People Who Self-Reported Testing HIV-Positive but Tested HIV-Negative: A Multi-Country Puzzle of Data, Serology, and Ethics, 2015–2021
Abstract
:1. Introduction
2. Materials and Methods
Analyses
3. Results
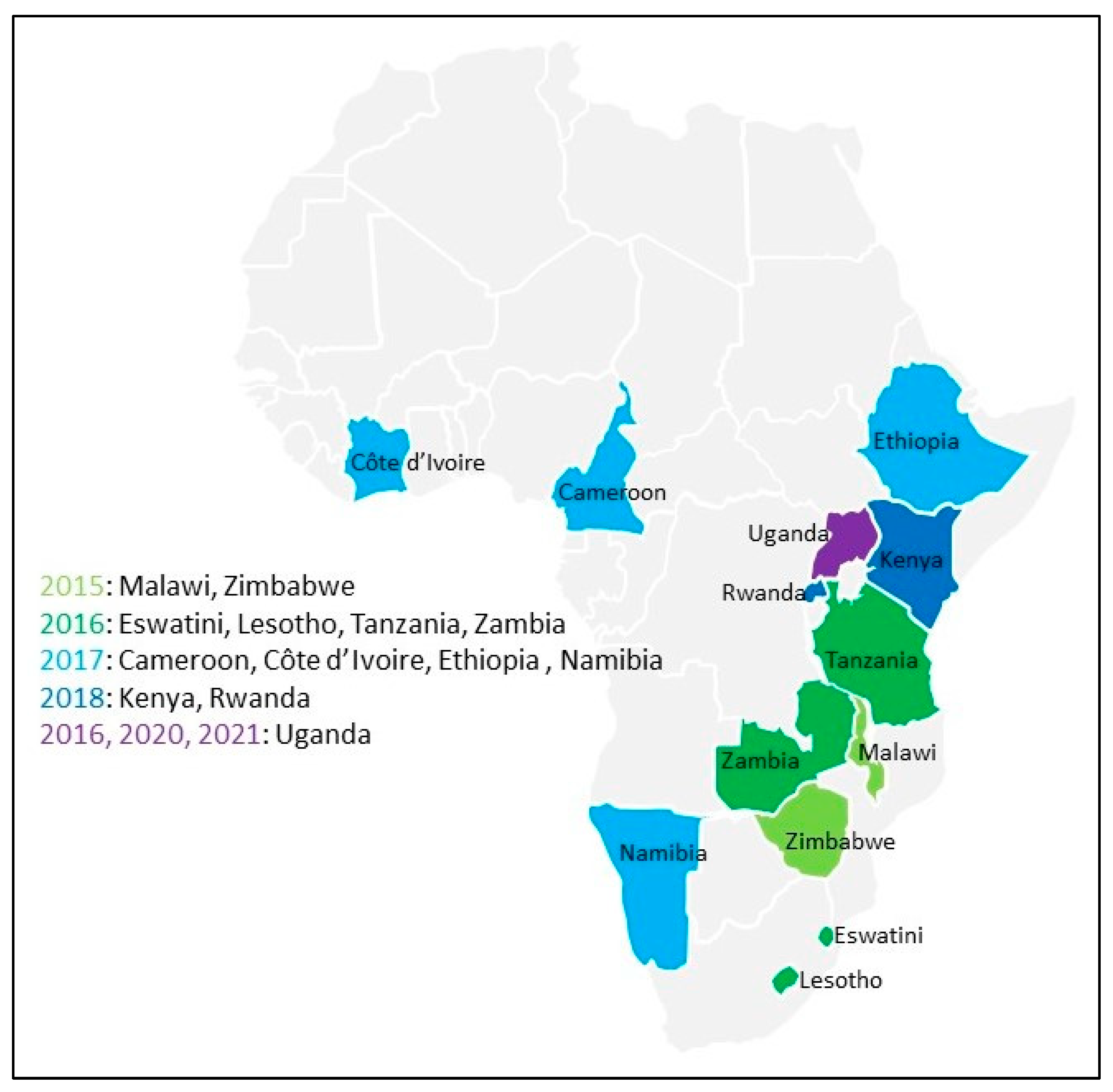
3.1. Participants Self-Reported as Positive across 15 Surveys
3.2. Participants Self-Reported as Positive and Self-Report Discrepancies in Uganda
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fishel, J.D.; Barrère, B.; Kishor, S. Validity of Data on Self-Reported HIV Status in Malawi and Uganda and Implications for Measurement of ARV Coverage; ICF International: Rockville, MD, USA, 2014. [Google Scholar]
- Rohr, J.K.; Gómez-Olivé, F.X.; Rosenberg, M.; Manne-Goehler, J.; Geldsetzer, P.; Wagner, R.G.; Houle, B.; Salomon, J.A.; Kahn, K.; Tollman, S.; et al. Performance of Self-Reported HIV Status in Determining True HIV Status among Older Adults in Rural South Africa: A Validation Study. J. Int. AIDS Soc. 2017, 20, 21691. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.H.; Kabudula, C.W.; West, R.L.; Dufour, M.-S.K.; Julien, A.; Masilela, N.G.; Tollman, S.M.; Pettifor, A.; Kahn, K.; Lippman, S.A. Estimating the Prevalence of Over- and Under-Reporting in HIV Testing, Status and Treatment in Rural Northeast South Africa: A Comparison of a Survey and Clinic Records. AIDS Behav. 2023, 3248–3257. [Google Scholar] [CrossRef] [PubMed]
- Chasimpha, S.J.D.; Mclean, E.M.; Dube, A.; McCormack, V.; dos-Santos-Silva, I.; Glynn, J.R. Assessing the Validity of and Factors That Influence Accurate Self-Reporting of HIV Status after Testing: A Population-Based Study. AIDS 2020, 34, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Fuente-Soro, L.; Lopez-Varela, E.; Augusto, O.; Sacoor, C.; Nhacolo, A.; Honwana, N.; Karajeanes, E.; Vaz, P.; Naniche, D. Monitoring Progress towards the First UNAIDS Target: Understanding the Impact of People Living with HIV Who Re-Test during HIV-Testing Campaigns in Rural Mozambique. J. Int. AIDS Soc. 2018, 21, e25095. [Google Scholar] [CrossRef]
- Jahun, I.; Ehoche, A.; Bamidele, M.; Yakubu, A.; Bronson, M.; Dalhatu, I.; Greby, S.; Agbakwuru, C.; Baffa, I.; Iwara, E.; et al. Evaluation of Accuracy and Performance of Self-Reported HIV and Antiretroviral Therapy Status in the Nigeria AIDS Indicator and Impact Survey (2018). PLoS ONE 2022, 17, e0273748. [Google Scholar] [CrossRef]
- Logan, N.Z.; Kilmarx, P.H.; Rolle, I.; Patel, H.K.; Duong, Y.T.; Lee, K.; Shang, J.D.; Bodika, S.; Koui, I.T.; Balachandra, S.; et al. Brief Report: Self-Reported HIV-Positive Status but Subsequent HIV-Negative Test Results in Population-Based HIV Impact Assessment Survey Participants—11 Sub-Saharan African Countries, 2015–2018. JAIDS J. Acquir. Immune Defic. Syndr. 2024, 95, 313–317. [Google Scholar] [CrossRef]
- PHIA Project. PHIA Project—Guiding the Global HIV Response. Available online: https://phia.icap.columbia.edu/ (accessed on 23 May 2022).
- Justman, J.E.; Mugurungi, O.; El-Sadr, W.M. HIV Population Surveys—Bringing Precision to the Global Response. N. Engl. J. Med. 2018, 378, 1859–1861. [Google Scholar] [CrossRef]
- Nsanzimana, S.; Rwibasira, G.N.; Malamba, S.S.; Musengimana, G.; Kayirangwa, E.; Jonnalagadda, S.; Rezende, E.F.; Eaton, J.W.; Mugisha, V.; Remera, E.; et al. HIV Incidence and Prevalence among Adults Aged 15–64 Years in Rwanda: Results from the Rwanda Population-Based HIV Impact Assessment (RPHIA) and District-Level Modeling, 2019. Int. J. Infect. Dis. 2022, 116, 245–254. [Google Scholar] [CrossRef]
- Cameroon Final Report (En, Fr) 2017–2018. PHIA Project. Available online: https://phia.icap.columbia.edu/cameroon-final-report/ (accessed on 1 April 2024).
- Cote d’Ivoire Final Report (En, Fr) 2017–2018. PHIA Project. Available online: https://phia.icap.columbia.edu/cote-divoire-final-report-en-fr/ (accessed on 1 April 2024).
- Lulseged, S.; Belete, W.; Ahmed, J.; Gelibo, T.; Teklie, H.; West, C.W.; Melaku, Z.; Demissie, M.; Farhani, M.; Eshetu, F.; et al. Factors Associated with Unawareness of HIV-Positive Status in Urban Ethiopia: Evidence from the Ethiopia Population-Based HIV Impact Assessment 2017–2018. PLoS ONE 2021, 16, e0255163. [Google Scholar] [CrossRef]
- Mwangi, J.; Miruka, F.; Mugambi, M.; Fidhow, A.; Chepkwony, B.; Kitheka, F.; Ngugi, E.; Aoko, A.; Ngugi, C.; Waruru, A. Characteristics of Users of HIV Self-Testing in Kenya, Outcomes, and Factors Associated with Use: Results from a Population-Based HIV Impact Assessment, 2018. BMC Public Health 2022, 22, 643. [Google Scholar] [CrossRef]
- Sachathep, K.; Radin, E.; Hladik, W.; Hakim, A.; Saito, S.; Burnett, J.; Brown, K.; Phillip, N.; Jonnalagadda, S.; Low, A.; et al. Population-Based HIV Impact Assessments Survey Methods, Response, and Quality in Zimbabwe, Malawi, and Zambia. JAIDS J. Acquir. Immune Defic. Syndr. 2021, 87, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Low, A.; Sachathep, K.; Rutherford, G.; Nitschke, A.-M.; Wolkon, A.; Banda, K.; Miller, L.A.; Solmo, C.; Jackson, K.; Patel, H.; et al. Migration in Namibia and Its Association with HIV Acquisition and Treatment Outcomes. PLoS ONE 2021, 16, e0256865. [Google Scholar] [CrossRef] [PubMed]
- Uganda Summary Sheet 2020–2021. PHIA Project. Available online: https://phia.icap.columbia.edu/uganda-summary-sheet-2020-2021/ (accessed on 1 April 2024).
- Uganda Refugee Population-based HIV Impact Assessment 2021. PHIA Project. Available online: https://phia.icap.columbia.edu/uganda-refugee-summary-sheet-2020-2021-2/ (accessed on 1 April 2024).
- PHIA Project. About. Available online: https://phia.icap.columbia.edu/about/ (accessed on 1 April 2024).
- PHIA Data Manager. Available online: https://phia-data.icap.columbia.edu/ (accessed on 30 January 2022).
- Patel, H.K.; Duong, Y.T.; Birhanu, S.; Dobbs, T.; Lupoli, K.; Moore, C.; Detorio, M.; Sleeman, K.; Manjengwa, J.; Wray-Gordon, F.; et al. A Comprehensive Approach to Assuring Quality of Laboratory Testing in HIV Surveys: Lessons Learned from the Population-Based HIV Impact Assessment Project. JAIDS J. Acquir. Immune Defic. Syndr. 2021, 87, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Smith, R.; Mitchell, R.; Duong, Y.T.; Brown, K.; Kinchen, S.; Lee, K.; Ogollah, F.M.; Dzinamarira, T.; Maliwa, V.; et al. Data Architecture to Support Real-Time Data Analytics for the Population-Based HIV Impact Assessments. JAIDS J. Acquir. Immune Defic. Syndr. 2021, 87, S28–S35. [Google Scholar] [CrossRef]
- Final Reports. PHIA Project. Available online: https://phia.icap.columbia.edu/resource_categories/final-reports/ (accessed on 22 September 2023).
- Kaleebu, P.; Kitandwe, P.K.; Lutalo, T.; Kigozi, A.; Watera, C.; Nanteza, M.B.; Hughes, P.; Musinguzi, J.; Opio, A.; Downing, R.; et al. Evaluation of HIV-1 Rapid Tests and Identification of Alternative Testing Algorithms for Use in Uganda. BMC Infect. Dis. 2018, 18, 93. [Google Scholar] [CrossRef]
- World Health Organization. Consolidated Guidelines on HIV Testing Services (2015); Guidelines; WHO: Villars-sous-Yens, Switzerland, 2015; p. 163. Available online: https://www.who.int/publications-detail-redirect/9789241508926 (accessed on 19 October 2023).
- Gonsalves, L.; Hunter, E.C.; Brizuela, V.; Tucker, J.D.; Srinivas, M.L.; Gitau, E.; Mercer, C.H.; Bajos, N.; Collins, D. Cognitive Testing of a Survey Instrument to Assess Sexual Practices, Behaviours, and Health Outcomes: A Multi-Country Study Protocol. Reprod. Health 2021, 18, 249. [Google Scholar] [CrossRef]
- Uruena, A.; Cassetti, I.; Kashyap, N.; Deleage, C.; Estes, J.D.; Trindade, C.; Hammoud, D.A.; Burbelo, P.D.; Natarajan, V.; Dewar, R.; et al. Prolonged Posttreatment Virologic Control and Complete Seroreversion After Advanced Human Immunodeficiency Virus-1 Infection. Open Forum Infect. Dis. 2020, 8, ofaa613. [Google Scholar] [CrossRef]
- Etemad, B.; Esmaeilzadeh, E.; Li, J.Z. Learning from the Exceptions: HIV Remission in Post-Treatment Controllers. Front. Immunol. 2019, 10, 1749. [Google Scholar] [CrossRef]
- Renault, C.; Bolloré, K.; Pisoni, A.; Motto-Ros, C.; Van de Perre, P.; Reynes, J.; Tuaillon, E. Accuracy of Real-Time PCR and Digital PCR for the Monitoring of Total HIV DNA under Prolonged Antiretroviral Therapy. Sci. Rep. 2022, 12, 9323. [Google Scholar] [CrossRef]
- Johnson, C.C.; Fonner, V.; Sands, A.; Ford, N.; Obermeyer, C.M.; Tsui, S.; Wong, V.; Baggaley, R. To Err Is Human, to Correct Is Public Health: A Systematic Review Examining Poor Quality Testing and Misdiagnosis of HIV Status. J. Int. AIDS Soc. 2017, 20, 21755. [Google Scholar] [CrossRef]
- Shahar, E.; Shapiro, A.; Baskin, L.; Oz, Z.K. Antiretroviral Therapy-Induced Negative HIV Antibody Test Following Diagnosis of HIV Infection. AIDS 2019, 33, 1804–1805. [Google Scholar] [CrossRef] [PubMed]
- Are HIV Tests Accurate in People Taking HIV Treatment, PrEP or PEP? Available online: https://www.aidsmap.com/about-hiv/are-hiv-tests-accurate-people-taking-hiv-treatment-prep-or-pep (accessed on 19 February 2022).
- CDC. CDC’s Epidemic Intelligence Service (EIS). Centers for Disease Control and Prevention. Available online: https://archive.cdc.gov/#/details?q=Self-Reported%20HIV-Positive%20Status%20but%20Subsequent%20HIV-NegativeTest%20&start=0&rows=10&url=https://www.cdc.gov/eis/conference/dpk/HIV_Positive_but_Subsequent_HIV_Negative.html (accessed on 9 February 2024).
- Kolakowska, A.; Maresca, A.F.; Collins, I.J.; Cailhol, J. Update on Adverse Effects of HIV Integrase Inhibitors. Curr. Treat. Options Infect. Dis. 2019, 11, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.Y.; Kaul, M. Beneficial and Adverse Effects of cART Affect Neurocognitive Function in HIV-1 Infection: Balancing Viral Suppression against Neuronal Stress and Injury. J. Neuroimmune Pharmacol. 2021, 16, 90–112. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, Y.; Zhou, R.; Jiang, T.; Zhang, H.; Li, Z.; Liu, A.; Shao, Y.; Hua, W.; Zhang, T.; et al. Efficacy and Tolerability of Lopinavir/Ritonavir- and Efavirenz-Based Initial Antiretroviral Therapy in HIV-1-Infected Patients in a Tertiary Care Hospital in Beijing, China. Front. Pharmacol. 2019, 10, 1472. [Google Scholar] [CrossRef]
- Mandavilli, A. A Woman May Have Been Cured of H.I.V. Without Medical Treatment. The New York Times, 26 August 2020. Available online: https://www.nytimes.com/2020/08/26/health/hiv-cure.html (accessed on 16 February 2022).
- Mandavilli, A. A Woman Is Cured of H.I.V. Using a Novel Treatment. The New York Times, 15 February 2022. Available online: https://www.nytimes.com/2022/02/15/health/hiv-cure-cord-blood.html (accessed on 16 February 2022).
- Jiang, C.; Lian, X.; Gao, C.; Sun, X.; Einkauf, K.B.; Chevalier, J.M.; Chen, S.M.Y.; Hua, S.; Rhee, B.; Chang, K.; et al. Distinct Viral Reservoirs in Individuals with Spontaneous Control of HIV-1. Nature 2020, 585, 261–267. [Google Scholar] [CrossRef]
- Kassutto, S.; Johnston, M.N.; Rosenberg, E.S. Incomplete HIV Type 1 Antibody Evolution and Seroreversion in Acutely Infected Individuals Treated with Early Antiretroviral Therapy. Clin. Infect. Dis. 2005, 40, 868–873. [Google Scholar] [CrossRef]
- Xia, Y.; Milwid, R.M.; Godin, A.; Boily, M.-C.; Johnson, L.F.; Marsh, K.; Eaton, J.W.; Maheu-Giroux, M. Accuracy of Self-Reported HIV-Testing History and Awareness of HIV-Positive Status in Four Sub-Saharan African Countries. AIDS 2021, 35, 503–510. [Google Scholar] [CrossRef]
- Yorlets, R.R.; Lurie, M.N.; Ginsburg, C.; Hogan, J.W.; Joyce, N.R.; Harawa, S.; Collinson, M.A.; Gómez-Olivé, F.X.; White, M.J. Validity of Self-Report for Ascertaining HIV Status Among Circular Migrants and Permanent Residents in South Africa: A Cross-Sectional, Population-Based Analysis. AIDS Behav. 2023, 27, 919–927. [Google Scholar] [CrossRef]
- Latkin, C.A.; Vlahov, D. Socially Desirable Response Tendency as a Correlate of Accuracy of Self-Reported HIV Serostatus for HIV Seropositive Injection Drug Users. Addiction 1998, 93, 1191–1197. [Google Scholar] [CrossRef]
- Bignami-Van Assche, S.; Chao, L.-W.; Anglewicz, P.; Chilongozi, D.; Bula, A. The Validity of Self-Reported Likelihood of HIV Infection among the General Population in Rural Malawi. Sex. Transm. Infect. 2007, 83, 35–40. [Google Scholar] [CrossRef]
- Botswana HIV/AIDS Impact Survey Report|UNICEF Botswana. Available online: https://www.unicef.org/botswana/documents/botswana-hivaids-impact-survey-report-0 (accessed on 26 November 2023).
- Nardell, M.F.; Hedt-Gauthier, B.; Earnshaw, V.A.; Bogart, L.M.; Dietrich, J.J.; Courtney, I.; Tshabalala, G.; Bor, J.; Orrell, C.; Gray, G.; et al. Understanding Repeat Positive HIV Testing in South Africa Under Changing Treatment Guidelines. AIDS Behav. 2022, 26, 1366–1376. [Google Scholar] [CrossRef] [PubMed]



| Question | Uganda 2016 | Uganda 2020 and Uganda Refugees 2021 |
|---|---|---|
| What month and year was your last HIV test? What was the result of that HIV test? | included | not included |
| When was your last HIV test? Please give the month and year if you can. What was the result of your last HIV test? | not included | included |
| Has a health care provider ever told you that you have HIV? | not included | included |
| Did you test positive for HIV before your pregnancy with [child’s name]? | included 1 | included 1 |
| What was the result of your last HIV test during your last pregnancy with [child’s name]? | included 1 | included 1 |
| Did you test for HIV during labor? What was the result of that test? | included 1,2 | not included |
| What was the result of the HIV test that you received after the delivery of your last pregnancy with [child’s name]? | not included | included 1 |
| Self-Report Is True | Self-Report Is False | |
|---|---|---|
| Test is correct |
|
|
| Test is incorrect |
| Not applicable |
| Country | Survey | Year Started | Participants | Tested HIV-Positive | Viral Load Suppressed 1 | PSRP | PSRP-CN, % of Participants | PSRP-CN, % of PSRP |
|---|---|---|---|---|---|---|---|---|
| Cameroon | CAMPHIA | 2017 | 25,279 | 944 | 406 | 432 | 10/25,279 (0.04%) | 10/432 (2.31%) |
| Côte d’Ivoire | CIPHIA | 2017 | 17,319 | 417 | 181 | 161 | 7/17,319 (0.04%) | 7/161 (4.35%) |
| Eswatini | SHIMS2 | 2016 | 9744 | 2839 | 2096 | 2486 | 40/9744 (0.41%) | 40/2486 (1.61%) |
| Ethiopia 2 | EPHIA | 2017 | 18,563 | 593 | 417 | 453 | 4/18,563 (0.02%) | 4/453 (0.88%) |
| Kenya | KENPHIA | 2018 | 27,139 | 1482 | 1068 | 1097 | 14/27,139 (0.05%) | 14/1097 (1.28%) |
| Lesotho | LEPHIA | 2016 | 11,867 | 3239 | 2238 | 2582 | 23/11,867 (0.19%) | 23/2582 (0.89%) |
| Malawi | MPHIA | 2015 | 16,869 | 2176 | 1482 | 1606 | 11/16,869 (0.07%) | 11/1606 (0.68%) |
| Namibia | NAMPHIA | 2017 | 16,292 | 2353 | 1818 | 1921 | 14/16,292 (0.09%) | 14/1921 (0.73%) |
| Rwanda | RPHIA | 2018 | 29,476 | 888 | 680 | 661 | 13/29,476 (0.04%) | 13/661 (1.97%) |
| Tanzania | THIS | 2016 | 29,287 | 1771 | 924 | 1055 | 10/29,287 (0.03%) | 10/1055 (0.95%) |
| Uganda 2016 | UPHIA | 2016 | 28,593 | 1735 | 1051 | 1201 | 42/28,593 (0.15%) | 42/1201 (3.50%) |
| Uganda 2020 | UPHIA | 2020 | 23,148 | 1388 | 1055 | 1081 | 33/23,148 (0.14%) | 33/1081 (3.05%) |
| Uganda Refugees 3 | RUPHIA | 2021 | 2509 | 44 | 34 | 37 | 5/2509 (0.20%) | 5/37 (13.51%) |
| Zambia | ZAMPHIA | 2016 | 19,592 | 2527 | 1508 | 1738 | 15/19,592 (0.08%) | 15/1738 (0.86%) |
| Zimbabwe | ZIMPHIA | 2015 | 19,970 | 3293 | 2057 | 2515 | 15/19,970 (0.08%) | 15/2515 (0.60%) |
| Overall | - | - | 295,647 | 25,689 | 17,015 | 19,026 | 256/295,647 (0.09%) | 256/19,026 (1.35%) |
| Survey | Survey Prevalence 1 (95% Confidence Interval) | Conditional Prevalence 2 (95% Confidence Interval) | Percentage Point Increase (Absolute Change) | Increase as a Percent of Prevalence (Relative Change) |
|---|---|---|---|---|
| Cameroon | 3.67% (3.32–4.02%) | 3.71% (3.36–4.06%) | 0.04% | 1.06% |
| Côte d’Ivoire | 2.70% (2.37–3.03%) | 2.77% (2.42–3.11%) | 0.07% | 2.36% |
| Eswatini | 27.92% (26.54–29.30%) | 28.35% (26.96–29.73%) | 0.43% | 1.53% |
| Ethiopia 3 | 3.01% (2.60–3.42%) | 3.03% (2.62–3.45%) | 0.02% | 0.67% |
| Kenya | 4.86% (4.47–5.24%) | 4.89% (4.50–5.28%) | 0.03% | 0.68% |
| Lesotho | 25.56% (24.68–26.44%) | 25.74% (24.86–26.62%) | 0.18% | 0.72% |
| Malawi | 10.52% (9.88–11.16%) | 10.56% (9.92–11.20%) | 0.04% | 0.37% |
| Namibia | 12.56% (11.68–13.45%) | 12.65% (11.76–13.53%) | 0.09% | 0.68% |
| Rwanda | 2.96% (2.62–3.31%) | 3.02% (2.67–3.36%) | 0.06% | 1.72% |
| Tanzania | 5.01% (4.66–5.37%) | 5.03% (4.68–5.39%) | 0.02% | 0.41% |
| Uganda 2016 | 6.25% (5.82–6.67%) | 6.39% (5.96–6.82%) | 0.14% | 2.26% |
| Uganda 2020 | 5.89% (5.40–6.39%) | 6.02% (5.53–6.52%) | 0.13% | 2.18% |
| Uganda Refugees 4 | 1.47% (0.83–2.11%) | 1.57% (0.93–2.21%) | 0.10% | 6.79% |
| Zambia | 12.00% (11.34–12.66%) | 12.08% (11.42–12.74%) | 0.08% | 0.68% |
| Zimbabwe | 14.08% (13.42–14.75%) | 14.15% (13.49–14.82%) | 0.07% | 0.50% |
| Overall 5 | 9.23% (N/A) | 9.33% (N/A) | 0.10% | 1.08% |
| Survey | Survey Population VLS 1 (95% Confidence Interval) | Conditional Population VLS 2 (95% Confidence Interval) | Percentage Point Increase (Absolute Change) | Increase as a Percent of Population VLS (Relative Change) |
|---|---|---|---|---|
| Cameroon | 43.99% (39.97–48.02%) | 44.58% (40.65–48.51%) | 0.59% | 1.34% |
| Côte d’Ivoire | 39.27% (32.07–46.48%) | 40.68% (33.62–47.74%) | 1.41% | 3.57% |
| Eswatini | 72.37% (70.43–74.30%) | 72.78% (70.87–74.69%) | 0.41% | 0.57% |
| Ethiopia 3 | 70.26% (65.43–75.09%) | 70.45% (65.64–75.27%) | 0.19% | 0.28% |
| Kenya | 70.81% (67.92–73.69%) | 71.00% (68.12–73.88%) | 0.19% | 0.28% |
| Lesotho | 67.57% (65.68–69.45%) | 67.80% (65.94–69.65%) | 0.23% | 0.34% |
| Malawi | 67.74% (65.39–70.08%) | 67.85% (65.52–70.19%) | 0.11% | 0.17% |
| Namibia | 76.89% (74.26–79.52%) | 77.04% (74.42–79.67%) | 0.15% | 0.20% |
| Rwanda | 75.66% (71.55–79.77%) | 76.07% (72.03–80.12%) | 0.41% | 0.54% |
| Tanzania | 51.91% (48.51–55.30%) | 52.10% (48.73–55.48%) | 0.19% | 0.38% |
| Uganda 2016 | 59.31% (56.39–62.24%) | 60.21% (57.31–63.12%) | 0.90% | 1.52% |
| Uganda 2020 | 74.52% (71.63–77.42%) | 75.07% (72.24–77.90%) | 0.55% | 0.73% |
| Uganda Refugees 3 | 73.82% (60.21–87.43%) | 75.48% (62.67–88.30%) | 1.66% | 2.26% |
| Zambia | 57.80% (55.28–60.31%) | 58.08% (55.59–60.57%) | 0.28% | 0.49% |
| Zimbabwe | 58.96% (56.75–61.16%) | 59.16% (56.97–61.35%) | 0.20% | 0.34% |
| Overall 4 | 64.06% (N/A) | 64.56% (N/A) | 0.50% | 0.78% |
| Surveys Overall 1 | PSRP | Discrepancy | Concluded to Be Negative | ||
|---|---|---|---|---|---|
| Total participants | 54,250 | 2319 | 107 | 80 | |
| Male | 22,764 (42.0%) | 685 (29.5%) | 45 (42.1%) | 34 (42.5%) | |
| Female | 31,486 (58.0%) | 1634 (70.5%) | 62 (57.9%) | 46 (57.5%) | |
| ARVs taken ever (only asked of PSRP) | Yes | N/A | 2125 (91.6%) | 62 (57.9%) | 38 (47.5%) |
| No | N/A | 125 (5.4%) | 22 (20.6%) | 19 (23.8%) | |
| No answer 2 | N/A | 69 (3.0%) | 23 (21.5%) | 23 (28.8%) | |
| ARVs taken currently (if ARVs taken ever, Yes) | Yes | N/A | 2075 (97.6%) | 55 (88.7%) | 31 (81.6%) |
| No | N/A | 50 (2.4%) | 7 (11.3%) | 7 (18.4%) | |
| No answer 2 | N/A | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| ARVs detected 3 | Tested | N/A | 2274 | 65 | 38 |
| Detected | N/A | 1910 (84.0%) | 34 (52.3%) | 10 (26.3%) | |
| Not detected | N/A | 364 (16.0%) | 31 (47.7%) | 28 (73.7%) | |
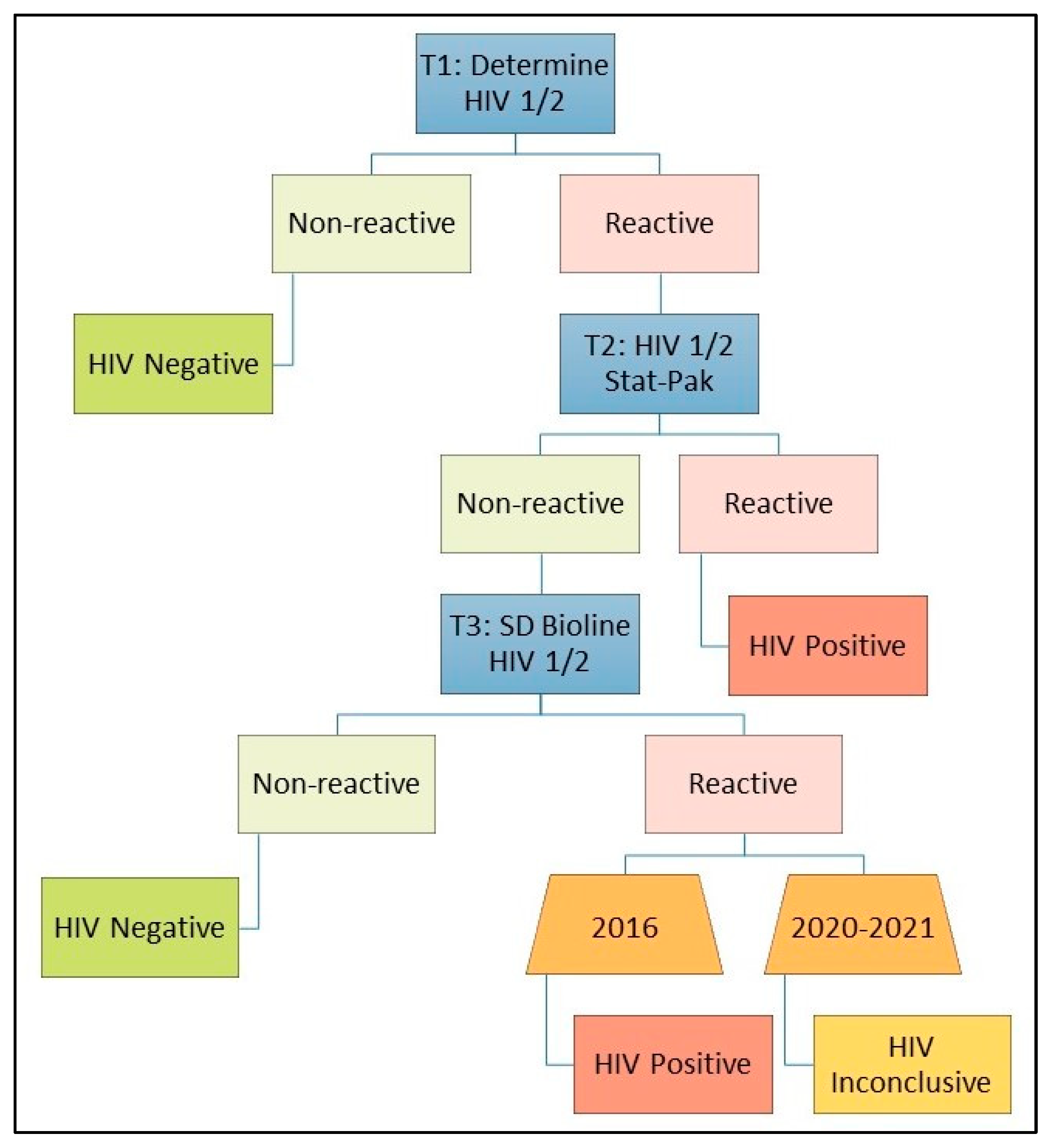
| Survey | PSRP | Participants with Non-Positive Results for Each Test n/N (%) | PSRP-CN among PSRP | PSRP-CN among PSRD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Household RTs | Quality Assurance RTs 1 | Geenius 2 | TNA PCR 3 | Western Blot 4 | Any Test (PSRD) | All Tests 5 | ||||
| Uganda 2016 | 1201 | 43/1201 (3.6%) | 41/1183 (3.5%) | N/A | 40/43 (93.0%) | N/A | 50/1201 (4.2%) | 36/1201 (3.0%) | 42/1201 (3.5%) | 42/50 (84.0%) |
| Uganda 2020 | 1081 | 46/1080 (4.3%) | 15/119 (12.6%) | 39/1081 (3.6%) | 36/44 (81.8%) | 14/17 (82.4%) | 49/1081 (4.5%) | 32/1081 (3.0%) | 33/1081 (3.1%) | 33/49 (67.3%) |
| Uganda Refugees 6 | 37 | 7/37 (18.9%) | 6/13 (46.2%) | 8/37 (21.6%) | 7/8 (87.5%) | N/A | 8/37 (21.6%) | 6/37 (16.2%) | 5/37 (13.5%) | 5/8 (62.5%) |
| Total | 2319 | 96/2318 (4.1%) | 62/1315 (4.7%) | 47/1118 (4.2%) | 83/95 (87.4%) | 14/17 (82.4%) | 107/2319 (4.6%) | 74/2319 (3.2%) | 80/2319 (3.4%) | 80/107 (74.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metz, M.; Among, V.H.; Dzinamarira, T.; Ussery, F.; Nkurunziza, P.; Bahizi, J.; Biraro, S.; Ogollah, F.M.; Musinguzi, J.; Kirungi, W.; et al. People Who Self-Reported Testing HIV-Positive but Tested HIV-Negative: A Multi-Country Puzzle of Data, Serology, and Ethics, 2015–2021. Trop. Med. Infect. Dis. 2024, 9, 220. https://doi.org/10.3390/tropicalmed9090220
Metz M, Among VH, Dzinamarira T, Ussery F, Nkurunziza P, Bahizi J, Biraro S, Ogollah FM, Musinguzi J, Kirungi W, et al. People Who Self-Reported Testing HIV-Positive but Tested HIV-Negative: A Multi-Country Puzzle of Data, Serology, and Ethics, 2015–2021. Tropical Medicine and Infectious Disease. 2024; 9(9):220. https://doi.org/10.3390/tropicalmed9090220
Chicago/Turabian StyleMetz, Melissa, Vivian Hope Among, Tafadzwa Dzinamarira, Faith Ussery, Peter Nkurunziza, Janet Bahizi, Samuel Biraro, Francis M. Ogollah, Joshua Musinguzi, Wilford Kirungi, and et al. 2024. "People Who Self-Reported Testing HIV-Positive but Tested HIV-Negative: A Multi-Country Puzzle of Data, Serology, and Ethics, 2015–2021" Tropical Medicine and Infectious Disease 9, no. 9: 220. https://doi.org/10.3390/tropicalmed9090220
APA StyleMetz, M., Among, V. H., Dzinamarira, T., Ussery, F., Nkurunziza, P., Bahizi, J., Biraro, S., Ogollah, F. M., Musinguzi, J., Kirungi, W., Naluguza, M., Mwangi, C., Birhanu, S., Nelson, L. J., Longwe, H., Winterhalter, F. S., Voetsch, A. C., Parekh, B. S., Patel, H. K., ... Farley, S. M. (2024). People Who Self-Reported Testing HIV-Positive but Tested HIV-Negative: A Multi-Country Puzzle of Data, Serology, and Ethics, 2015–2021. Tropical Medicine and Infectious Disease, 9(9), 220. https://doi.org/10.3390/tropicalmed9090220







