Effects of Five Years of Treatment of Onchocerciasis with Ivermectin under Community Guidelines in Resurgent Areas of Burkina Faso: A before-and-after Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design
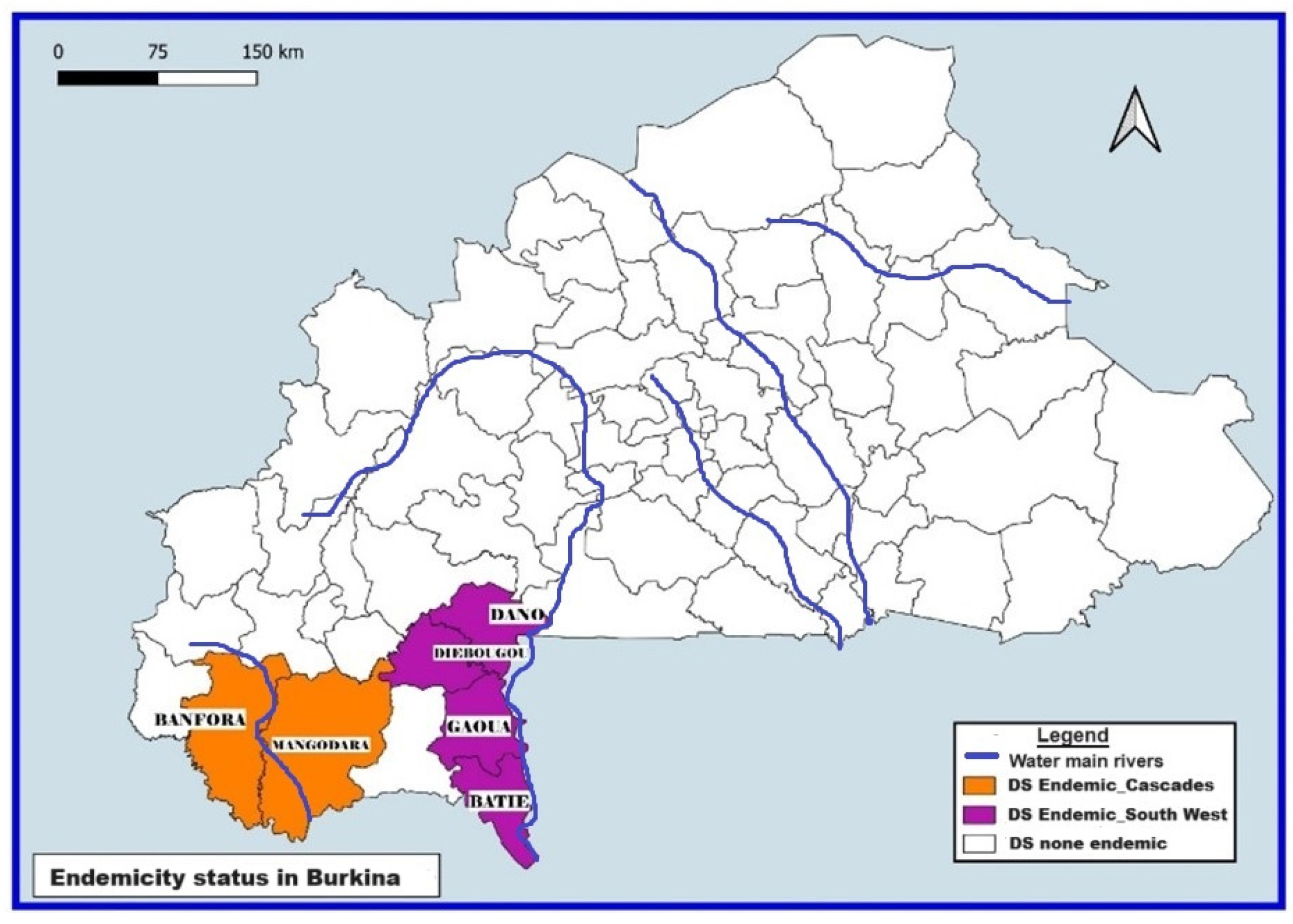
2.2. Study Setting
2.3. Implementation of the CDTI by the National Program for the Fight against NTDs in Burkina Faso
2.4. Data Sources: Epidemiological Surveys Implemented in the Framework of Onchocerciasis Surveillance
2.5. Study Population
2.6. Study Variables
2.7. Data Analysis
2.8. Ethical Considerations
3. Results
3.1. Description of the Sample
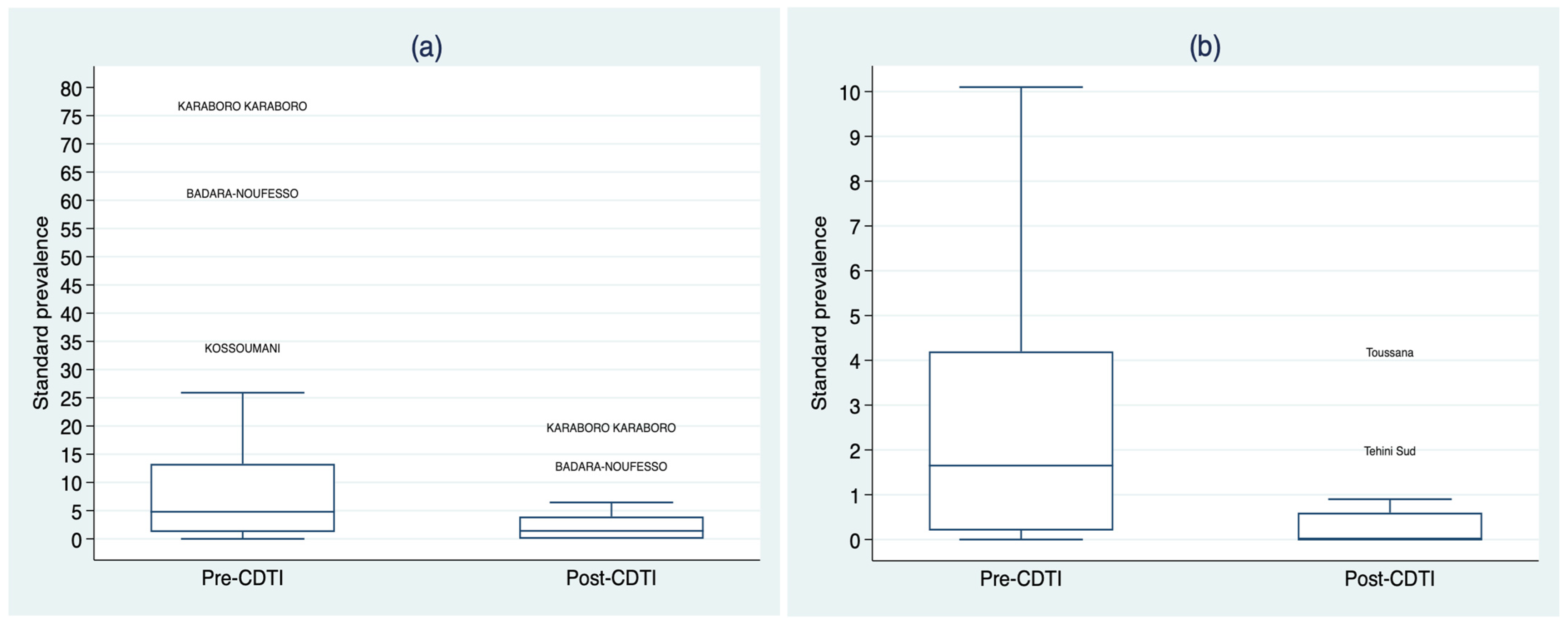
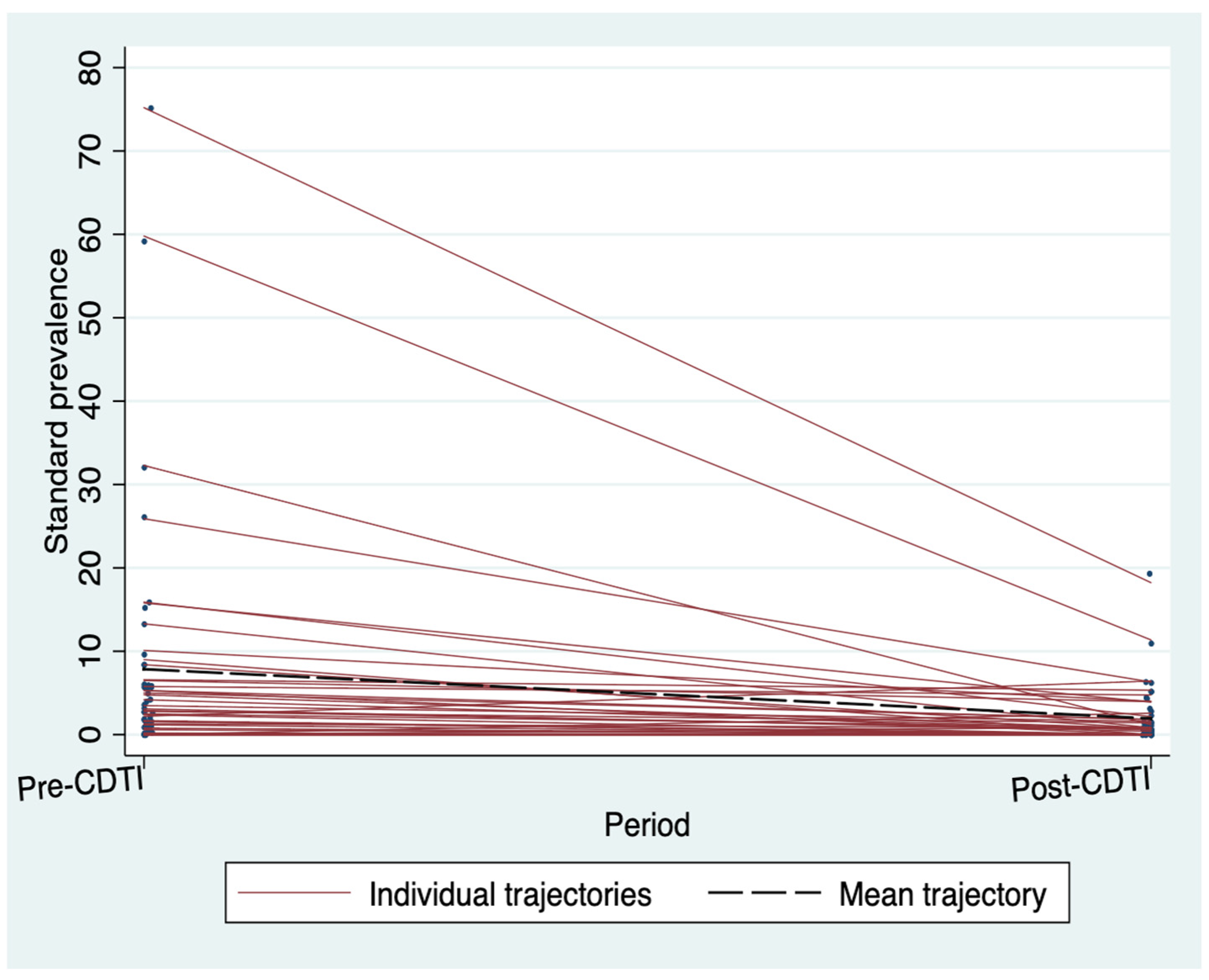
3.2. Standardized Microfilarodermia Prevalence before and after the CDTI
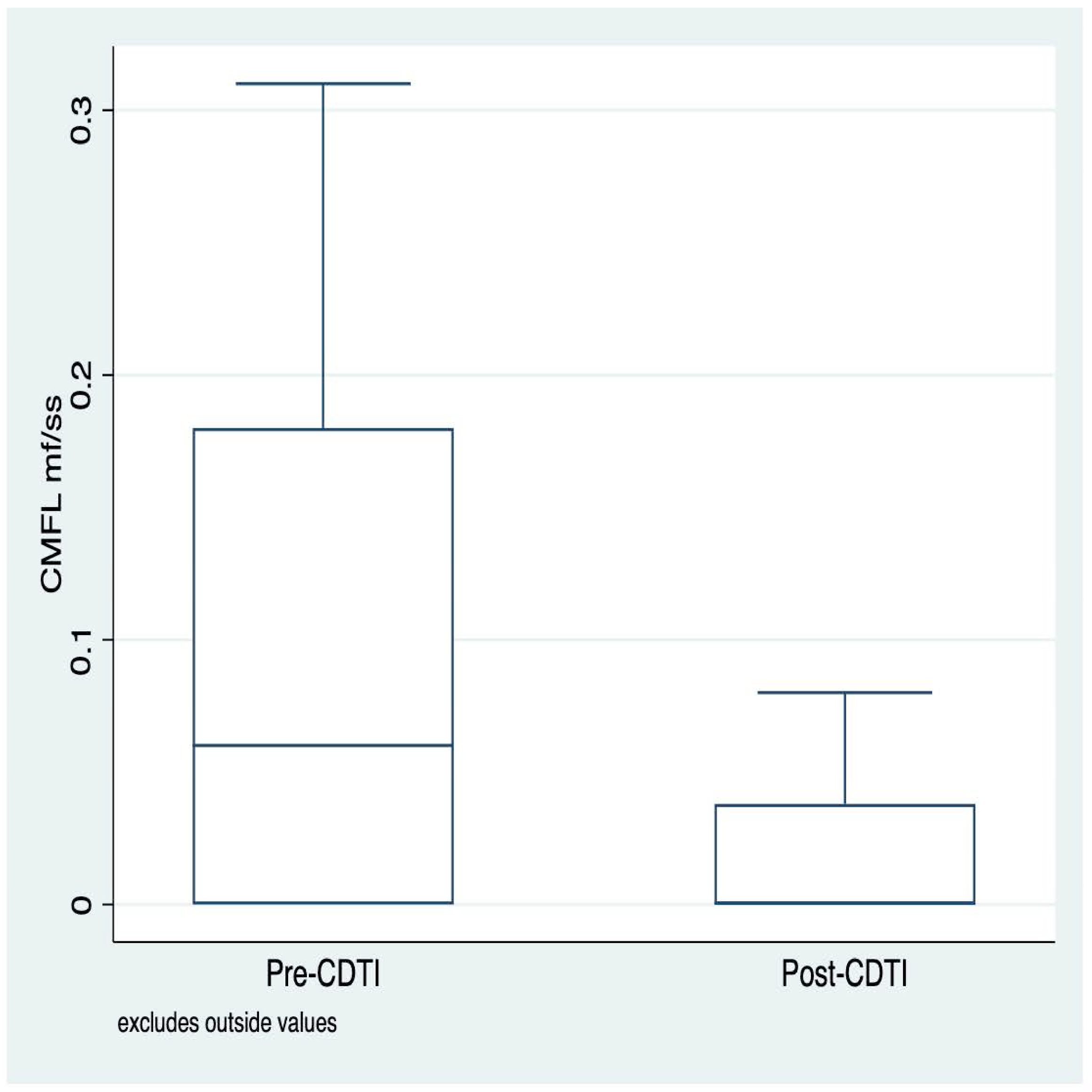
3.3. Distribution of Community Micofilarial Load (CMFL) before and after CDTI
3.4. The Effects of CDTI on Standardized Microfilaria Prevalence and CMFL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elimination of Human Onchocerciasis: Progress Report. 2021. Available online: https://www.who.int/publications/i/item/who-wer9746-591-598 (accessed on 19 December 2022).
- Controlling Neglected Tropical Diseases to Achieve the Sustainable Development Goals: A Sustainability Framework for Action on Neglected Tropical Diseases 2021–2030. Available online: https://www.who.int/fr/publications-detail/9789240019027 (accessed on 20 December 2022).
- World Health Organization. Integrating Neglected Tropical Diseases into the Global Health and Development Agenda: The Fourth WHO Report on Neglected Tropical Diseases; Policy Brief. Retrieved from Geneve; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Dadzie, Y.; Amazigo, U.V.; Boatin, B.A.; Sékétéli, A. Is onchocerciasis elimination in Africa feasible by 2025: A perspective based on lessons learned from the African control programmes. Infect. Dis. Poverty 2018, 7, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Cupp, E.; Sauerbrey, M.; Cama, V.; Eberhard, M.; Lammie, P.J.; Unnasch, T.R. Elimination of onchocerciasis in Africa by 2025: The need for a broad perspective. Infect. Dis. Poverty 2019, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Dadzie, Y.; Neira, M.; Hopkins, D. Final report of the Conference on the eradicability of Onchocerciasis. Filaria J. 2003, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Diawara, L.; Traoré, M.O.; Badji, A.; Bissan, Y.; Doumbia, K.; Goita, S.F.; Konaté, L.; Mounkoro, K.; Sarr, M.D.; Seck, A.F.; et al. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: First evidence from studies in Mali and Senegal. PLoS Negl. Trop. Dis. 2009, 3, e497. [Google Scholar] [CrossRef] [PubMed]
- Gebrezgabiher, G.; Mekonnen, Z.; Yewhalaw, D.; Hailu, A. Status of parasitological indicators and morbidity burden of onchocerciasis after years of successive implementation of mass distribution of ivermectin in selected communities of Yeki and Asosa districts, Ethiopia. BMC Public Health 2020, 20, 1233. [Google Scholar] [CrossRef] [PubMed]
- Tekle, A.H.; Zouré, H.G.; Noma, M.; Boussinesq, M.; Coffeng, L.E.; Stolk, W.A.; Remme, J.H. Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: Epidemiological evaluation results. Infect. Dis. Poverty 2016, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, A.; Achukwi, M.D.; Renz, A. Ongoing Transmission of Onchocerca volvulus after 25 Years of Annual Ivermectin Mass Treatments in the Vina du Nord River Valley, in North Cameroon. PLoS Negl. Trop. Dis. 2016, 10, e0004392. [Google Scholar] [CrossRef] [PubMed]
- Forrer, A.; Wanji, S.; Obie, E.D.; Nji, T.M.; Hamill, L.; Ozano, K.; Piotrowski, H.; Dean, L.; Njouendou, A.J.; Ekanya, R.; et al. Why onchocerciasis transmission persists after 15 annual ivermectin mass drug administrations in South-West Cameroon. BMJ Glob. Health 2021, 6, e003248. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.; Krit, M.; Pfarr, K.; Laemmer, C.; De Witte, J.; Nwane, P.; Kamgno, J.; Nana-Djeunga, H.C.; Boussinesq, M.; Dujardin, J.C.; et al. Onchocerca volvulus transmission in the Mbam valley of Cameroon following 16 years of annual community-directed treatment with ivermectin, and the description of a new cytotype of Simulium squamosum. Parasit. Vectors 2021, 14, 563. [Google Scholar] [CrossRef] [PubMed]
- Komlan, K.; Vossberg, P.S.; Gantin, R.G.; Solim, T.; Korbmacher, F.; Banla, M.; Padjoudoum, K.; Karabou, P.; Köhler, C.; Soboslay, P.T. Onchocerca volvulus infection and serological prevalence, ocular onchocerciasis and parasite transmission in northern and central Togo after decades of Simulium damnosum s.l. vector control and mass drug administration of ivermectin. PLoS Negl. Trop. Dis. 2018, 12, e0006312. [Google Scholar] [CrossRef] [PubMed]
- Nikièma, A.S.; Koala, L.; Post, R.J.; Paré, A.B.; Kafando, C.M.; Drabo, F.; Belem, A.M.; Dabiré, R.K.; Traoré, S. Onchocerciasis prevalence, human migration and risks for onchocerciasis elimination in the Upper Mouhoun, Nakambé and Nazinon river basins in Burkina Faso. Acta Trop. 2018, 185, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Koala, L.; Nikiema, A.; Post, R.J.; Paré, A.B.; Kafando, C.M.; Drabo, F.; Traoré, S. Recrudescence of onchocerciasis in the Comoé valley in Southwest Burkina Faso. Acta Trop. 2017, 166, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Koala, L.; Nikièma, A.S.; Paré, A.B.; Drabo, F.; Toé, L.D.; Belem, A.M.; Boakye, D.A.; Traoré, S.; Dabiré, R.K. Entomological assessment of the transmission following recrudescence of onchocerciasis in the Comoé Valley, Burkina Faso. Parasits Vectors 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.; Belay, T.; Yehalaw, D.; Taha, M.; Zemene, E.; Zeynudin, A. Impact of six years community directed treatment with ivermectin in the Control of Onchocerciasis, western Ethiopia. PLoS ONE. 2016, 11, e0141029. [Google Scholar] [CrossRef] [PubMed]
- Opara, K.N.; Fagbemi, B.O.; Atting, I.A.; Oyene, U.E.; Okenu, D.M.N. Status of forest onchocerciasis in the Lower Cross River Basin, Nigeria: Change in clinical and parasitological indices after 6 years of ivermectin intervention. Public Health 2007, 121, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ministère de l’économie, des finances et de la prospective. Journal Burkinabè de la Statistique: Trimestriel d’Information du Sys-tème Statistique National. Available online: https://www.insd.bf/sites/default/files/journal-2023-07/JBS%20009%20T2%20INSD.pdf (accessed on 8 August 2024).
- Institut National de la Statistique et de la Démographie. Cinquieme Recensement General de la Population et de l’habitat du Burkina Faso, Synthèse des Résultats Définitifs; Institut National de la Statistique et de la Démographie: Ouagadougou, Burkina Faso, 2022. [Google Scholar]
- Etude de l’évolution Climatique au Burkina Faso de 1983 a 2012: Cas des Villes de Bobo Dioulasso, Ouagadougou et Dori. Available online: https://www.researchgate.net/publication/323068909_etude_de_l’évolution_climatique_au_Burkina_Faso_de_1983_a_2012_cas_des_villes_de_Bobo_Dioulasso_Ouagadou_et_Dori (accessed on 20 December 2022).
- Ministère de la santé du Burkina Faso. Plan Stratégique de lutte Contre les Maladies Tropicales Négligées 2016–2020, Burkina Faso; Ministère de la santé: Burkina Faso, Ouagadougou, 2016. [Google Scholar]
- Ministère de la santé du Burkina Faso. Rapport Annuel D’activités 2010 du Programme National de lutte Contre L’onchocercose; Ministère de la santé: Burkina Faso, Ouagadougou, 2010. [Google Scholar]
- Ministère de la santé du Burkina Faso. Actualisation de la Cartographie de L’onchocercose au Burkina Faso; Ministère de la santé: Burkina Faso, Ouagadougou, 2011. [Google Scholar]
- Ministère de la santé du Burkina Faso. Rapport de L’évaluation épidémiologique de L’onchocercose dans la région des Cascades; Ministère de la santé: Burkina Faso, Ouagadougou, 2016. [Google Scholar]
- Ministère de la santé du Burkina Faso. Rapport de L’étude D’Impact de cinq Années de Traitement de L’ivermectine Contre L’onchocercose sur les Indicateurs Parasitologiques Enregistrés chez les Populations Vivant dans les Villages Situés le Long des Fleuves Bougouriba et Mouhoun (Région du Sud-Ouest, Burkina Faso); Ministère de la santé: Burkina Faso, Ouagadougou, 2018. [Google Scholar]
- Control of Neglected Tropical Diseases (NTD), Global Onchocerciasis Network for Elimination (GONE), Guidelines Review Committee. Guidelines for Stopping Mass Drug Administration and Verifying Elimination of Human Onchocerciasis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Organisation mondiale de la santé. Dix Années de lutte Contre L’onchocercose en Afrique de L’Ouest; Organisation mondiale de la santé: Geneva, Switzerland, 1985. [Google Scholar]
- Nikièma, A.S.; Koala, L.; Sondo, A.K.; Post, R.J.; Paré, A.B.; Kafando, C.M.; Kambiré, R.S.; Sow, B.; Bougouma, C.; Dabiré, R.K.; et al. The impact of ivermectin on onchocerciasis in villages co-endemic for lymphatic filariasis in an area of onchocerciasis recrudescence in Burkina Faso. PLoS Negl. Trop. Dis. 2021, 15, e0009117. [Google Scholar] [CrossRef] [PubMed]
- Cupp, E.W.; Sauerbrey, M.; Richards, F. Elimination of human onchocerciasis: History of progress and current feasibility using ivermectin (Mectizan®) monotherapy. Acta Trop. 2011, 120, S100–S108. [Google Scholar] [CrossRef] [PubMed]
- Zarroug, I.M.; Hashim, K.; ElMubark, W.A.; Shumo, Z.A.; Salih, K.A.; ElNojomi, N.A.; Awad, H.A.; Aziz, N.; Katabarwa, M.; Hassan, H.K.; et al. The First Confirmed Elimination of an Onchocerciasis Focus in Africa: Abu Hamed, Sudan. Am. J. Trop. Med. Hyg. 2016, 95, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Boatin, B. The onchocerciasis Control Program in West Africa (OCP). Ann. Trop. Med. Parasitol. 2008, 102 (Suppl. S1), 13–17. [Google Scholar] [CrossRef] [PubMed]
| Region | Health District | Number of OV Endemic Villages | Number of Villages Selected for Survey |
|---|---|---|---|
| Cascades | Banfora | 103 | 20 |
| Mangodara | 20 | 5 | |
| Southwest | Batié | 111 | 8 |
| Dano | 108 | 3 | |
| Diébougou | 47 | 5 | |
| Gaoua | 11 | 2 |
| Pre-CDTI Period | Post-CDTI Period | p-Value * | |
|---|---|---|---|
| Cascades and Southwest (N. of villages) | 43 | 43 | |
| Standardized microfilarodermia prevalence | 2.8 (0.2–6.6) | 0.72 (0.0–2.17) | <0.0001 |
| Community microfilarial load | 0.06 (0.0–0.18) | 0.0005 (0.0–0.038) | <0.0001 |
| Cascades (N. of villages) | 25 | 25 | |
| Standardized microfilarodermia prevalence | 4.8 (1.2–13.3) | 1.41 (0.0–3.96) | 0.0008 |
| Community microfilarial load | 0.08 (0.0–0.23) | 0.021 (0.0–0.054) | 0.0001 |
| Southwest (N. of villages) | 18 | 18 | |
| Standardized microfilarodermia prevalence | 1.65 (0.2–4.2) | 0.0 (0.0–0.6) | 0.0011 |
| Community microfilarial load | 0.04 (0.0–0.14) | 0.0 (0.0–0.01) | 0.0009 |
| Less than 5 km from river (N. of villages) | 32 | 32 | |
| Standardized microfilarodermia prevalence | 3.85 (1.0–9.55) | 0.77 (0.0–3.035) | <0.0001 |
| Community microfilarial load | 0.075 (0.015–0.235) | 0.005 (0.0–0.044) | <0.0001 |
| 5 km and more from river (N. of villages) | 11 | 11 | |
| Standardized microfilarodermia prevalence | 2.5 (0.0–3.1) | 0.6 (0.0–1.41) | 0.0816 |
| Community microfilarial load | 0.04 (0.0–0.08) | 0.0005 (0.0–0.028) | 0.0542 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouedraogo, M.O.; Meda, I.B.; Kourouma, K.; Wienne, F.Y.; Nare, D.; Bougouma, C.; Compaore, J.; Kouanda, S. Effects of Five Years of Treatment of Onchocerciasis with Ivermectin under Community Guidelines in Resurgent Areas of Burkina Faso: A before-and-after Analysis. Trop. Med. Infect. Dis. 2024, 9, 207. https://doi.org/10.3390/tropicalmed9090207
Ouedraogo MO, Meda IB, Kourouma K, Wienne FY, Nare D, Bougouma C, Compaore J, Kouanda S. Effects of Five Years of Treatment of Onchocerciasis with Ivermectin under Community Guidelines in Resurgent Areas of Burkina Faso: A before-and-after Analysis. Tropical Medicine and Infectious Disease. 2024; 9(9):207. https://doi.org/10.3390/tropicalmed9090207
Chicago/Turabian StyleOuedraogo, Micheline O., Ivlabèhirè Bertrand Meda, Karifa Kourouma, Fanny Yago Wienne, Dieudonné Nare, Clarisse Bougouma, Justin Compaore, and Seni Kouanda. 2024. "Effects of Five Years of Treatment of Onchocerciasis with Ivermectin under Community Guidelines in Resurgent Areas of Burkina Faso: A before-and-after Analysis" Tropical Medicine and Infectious Disease 9, no. 9: 207. https://doi.org/10.3390/tropicalmed9090207
APA StyleOuedraogo, M. O., Meda, I. B., Kourouma, K., Wienne, F. Y., Nare, D., Bougouma, C., Compaore, J., & Kouanda, S. (2024). Effects of Five Years of Treatment of Onchocerciasis with Ivermectin under Community Guidelines in Resurgent Areas of Burkina Faso: A before-and-after Analysis. Tropical Medicine and Infectious Disease, 9(9), 207. https://doi.org/10.3390/tropicalmed9090207







