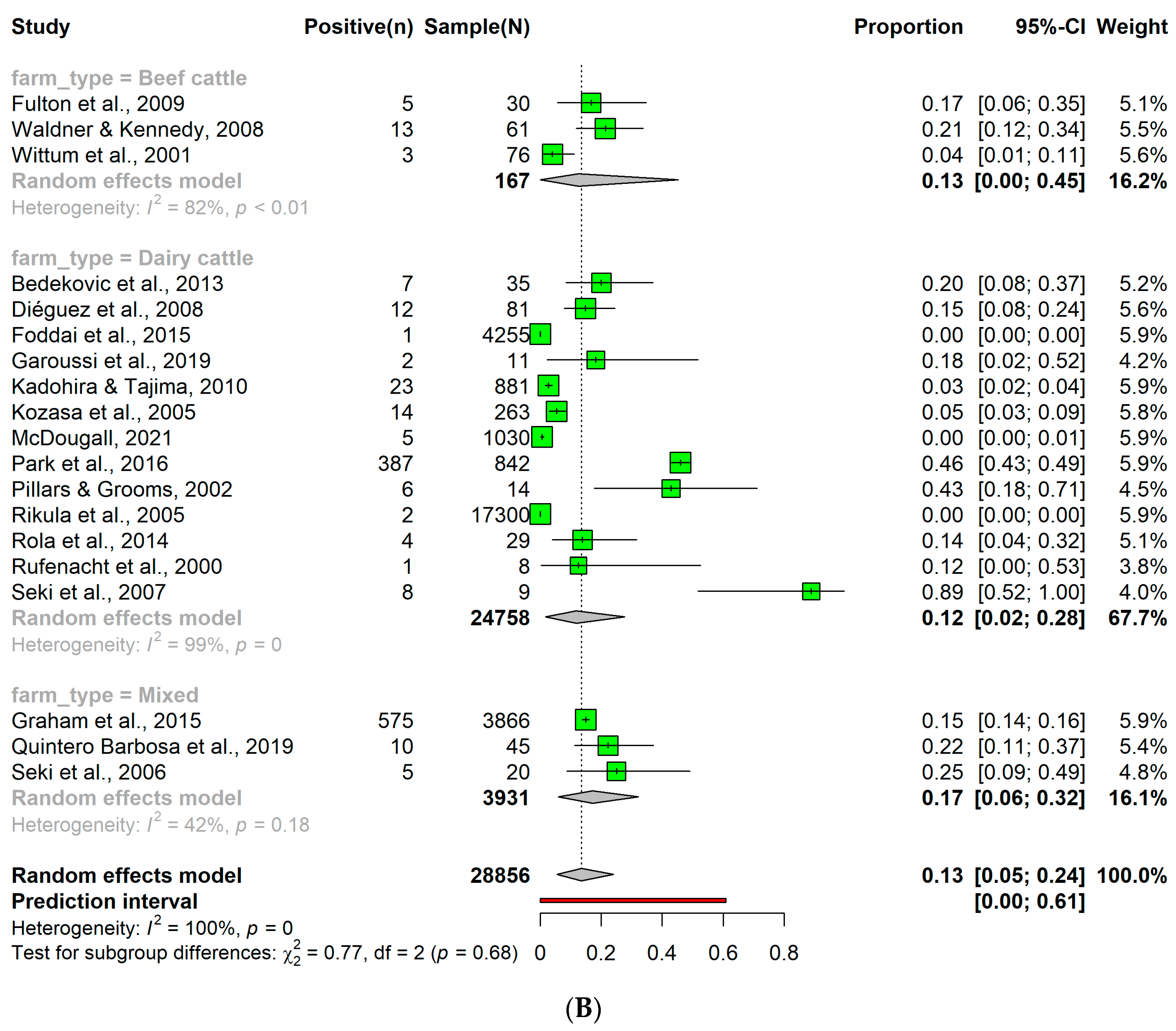
3.5. Detection Rates of Persistent Bovine Viral Diarrhea Virus Infection
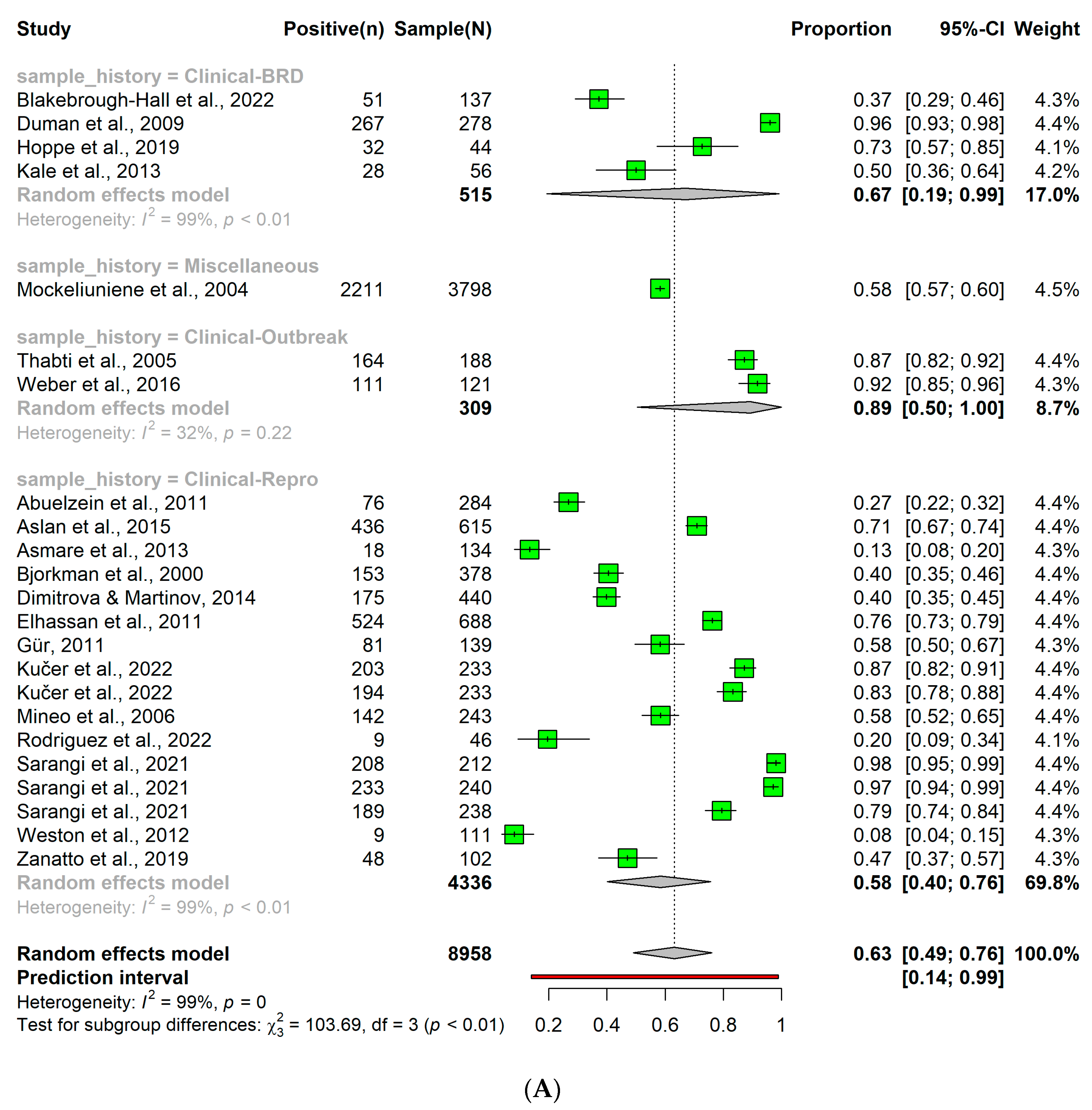
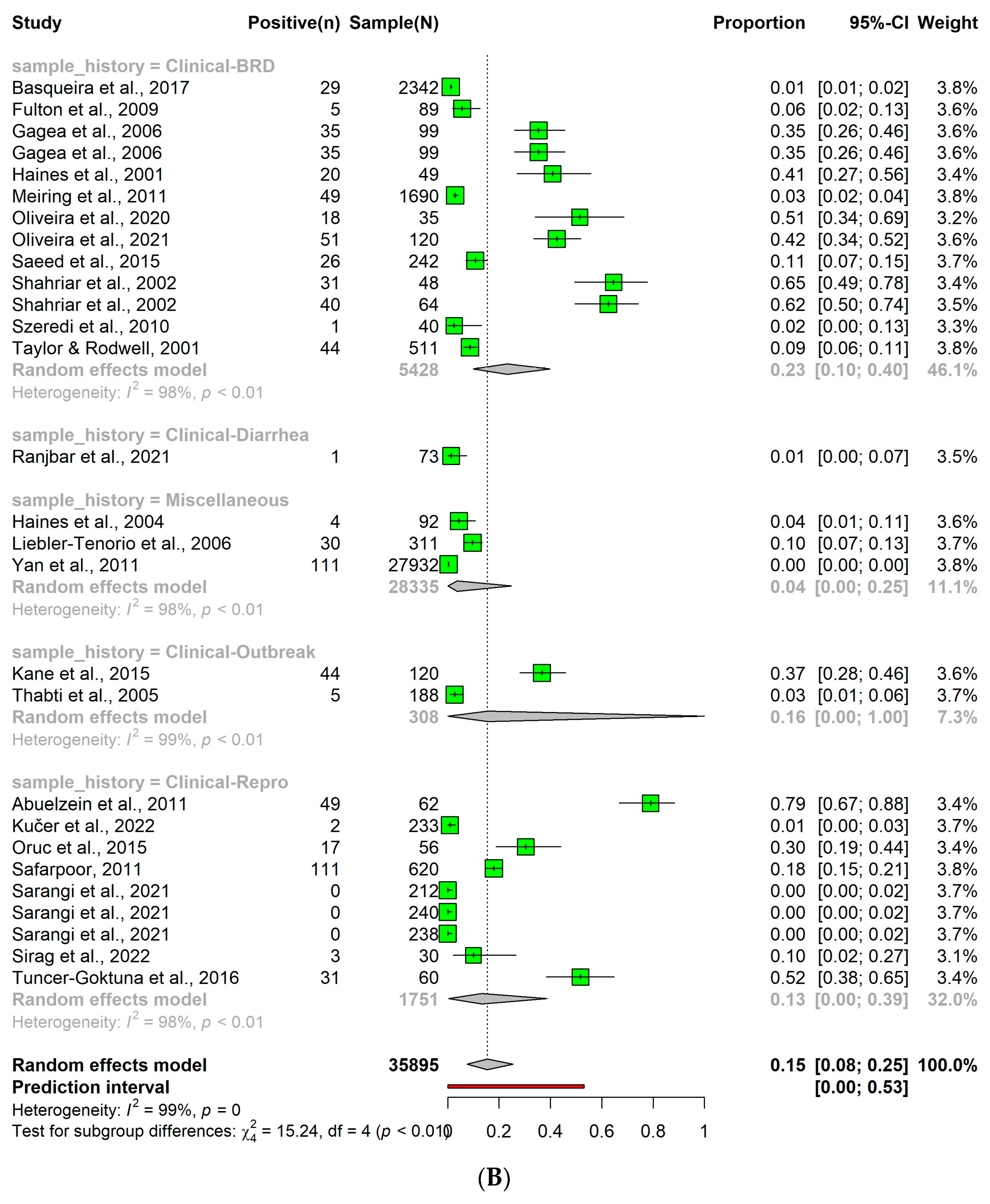
3.6. Detection of Bovine Viral Diarrhea Virus in Clinical Samples
3.7. Risk Factors and Covariates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnaiz, I.; Cervino, M.; Martinez, S.; Fouz, R.; Dieguez, F.J. Bovine viral diarrhea virus (BVDV) infection: Effect on reproductive performance and milk yield in dairy herds. Vet. J. 2021, 277, 105747. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Fulton, R.W.; Bauermann, F.V.; Falkenberg, S.M.; Welch, J.; Confer, A.W. Sequential exposure to bovine viral diarrhea virus and bovine coronavirus results in increased respiratory disease lesions: Clinical, immunologic, pathologic, and immunohistochemical findings. J. Vet. Diagn. Investig. 2020, 32, 513–526. [Google Scholar] [CrossRef]
- Rajput, M.K.S.; Darweesh, M.F.; Braun, L.J.; Mansour, S.M.G.; Chase, C.C.L. Comparative humoral immune response against cytopathic or non-cytopathic bovine viral diarrhea virus infection. Res. Vet. Sci. 2020, 129, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Burciaga-Robles, L.O.; Krehbiel, C.R.; Step, D.L.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Confer, A.W.; Fulton, R.W. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and Mannheimia haemolytica challenge on animal performance, nitrogen balance, and visceral organ mass in beef steers. J. Anim. Sci. 2010, 88, 2179–2188. [Google Scholar] [CrossRef]
- Falkenberg, S.M.; Dassanayake, R.P.; Neill, J.D.; Ridpath, J.F. Evaluation of bovine viral diarrhea virus transmission potential to naive calves by direct and indirect exposure routes. Vet. Microbiol. 2018, 217, 144–148. [Google Scholar] [CrossRef]
- Tiwari, A.; Vanleeuwen, J.A.; Dohoo, I.R.; Keefe, G.P.; Haddad, J.P.; Tremblay, R.; Scott, H.M.; Whiting, T. Production effects of pathogens causing bovine leukosis, bovine viral diarrhea, paratuberculosis, and neosporosis. J. Dairy Sci. 2007, 90, 659–669. [Google Scholar] [CrossRef]
- Al-Kubati, A.A.; Hussen, J.; Kandeel, M.; Al-Mubarak, A.I.; Hemida, M.G. Recent advances on the bovine viral diarrhea virus molecular pathogenesis, immune response, and vaccines development. Front. Vet. Sci. 2021, 8, 665128. [Google Scholar] [CrossRef]
- Shortall, O. Lessons learned for animal health governance from bovine viral diarrhea eradication schemes in Scotland and Ireland. Front. Vet. Sci. 2022, 9, 956635. [Google Scholar] [CrossRef]
- Moennig, V.; Becher, P. Control of Bovine Viral Diarrhea. Pathogens 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- VanderLey, B.; Ridpath, J.; Sweiger, S. Comparison of detection of Bovine virus diarrhea virus antigen in various types of tissue and fluid samples collected from persistently infected cattle. J. Vet. Diagn. Investig. 2011, 23, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, R.W.; Ray, R.; Dubovi, E.J. Comparison of virus isolation and reverse transcription polymerase chain reaction assay for detection of bovine viral diarrhea virus in bulk milk tank samples. J. Vet. Diagn. Investig. 2000, 12, 184–186. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Tan, B.; Li, P.; Wang, F.X.; Guo, L.; Yang, Y.; Sun, N.; Zhu, H.W.; Wen, Y.J.; Cheng, S.P. Comparison of conventional RT-PCR, reverse-transcription loop-mediated isothermal amplification, and SYBR green I-based real-time RT-PCR in the rapid detection of bovine viral diarrhea virus nucleotide in contaminated commercial bovine sera batches. J. Virol. Methods 2014, 207, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Saliki, J.T.; Dubovi, E.J. Laboratory diagnosis of bovine viral diarrhea virus infections. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 69–83. [Google Scholar] [CrossRef]
- Smithgall, M.C.; Dowlatshahi, M.; Spitalnik, S.L.; Hod, E.A.; Rai, A.J. Types of Assays for SARS-CoV-2 Testing: A Review. Lab. Med. 2020, 51, e59–e65. [Google Scholar] [CrossRef]
- Lanyon, S.R.; Anderson, M.L.; Bergman, E.; Reichel, M.P. Validation and evaluation of a commercially available ELISA for the detection of antibodies specific to bovine viral diarrhoea virus (bovine pestivirus). Aust. Vet. J. 2013, 91, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Jalal, S.; Hwang, S.Y.; Kim, C.M.; Kim, D.M.; Yun, N.R.; Seo, J.W.; Young Kim, D.; Jung, S.I.; Kim, U.J.; Kim, S.E.; et al. Comparison of RT-PCR, RT-nested PCRs, and real-time PCR for diagnosis of severe fever with thrombocytopenia syndrome: A prospective study. Sci. Rep. 2021, 11, 16764. [Google Scholar] [CrossRef]
- Edmondson, M.A.; Givens, M.D.; Walz, P.H.; Gard, J.A.; Stringfellow, D.A.; Carson, R.L. Comparison of tests for detection of bovine viral diarrhea virus in diagnostic samples. J. Vet. Diagn. Investig. 2007, 19, 376–381. [Google Scholar] [CrossRef]
- Zirra-Shallangwa, B.; Gordon, L.G.; Hernandez-Castro, L.E.; Cook, E.A.J.; Bronsvoort, B.M.C.; Kelly, R.F. The Epidemiology of Bovine Viral Diarrhea Virus in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2022, 9, 947515. [Google Scholar] [CrossRef]
- Su, N.; Wang, Q.; Liu, H.Y.; Li, L.M.; Tian, T.; Yin, J.Y.; Zheng, W.; Ma, Q.X.; Wang, T.T.; Li, T.; et al. Prevalence of bovine viral diarrhea virus in cattle between 2010 and 2021: A global systematic review and meta-analysis. Front. Vet. Sci. 2022, 9, 1086180. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, K. NVivo. J. Med. Libr. Assoc. 2022, 110, 270–272. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Olkin, I.; Dahabreh, I.J.; Trikalinos, T.A. GOSH—A graphical display of study heterogeneity. Res. Synth. Methods 2012, 3, 214–223. [Google Scholar] [CrossRef]
- Ester, M.; Kriegel, H.-P.; Sander, J.; Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the KDD’96: Proceedings of the Second International Conference on Knowledge Discovery and Data Mining, Portland, OR, USA, 2–4 August 1996; pp. 226–231. [Google Scholar]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M.; Moher, D. Addressing reporting biases. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; Wiley: Hoboken, NJ, USA, 2008; pp. 297–333. [Google Scholar]
- Hashemi, M.; Bakhshesh, T.; Khezri, M.; Gharagouzlouian, M.M.; Tavakoli, G. A two-year serological study of bovine viral diarrhea virus, bovine alphaherpesvirus 1 and bovine parainfluenza virus type 3 in Qazvin dairy cattle farms, Northwestern of Iran. Vet. Arhiv 2022, 92, 1–10. [Google Scholar] [CrossRef]
- Demil, E.; Fentie, T.; Vidal, G.; Jackson, W.; Lane, J.; Mekonnen, S.A.; Smith, W. Prevalence of bovine viral diarrhea virus antibodies and risk factors in dairy cattle in Gondar city, Northwest Ethiopia. Prev. Vet. Med. 2021, 191, 105363. [Google Scholar] [CrossRef] [PubMed]
- Lancheros-Buitrago, D.J.; Bulla-Castaneda, D.M.; Pulido-Medellin, M.O.; Lopez Buitrago, H.A.; Diaz-Anaya, A.M.; Garcia-Corredor, D.J. Serodiagnosis and Risk Factors Associated with Infectious Agents of Reproductive Diseases in Bovines of Chiquinquira, District of Boyaca (Colombia). Vet. Med. Int. 2022, 2022, 7436651. [Google Scholar] [CrossRef]
- Hanon, J.B.; De Baere, M.; de la Ferte, C.; Roelandt, S.; Guillot, G.; Van der Stede, Y.; Cay, B. Serological monitoring on milk and serum samples in a BVD eradication program: A field study in Belgium showing antibody ELISA performances and epidemiological aspects. Prev. Vet. Med. 2018, 160, 136–144. [Google Scholar] [CrossRef]
- Daves, L.; Yimer, N.; Arshad, S.S.; Sarsaifi, K.; Omar, M.A.; Yusoff, R.; Haron, A.W.; Abdullah, F.F.J. Seroprevalence of Bovine Viral Diarrhea Virus Infection and Associated Risk Factors in Cattle in Selangor, Malaysia. Vet. Med.-Open J. 2016, 1, 22–28. [Google Scholar] [CrossRef]
- Baillargeon, P.; Arango-Sabogal, J.C.; Wellemans, V.; Fecteau, G. Determining bovine viral diarrhea and infectious bovine rhinotracheitis infections in dairy cattle using precolostral blood. Can. Vet. J. 2017, 58, 360–364. [Google Scholar]
- Noaman, V.; Nabinejad, A.R. Seroprevalence and risk factors assessment of the three main infectious agents associated with abortion in dairy cattle in Isfahan province, Iran. Trop. Anim. Health Prod. 2020, 52, 2001–2009. [Google Scholar] [CrossRef]
- VanLeeuwen, J.; Muraya, J.; Gitau, G.; Makau, D.; Crane, B.; McKenna, S.; Wichtel, J. Seroprevalence and risk factors of Neospora caninum and Bovine Viral Diarrhoea Virus in smallholder dairy cattle in Kenya. East Afr. J. Sci. Technol. Innov. 2021, 3, 1–17. [Google Scholar] [CrossRef]
- Marques, A.L.A.; Assis, A.C.D.; Simoes, S.V.D.; Tolentino, M.L.D.D.; de Azevedo, S.S. Risk factors associated with Bovine Viral Diarrhea Virus (BVDV) infection in the semiarid of the state of Paraiba, in the northeast region of Brazil. Semin.-Cienc. Agrar. 2016, 37, 3095–3105. [Google Scholar] [CrossRef]
- Fernandes, L.G.; Nogueira, A.H.; De Stefano, E.; Pituco, E.M.; Ribeiro, C.P.; Alves, C.J.; Oliveira, T.S.; Clementino, I.J.; de Azevedo, S.S. Herd-level prevalence and risk factors for bovine viral diarrhea virus infection in cattle in the State of Paraiba, Northeastern Brazil. Trop. Anim. Health Prod. 2016, 48, 157–165. [Google Scholar] [CrossRef]
- Hashemi, M.; Bakhshesh, M.; Manavian, M. Bovine Viral Diarrhea Virus and Bovine Herpes Virus-1 in Dairy Cattle Herds in Fars Province, Southern Iran: Seroprevalence and Evaluation of Risk Factors. Arch. Razi Inst. 2022, 77, 1621–1629. [Google Scholar] [CrossRef]
- Rêgo, M.J.P.; Filho, A.F.B.B.; Oliveira, P.R.F.d.; Borges, J.D.M.; França, C.A.B.d.; Ribeiro, C.P.; Pituco, E.M.; Pinheiro Junior, J.W. Epidemiological analysis of infection by the bovine viral diarrhea virus on family farms in Brazil. Semin. Ciênc. Agrár. 2016, 37, 4119–4130. [Google Scholar] [CrossRef]
- Machado, G.; Egocheaga, R.M.; Hein, H.E.; Miranda, I.C.; Neto, W.S.; Almeida, L.L.; Canal, C.W.; Stein, M.C.; Corbellini, L.G. Bovine Viral Diarrhoea Virus (BVDV) in Dairy Cattle: A Matched Case-Control Study. Transbound. Emerg. Dis. 2016, 63, e1–e13. [Google Scholar] [CrossRef]
- Souza, F.O.; Santos, G.A.d.; Souza, B.C.d.S.; Moreira, M.A.S.; Silva-Júnior, A.; Peixoto, R.d.M.; Costa, M.M.d. Frequency and risk factors associated with the bovine viral diarrhea virus in herds in the semiarid region of the states of Bahia and Pernambuco, Brazil. Acta Vet. Bras. 2019, 13, 163–169. [Google Scholar] [CrossRef]
- Almeida, L.L.; Miranda, I.C.; Hein, H.E.; Neto, W.S.; Costa, E.F.; Marks, F.S.; Rodenbusch, C.R.; Canal, C.W.; Corbellini, L.G. Herd-level risk factors for bovine viral diarrhea virus infection in dairy herds from Southern Brazil. Res. Vet. Sci. 2013, 95, 901–907. [Google Scholar] [CrossRef]
- Thapa, A.; Acharya, M.; Raut, R.; Rimal, S. Seroprevalence and Risk Factors of Bovine Viral Diarrhea in Improved Cattle of Chitwan, Nawalpur and Rupandehi Districts of Nepal. Nepal. Vet. J. 2019, 36, 93–97. [Google Scholar] [CrossRef]
- Asnake, P.; Lemma, A.; Tesfaye, A.; Gizaw, D.; Guta, S.; Dima, C.; Tadesse, F. Seroprevalence of Bovine Viral Diarrhea Virus (BVDV) and Its Associated Risk Factors in Dairy Cattle in and Around Assela Town, South East Ethiopia. Res. Sq. 2020. preprint. [Google Scholar] [CrossRef]
- Aragaw, K.; Sibhat, B.; Ayelet, G.; Skjerve, E.; Gebremedhin, E.Z.; Asmare, K. Seroprevalence and factors associated with bovine viral diarrhea virus (BVDV) infection in dairy cattle in three milksheds in Ethiopia. Trop. Anim. Health Prod. 2018, 50, 1821–1827. [Google Scholar] [CrossRef]
- Wittum, T.E.; Grotelueschen, D.M.; Brock, K.V.; Kvasnicka, W.G.; Floyd, J.G.; Kelling, C.L.; Odde, K.G. Persistent bovine viral diarrhoea virus infection in US beef herds. Prev. Vet. Med. 2001, 49, 83–94. [Google Scholar] [CrossRef]
- Fulton, R.W.; Purdy, C.W.; Confer, A.W.; Saliki, J.T.; Loan, R.W.; Briggs, R.E.; Burge, L.J. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: Interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can. J. Vet. Res. 2000, 64, 151–159. [Google Scholar]
- Erfani, A.M.; Bakhshesh, M.; Fallah, M.H.; Hashemi, M. Seroprevalence and risk factors associated with bovine viral diarrhea virus and bovine herpes virus-1 in Zanjan Province, Iran. Trop. Anim. Health Prod. 2019, 51, 313–319. [Google Scholar] [CrossRef]
- Ortega, D.O.; Sarmiento, R.A.M.; Torreglosa, J.C.T.; Rocha, J.F. Prevalence and risk factors of bovine viral diarrhea in Colombian cattle. Vet. World 2020, 13, 1487–1494. [Google Scholar] [CrossRef]
- Barrett, D.; Parr, M.; Fagan, J.; Johnson, A.; Tratalos, J.; Lively, F.; Diskin, M.; Kenny, D. Prevalence of Bovine Viral Diarrhoea Virus (BVDV), Bovine Herpes Virus 1 (BHV 1), Leptospirosis and Neosporosis, and associated risk factors in 161 Irish beef herds. BMC Vet. Res. 2018, 14, 8. [Google Scholar] [CrossRef]
- Olmo, L.; Reichel, M.P.; Nampanya, S.; Khounsy, S.; Wahl, L.C.; Clark, B.A.; Thomson, P.C.; Windsor, P.A.; Bush, R.D. Risk factors for Neospora caninum, bovine viral diarrhoea virus, and Leptospira interrogans serovar Hardjo infection in smallholder cattle and buffalo in Lao PDR. PLoS ONE 2019, 14, e0220335. [Google Scholar] [CrossRef]
- O’Connor, A.M.; Sorden, S.D.; Apley, M.D. Association between the existence of calves persistently infected with bovine viral diarrhea virus and commingling on pen morbidity in feedlot cattle. Am. J. Vet. Res. 2005, 66, 2130–2134. [Google Scholar] [CrossRef]
- Karimi, O.; Sani, M.B.; Bakhshesh, M.; Harofteh, J.Z.; Poormirzayee, H. Seroprevalence of bovine viral diarrhea virus antibodies and risk factors in dairy cattle from the central desert of Iran. Trop. Anim. Health Prod. 2022, 54, 176. [Google Scholar] [CrossRef]
- Humphry, R.W.; Brulisauer, F.; McKendrick, I.J.; Nettleton, P.F.; Gunn, G.J. Prevalence of antibodies to bovine viral diarrhoea virus in bulk tank milk and associated risk factors in Scottish dairy herds. Vet. Rec. 2012, 171, 445. [Google Scholar] [CrossRef]
- Solis-Calderon, J.J.; Segura-Correa, V.M.; Segura-Correa, J.C. Bovine viral diarrhoea virus in beef cattle herds of Yucatan, Mexico: Seroprevalence and risk factors. Prev. Vet. Med. 2005, 72, 253–262. [Google Scholar] [CrossRef]
- Raheem, A.; Ahmad, A.; Rabbani, M.; Ghafoor, A.; Ajnum, A.A.; Avais, M.; Qurat-ul-Ain; Ramizland, R.M.; Ur-Rehman, H. Determination of Sero-Prevalence and Associated Risk Factors of Bovine Viral Diarrhea Virus (Bvdv) in Bovine Population from Southern Punjab, Pakistan. J. Anim. Plant Sci. 2020, 30, 545–551. [Google Scholar] [CrossRef]
- Graham, D.A.; Clegg, T.A.; Lynch, M.; More, S.J. Herd-level factors associated with the presence of bovine viral diarrhoea virus in herds participating in the voluntary phase of the Irish national eradication programme. Prev. Vet. Med. 2013, 112, 99–108. [Google Scholar] [CrossRef]
- Rypula, K.; Ploneczka-Janeczko, K.; Czopowicz, M.; Klimowicz-Bodys, M.D.; Shabunin, S.; Siegwalt, G. Occurrence of BVDV Infection and the Presence of Potential Risk Factors in Dairy Cattle Herds in Poland. Animals 2020, 10, 230. [Google Scholar] [CrossRef]
- Talafha, A.Q.; Hirche, S.M.; Ababneh, M.M.; Al-Majali, A.M.; Ababneh, M.M. Prevalence and risk factors associated with bovine viral diarrhea virus infection in dairy herds in Jordan. Trop. Anim. Health Prod. 2009, 41, 499–506. [Google Scholar] [CrossRef]
- Bezerra, N.P.C.; Bezerra, D.C.; Santos, H.P.; Pereira, H.d.M.; Silva, A.L.A. Risk factors analysis applied to antibodies to Bovine Herpesvirus Type 1, Bovine Viral Diarrhea Virus, Bovine Leukemia Virus and Brucella abortus among cattle: A cross-sectional study. Acta Vet. Bras. 2019, 13, 5–12. [Google Scholar] [CrossRef]
- Akagami, M.; Seki, S.; Kashima, Y.; Yamashita, K.; Oya, S.; Fujii, Y.; Takayasu, M.; Yaguchi, Y.; Suzuki, A.; Ono, Y.; et al. Risk factors associated with the within-farm transmission of bovine viral diarrhea virus and the incidence of persistently infected cattle on dairy farms from Ibaraki prefecture of Japan. Res. Vet. Sci. 2020, 129, 187–192. [Google Scholar] [CrossRef]
- Byrne, A.W.; Guelbenzu-Gonzalo, M.; Strain, S.A.J.; McBride, S.; Graham, J.; Lahuerta-Marin, A.; Harwood, R.; Graham, D.A.; McDowell, S. Assessment of concurrent infection with bovine viral diarrhoea virus (BVDV) and Mycobacterium bovis: A herd-level risk factor analysis from Northern Ireland. Prev. Vet. Med. 2017, 141, 38–47. [Google Scholar] [CrossRef]
- Elam, N.A.; Thomson, D.U.; Gleghorn, J.F. Effects of long- or short-term exposure to a calf identified as persistently infected with bovine viral diarrhea virus on feedlot performance of freshly weaned, transport-stressed beef heifers. J. Anim. Sci. 2008, 86, 1917–1924. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, L.C.; Guzman-Barragan, B.L.; Ordonez, D.; Tafur-Gomez, G.A. Cattle seroprevalence and risk factors associated with bovine viral diarrhea in the northeastern of Colombia. Trop. Anim. Health Prod. 2021, 53, 377. [Google Scholar] [CrossRef]
- Sun, W.W.; Meng, Q.F.; Cong, W.; Shan, X.F.; Wang, C.F.; Qian, A.D. Herd-level prevalence and associated risk factors for Toxoplasma gondii, Neospora caninum, Chlamydia abortus and bovine viral diarrhoea virus in commercial dairy and beef cattle in eastern, northern and northeastern China. Parasitol. Res. 2015, 114, 4211–4218. [Google Scholar] [CrossRef]
- Kumar, S.K.; Palanivel, K.M.; Sukumar, K.; Ronald, B.S.M.; Selvaraju, G.; Ponnudurai, G. Herd-level risk factors for bovine viral diarrhea infection in cattle of Tamil Nadu. Trop. Anim. Health Prod. 2018, 50, 793–799. [Google Scholar] [CrossRef]
- Saa, L.R.; Perea, A.; Garcia-Bocanegra, I.; Arenas, A.J.; Jara, D.V.; Ramos, R.; Carbonero, A. Seroprevalence and risk factors associated with bovine viral diarrhea virus (BVDV) infection in non-vaccinated dairy and dual purpose cattle herds in Ecuador. Trop. Anim. Health Prod. 2012, 44, 645–649. [Google Scholar] [CrossRef]
- Graham, D.A.; Clegg, T.A.; Thulke, H.H.; O’Sullivan, P.; McGrath, G.; More, S.J. Quantifying the risk of spread of bovine viral diarrhoea virus (BVDV) between contiguous herds in Ireland. Prev. Vet. Med. 2016, 126, 30–38. [Google Scholar] [CrossRef]
- Leon, J.C.P.; Diaz, W.; Vasquez, M.C.; Tobon, J.C.; Sanchez, A.; Ortiz, D. Seroprevalence and risk factor associated with respiratory viral pathogens in dual-purpose cattle of Aguachica, Rio de Oro, and La Gloria municipalities in Cesar department, Colombia. Vet. World 2019, 12, 951–958. [Google Scholar] [CrossRef]
- Segura-Correa, J.C.; Zapata-Campos, C.C.; Jasso-Obregon, J.O.; Martinez-Burnes, J.; Lopez-Zavala, R. Seroprevalence and risk factors associated with bovine herpesvirus 1 and bovine viral diarrhea virus in North-Eastern Mexico. Open Vet. J. 2016, 6, 143–149. [Google Scholar] [CrossRef]
- Casey-Bryars, M.; Tratalos, J.A.; Graham, D.A.; Guelbenzu-Gonzalo, M.P.; Barrett, D.; O’Grady, L.; Madden, J.M.; McGrath, G.; More, S.J. Risk factors for detection of bovine viral diarrhoea virus in low-risk herds during the latter stages of Ireland’s eradication programme. Prev. Vet. Med. 2022, 201, 105607. [Google Scholar] [CrossRef]
- Sarrazin, S.; Veldhuis, A.; Meroc, E.; Vangeel, I.; Laureyns, J.; Dewulf, J.; Caij, A.B.; Piepers, S.; Hooyberghs, J.; Ribbens, S.; et al. Serological and virological BVDV prevalence and risk factor analysis for herds to be BVDV seropositive in Belgian cattle herds. Prev. Vet. Med. 2013, 108, 28–37. [Google Scholar] [CrossRef]
- Aasish, G.; Sulav, D.; Umesh, S.; Dojraj, K.; Krishna, K. Seroprevalence and its associated risk factors of Bovine Neosporosis and Bovine Viral Diarrhea in cattle of Tilottama municipality, Rupandehi, Nepal. Int. J. Vet. Sci. Res. 2022, 8, 127–132. [Google Scholar] [CrossRef]
- Booker, C.W.; Abutarbush, S.M.; Morley, P.S.; Guichon, P.T.; Wildman, B.K.; Jim, G.K.; Schunicht, O.C.; Pittman, T.J.; Perrett, T.; Ellis, J.A.; et al. The effect of bovine viral diarrhea virus infections on health and performance of feedlot cattle. Can. Vet. J. 2008, 49, 253–260. [Google Scholar] [PubMed]
- Scharnbock, B.; Roch, F.F.; Richter, V.; Funke, C.; Firth, C.L.; Obritzhauser, W.; Baumgartner, W.; Kasbohrer, A.; Pinior, B. A meta-analysis of bovine viral diarrhoea virus (BVDV) prevalences in the global cattle population. Sci. Rep. 2018, 8, 14420. [Google Scholar] [CrossRef]
- Spetter, M.J.; Uriarte, E.L.L.; Armendano, J.I.; Morrell, E.L.; Canton, G.J.; Verna, A.E.; Dorsch, M.A.; Pereyra, S.B.; Odeon, A.C.; Saliki, J.T.; et al. Detection methods and characterization of bovine viral diarrhea virus in aborted fetuses and neonatal calves over a 22-year period. Braz. J. Microbiol. 2020, 51, 2077–2086. [Google Scholar] [CrossRef]
- Hanon, J.B.; De Baere, M.; De la Ferte, C.; Roelandt, S.; Van der Stede, Y.; Cay, B. Evaluation of 16 commercial antibody ELISAs for the detection of bovine viral diarrhea virus-specific antibodies in serum and milk using well-characterized sample panels. J. Vet. Diagn. Investig. 2017, 29, 833–843. [Google Scholar] [CrossRef]
- Fulton, R.W.; Hessman, B.E.; Ridpath, J.F.; Johnson, B.J.; Burge, L.J.; Kapil, S.; Braziel, B.; Kautz, K.; Reck, A. Multiple diagnostic tests to identify cattle with Bovine viral diarrhea virus and duration of positive test results in persistently infected cattle. Can. J. Vet. Res. 2009, 73, 117–124. [Google Scholar] [PubMed]
- Masood, R.; Khushi, M.; Arfan, A. Diagnostic Approaches for Detection of Bvdv Persistency in Dairy Herds; LAP Lambert Academic Publishing: Saarland, Germany, 2011. [Google Scholar]
- Ahmad, A. Diagnostic Approaches for Detection of Bovine Viral Diarrhea Virus Persistent Infection. J. Infect. Mol. Biol. 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Nickell, J.S.; White, B.J.; Larson, R.L.; Renter, D.G.; Roque, J.; Hesse, R.; Oberst, R.; Peddireddi, L.; Anderson, G. Onset and Duration of Transient Infections Among Antibody- Diverse Beef Calves Exposed to a Bovine Viral Diarrhea Virus Persistently Infected Calf. Int. J. Appl. Res. Vet. Med. 2011, 9, 29–39. [Google Scholar]
- Brock, K.V.; Grooms, D.L.; Ridpath, J.; Bolin, S.R. Changes in levels of viremia in cattle persistently infected with bovine viral diarrhea virus. J. Vet. Diagn. Investig. 1998, 10, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A. Diagnostic efficacy of a reverse transcriptase-polymerase chain reaction assay to screen cattle for persistent bovine viral diarrhea virus infection. J. Am. Vet. Med. Assoc. 2006, 229, 1472–1474. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Sanderson, M.W.; Walz, P.H.; Givens, M.D. Sensitivity of polymerase chain reaction for detection of bovine viral diarrhea virus in pooled serum samples and use of pooled polymerase chain reaction to determine prevalence of bovine viral diarrhea virus in auction market cattle. J. Vet. Diagn. Investig. 2008, 20, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Mortimer, R.G.; Powers, B. Reverse transcription-polymerase chain reaction on pooled samples to detect bovine viral diarrhea virus by using fresh ear-notch-sample supernatants. J. Vet. Diagn. Investig. 2006, 18, 89–93. [Google Scholar] [CrossRef]
- Igloi, Z.; Leven, M.; Abdel-Karem Abou-Nouar, Z.; Weller, B.; Matheeussen, V.; Coppens, J.; Koopmans, M.; Molenkamp, R. Comparison of commercial realtime reverse transcription PCR assays for the detection of SARS-CoV-2. J. Clin. Virol. 2020, 129, 104510. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Lombardi, A.; De Vecchi, E.; Giuliani, G.; Bartolone, R.; Gismondo, M.R. Comparison of nested PCR and real time PCR of Herpesvirus infections of central nervous system in HIV patients. BMC Infect. Dis. 2004, 4, 55. [Google Scholar] [CrossRef]
- Greiser-Wilke, I.; Grummer, B.; Moennig, V. Bovine viral diarrhoea eradication and control programmes in Europe. Biologicals 2003, 31, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Antos, A.; Miroslaw, P.; Rola, J.; Polak, M.P. Vaccination Failure in Eradication and Control Programs for Bovine Viral Diarrhea Infection. Front. Vet. Sci. 2021, 8, 688911. [Google Scholar] [CrossRef] [PubMed]

| Criterion | Description |
|---|---|
| Inclusion | Study design: Cross-sectional, longitudinal, case reports investigating BVDV in cattle; |
| Population: Cattle of any region, breed, age, production system; | |
| Outcome measures: Prevalence/detection of BVDV, confirmed via laboratory tests; | |
| Language: English or other languages with translations; | |
| Sample size: More than 30; | |
| Publication type: Peer-reviewed articles. | |
| Exclusion | Study design: Case reports with <30 cases, reviews, editorials, commentaries, opinion articles; |
| Population: Noncattle species; | |
| Outcome measures: Studies without BVDV prevalence/detection or lab confirmation; | |
| Data quality: Studies with significant flaws, unclear reporting, or missing key information; | |
| Sample size: Less than 30; | |
| Missing information: On the origin and type of data used for analysis; | |
| Duplicate data: Studies reporting duplicate/overlapping data. |
| Detection Method | Number of Pooled Studies | Prevalence | 95% CI | Tau2 | Tau | Q | I2 |
|---|---|---|---|---|---|---|---|
| Antigen | 46 | 0.05 | [0.02; 0.08] | 0.01 | 0.12 | 10,585.38 | 99.6% |
| Antibody | 144 | 0.43 | [0.39; 0.48] | 0.05 | 0.22 | 44,267.72 | 99.7% |
| Nucleic acid | 50 | 0.08 | [0.04; 0.11] | 0.19 | 0.43 | 58,908.83 | 99.9% |
| Virus isolation | 3 | 0.22 | [0.03; 0.50] | 0.01 | 0.11 | 23.09 | 91.3% |
| Detection Method | Outlier Removal Status | Number of Studies | Prevalence | 95% CI | Tau2 | Tau | I2 |
|---|---|---|---|---|---|---|---|
| Antibody | Pre-outlier removal | 144 | 0.43 | [0.39; 0.48] | 0.05 | 0.22 | 0.997 |
| Post-outlier removal | 122 | 0.44 | [0.39; 0.49] | 0.05 | 0.21 | 0.996 | |
| Antibody (herd-level) | Pre-outlier removal | 40 | 0.66 | [0.54; 0.77] | 0.44 | 0.67 | 0.999 |
| Post-outlier removal | 36 | 0.71 | [0.62; 0.80] | 0.08 | 0.28 | 0.98 | |
| Antigen | Pre-outlier removal | 46 | 0.05 | [0.02; 0.08] | 0.01 | 0.12 | 0.996 |
| Post-outlier removal | 37 | 0.03 | [0.02; 0.05] | 0.01 | 0.09 | 0.989 | |
| Nucleic acid | Pre-outlier detection | 50 | 0.08 | [0.04; 0.11] | 0.19 | 0.43 | 0.999 |
| Post-outlier removal | 49 | 0.07 | [0.04; 0.10] | 0.02 | 0.13 | 0.987 |
| Risk Factors | Description | Reference |
|---|---|---|
| Age of cattle | Adult cattle have higher prevalence compared to calves. | [29,30,31,32,33] |
| Postcolostral serum samples yielded a higher prevalence compared to precolostral samples. | [34] | |
| Heifers have a lower seropositive rate compared to calves. | [35] | |
| Cows have higher odds of testing seropositive over heifers. | [36] | |
| Weaning age. | [37] | |
| Number of calves on a farm less than one year old. | [38] | |
| Breeding and reproduction | Natural or extensive breeding. | [37,39,40,41,42,43] |
| History of abortion is associated with a higher prevalence. | [44,45] | |
| Serostatus to BVDV is associated with repeat breeding. | [46] | |
| Pregnant and lactating cattle. | [33,47] | |
| Coinfection status | Calves with pneumonic Pasteurellosis. | [48] |
| Concurrent infection with BHV-1. | [35,39,49,50] | |
| Coinfection with and Neospora caninum. | [36,50,51,52] | |
| Cattle mixing | Commingling or cattle introduction. | [36,37,53,54,55] |
| Cattle purchase. | [38,56,57,58,59,60,61,62,63] | |
| Direct contact with neighboring farms. | [41] | |
| Exposure to persistently infected calves did not affect the prevalence. | [53,64] | |
| Farm management | Farm owner literacy level. | [39] |
| Isolation paddocks for ill cattle. | [41] | |
| Pastureland management. | [38,59,65] | |
| Not providing colostrum. | [40] | |
| Cattle management system including housing and hygiene. | [30,36,54,55,66,67] | |
| Farm size area and animal density. | [37,68] | |
| Exchange visits between adjacent farm workers. | [60] | |
| Geographical location and season | Season of the year. | [29] |
| BVDV infection in a nearby farm. | [62,69] | |
| Geographic location. | [39,59,68,70,71] | |
| Herd size and type | Breed. | [42,63,65] |
| Herd size. | [43,52,58,62,63,66,72,73,74] | |
| PI cattle in a pen. | [75] | |
| Distance from positive herds. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werid, G.M.; Hemmatzadeh, F.; Miller, D.; Reichel, M.P.; Messele, Y.E.; Petrovski, K. Comparative Analysis of the Prevalence of Bovine Viral Diarrhea Virus in Cattle Populations Based on Detection Methods: A Systematic Review and Meta-Analysis. Pathogens 2023, 12, 1067. https://doi.org/10.3390/pathogens12081067
Werid GM, Hemmatzadeh F, Miller D, Reichel MP, Messele YE, Petrovski K. Comparative Analysis of the Prevalence of Bovine Viral Diarrhea Virus in Cattle Populations Based on Detection Methods: A Systematic Review and Meta-Analysis. Pathogens. 2023; 12(8):1067. https://doi.org/10.3390/pathogens12081067
Chicago/Turabian StyleWerid, Gebremeskel Mamu, Farhid Hemmatzadeh, Darren Miller, Michael P. Reichel, Yohannes E. Messele, and Kiro Petrovski. 2023. "Comparative Analysis of the Prevalence of Bovine Viral Diarrhea Virus in Cattle Populations Based on Detection Methods: A Systematic Review and Meta-Analysis" Pathogens 12, no. 8: 1067. https://doi.org/10.3390/pathogens12081067









