Suitable Mouse Model to Study Dynamics of West Nile Virus Infection in Culex quinquefasciatus Mosquitoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice Lineages
2.2. Mosquito Lineages and Mosquito Rearing
2.3. Virus Propagation and Titration
2.4. Mice Inoculation with WNV
2.5. Mosquito Infection with WNV
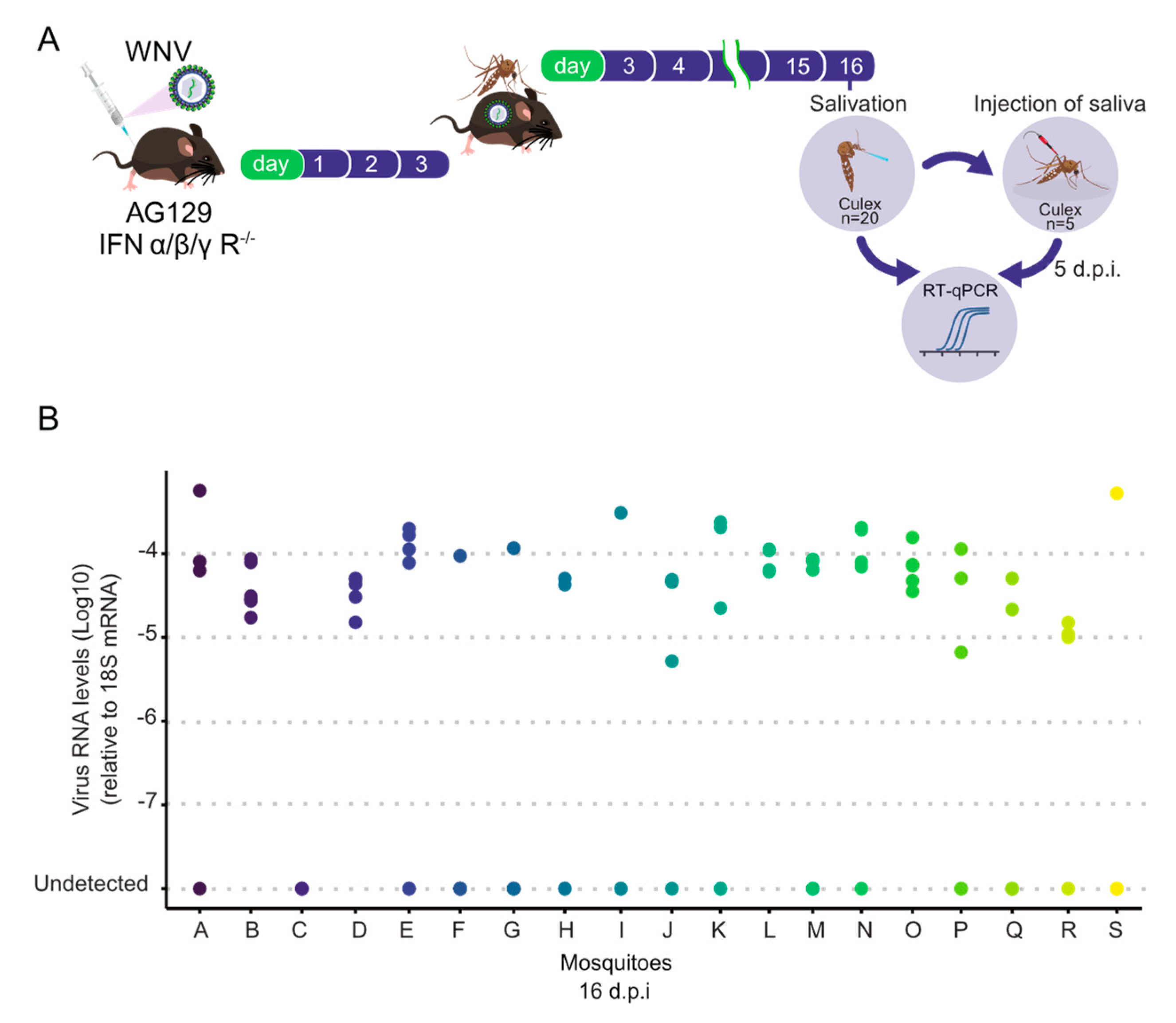
2.6. Mosquito Salivation and Saliva Infectivity Test
2.7. RNA Extraction and RT-qPCR
2.8. WNV Immunostaining and Microscopy
3. Results
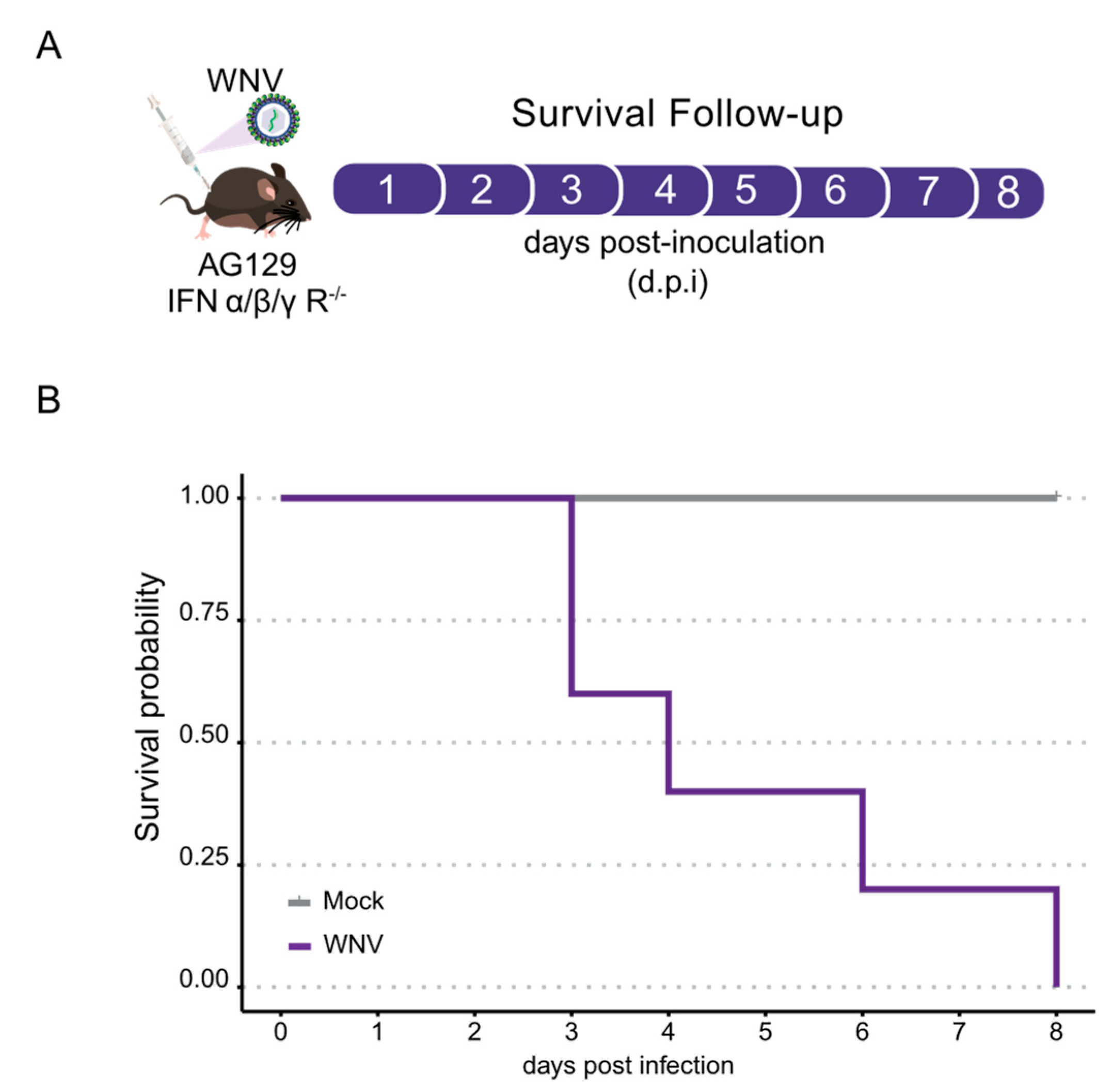
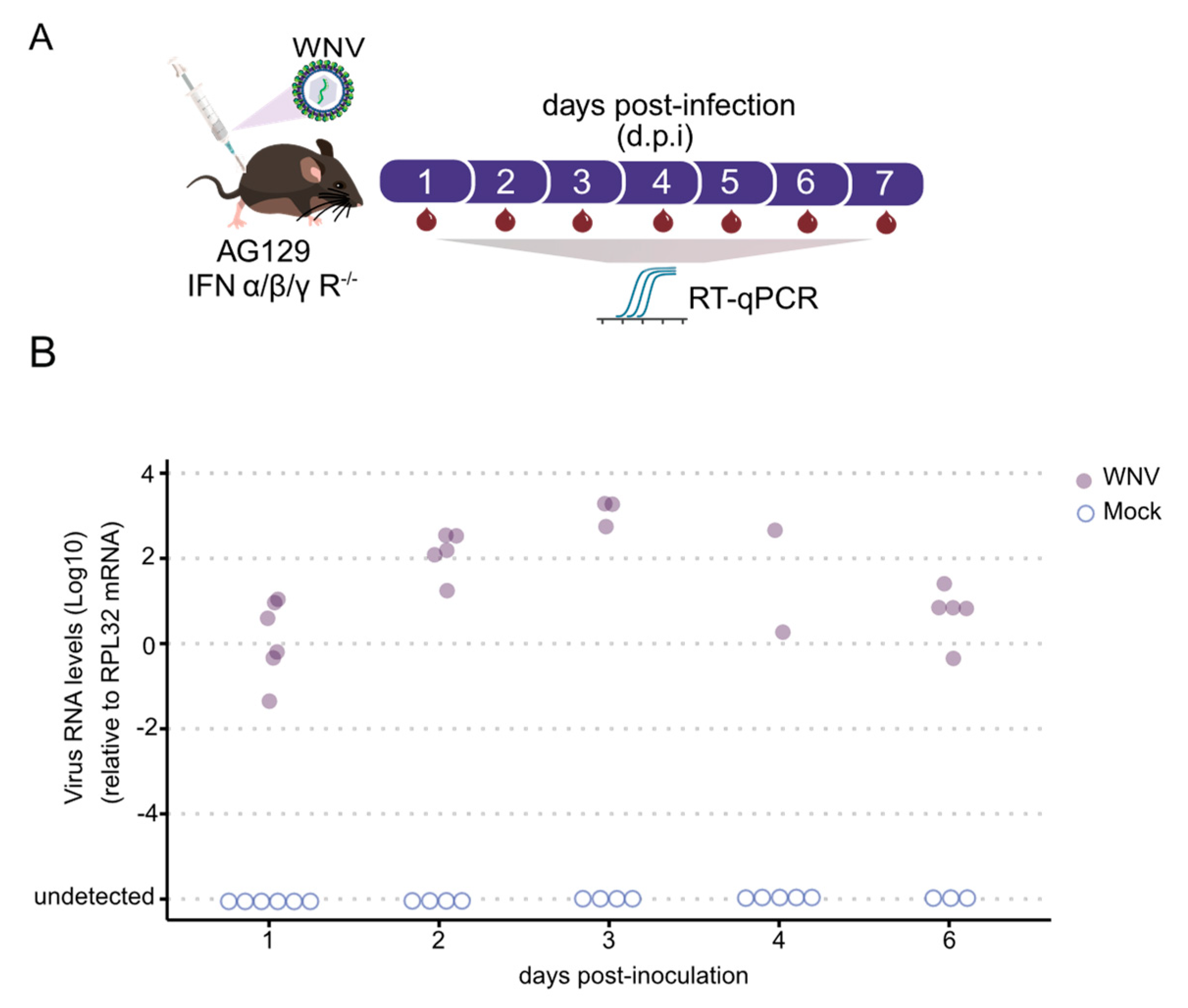
3.1. AG129 Mice Exhibit High Susceptibility to WNV Infection
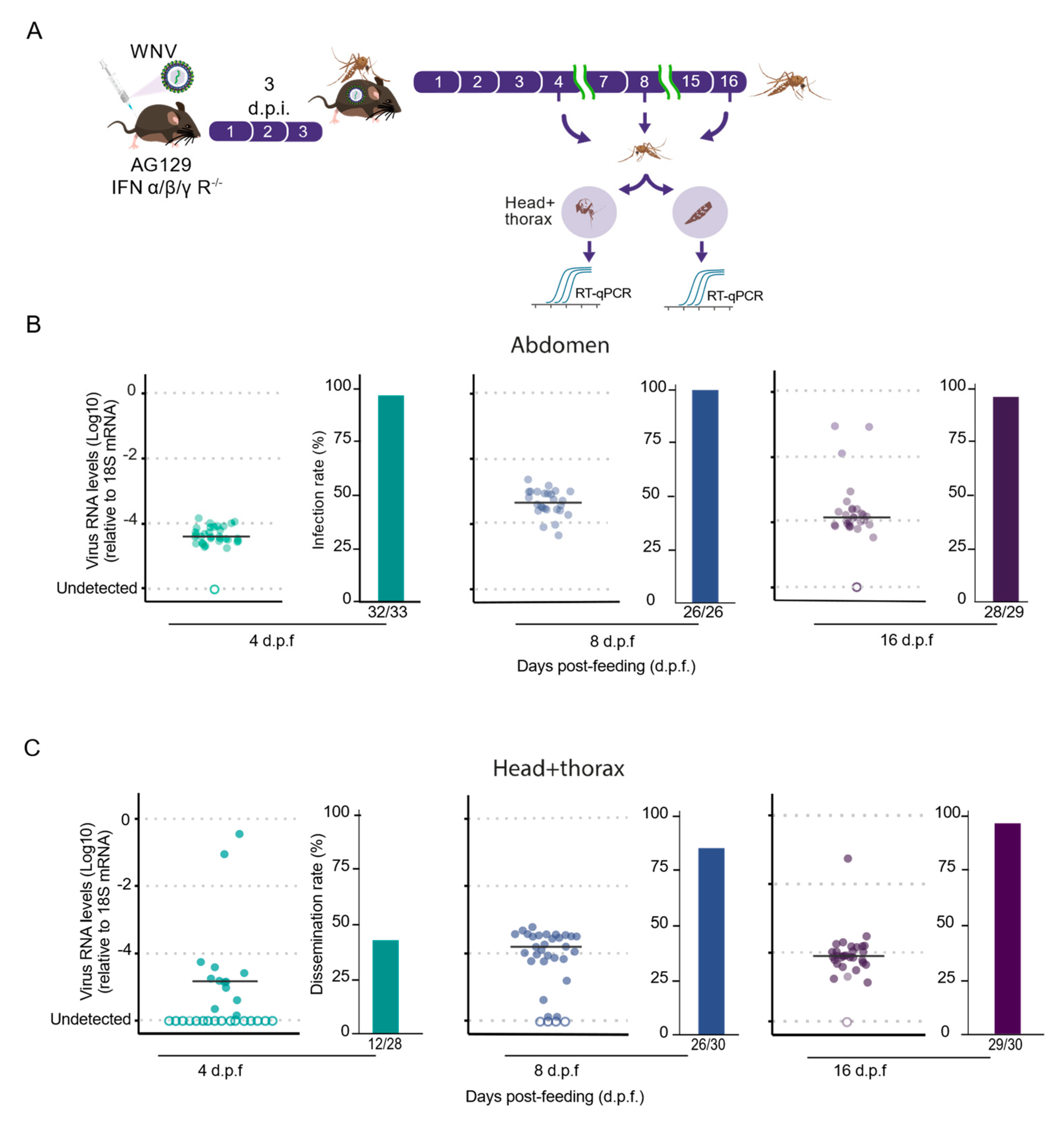
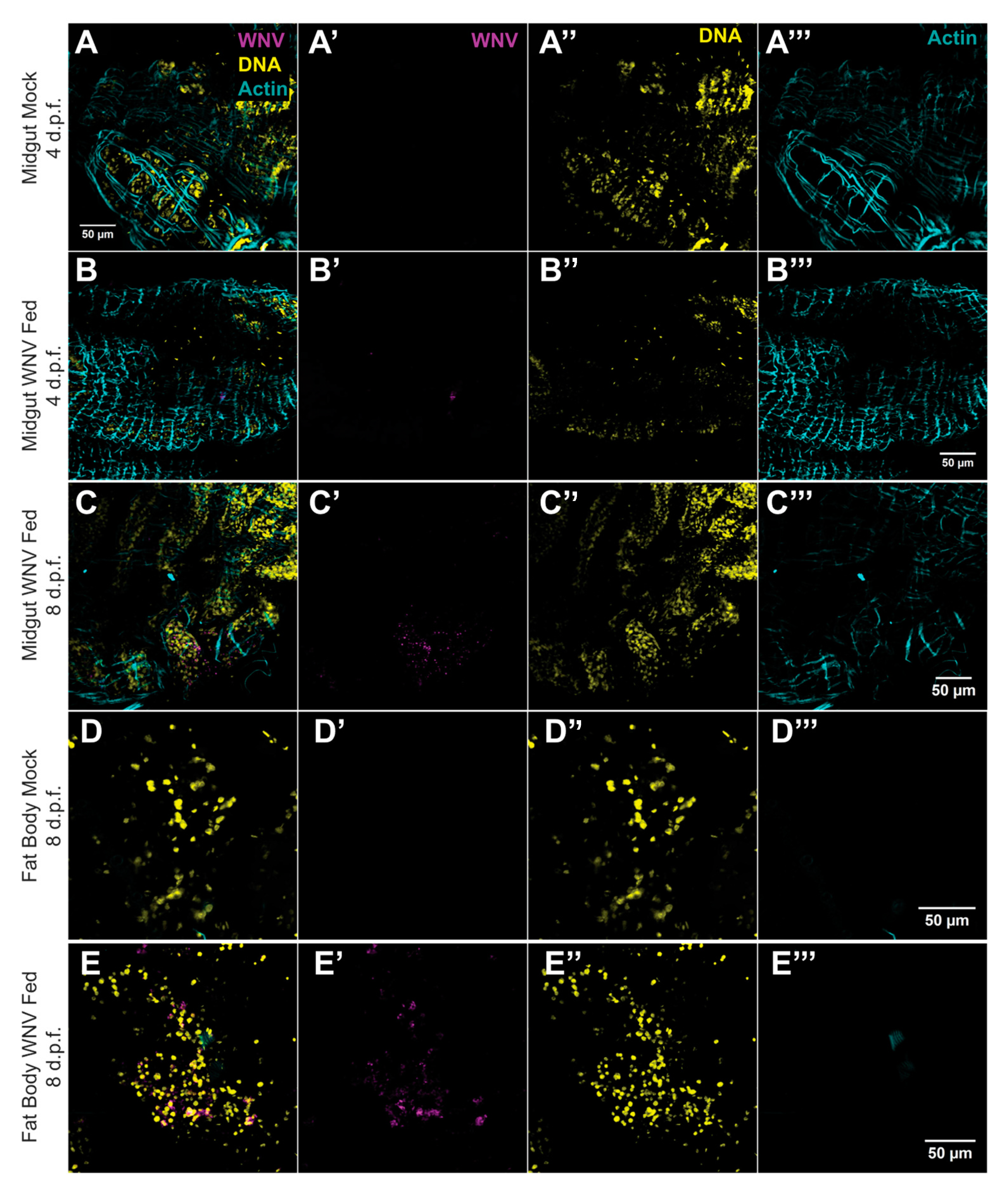
3.2. AG129 Mice Are Capable of Infecting Culex quinquefasciatus Mosquitoes with WNV through Blood Meals
3.3. High Transmission Efficiency of WNV by Culex quinquefasciatus Mosquitoes Following Blood Feeding on AG129 Infectious Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, H.; Sander, B.; Nelder, M.P. Long-Term Sequelae of West Nile Virus-Related Illness: A Systematic Review. Lancet Infect. Dis. 2015, 15, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Barrett, A.D.T. Transmission Cycles, Host Range, Evolution and Emergence of Arboviral Disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Ndione, M.H.D.; Silverj, A.; Diagne, M.M.; Curini, V.; Teodori, L.; Di Domenico, M.; Mbaye, R.; Leone, A.; Marcacci, M.; et al. Spatial and Temporal Dynamics of West Nile Virus between Africa and Europe. Nat. Commun. 2023, 14, 6440. [Google Scholar] [CrossRef] [PubMed]
- Erazo, D.; Grant, L.; Ghisbain, G.; Marini, G.; Colón-González, F.J.; Wint, W.; Rizzoli, A.; Van Bortel, W.; Vogels, C.B.F.; Grubaugh, N.D.; et al. Contribution of Climate Change to the Spatial Expansion of West Nile Virus in Europe. Nat. Commun. 2024, 15, 1196. [Google Scholar] [CrossRef] [PubMed]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-Year Historical Review of West Nile Virus since Its Initial Emergence in North America: Has West Nile Virus Become a Neglected Tropical Disease? PLoS Neglected Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef]
- Lorenz, C.; Chiaravalloti-Neto, F. Why Are There No Human West Nile Virus Outbreaks in South America? Lancet Reg. Health Am. 2022, 12, 100276. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A Structural Perspective of the Flavivirus Life Cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Samuel, M.A.; Diamond, M.S. Pathogenesis of West Nile Virus Infection: A Balance between Virulence, Innate and Adaptive Immunity, and Viral Evasion. J. Virol. 2006, 80, 9349–9360. [Google Scholar] [CrossRef]
- Kramer, L.D.; Li, J.; Shi, P.-Y. West Nile virus. Lancet Neurol. 2007, 6, 171–181. [Google Scholar] [CrossRef]
- Brinton, M.A. The Molecular Biology of West Nile Virus: A New Invader of the Western Hemisphere. Annu. Rev. Microbiol. 2002, 56, 371–402. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: Biology, Transmission, and Human Infection. Clin. Microbiol. Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef]
- Kramer, L.D.; Ciota, A.T.; Kilpatrick, A.M. Introduction, Spread, and Establishment of West Nile Virus in the Americas. J. Med. Entomol. 2019, 56, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W. Ecology of West Nile Virus in North America. Viruses 2013, 5, 2079–2105. [Google Scholar] [CrossRef]
- Komar, N. West Nile Virus: Epidemiology and Ecology in North America. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2003; Volume 61, pp. 185–234. ISBN 978-0-12-039861-4. [Google Scholar]
- Turell, M.J.; O’Guinn, M.L.; Dohm, D.J.; Jones, J.W. Vector Competence of North American Mosquitoes (Diptera: Culicidae) for West Nile Virus. J. Med. Entomol. 2001, 38, 130–134. [Google Scholar] [CrossRef]
- Godsey, M.S.; Nasci, R.; Savage, H.M.; Aspen, S.; King, R.; Powers, A.M.; Burkhalter, K.; Colton, L.; Charnetzky, D.; Lasater, S.; et al. West Nile Virus–Infected Mosquitoes, Louisiana, 2002. Emerg. Infect. Dis. 2005, 11, 1399–1404. [Google Scholar] [CrossRef]
- Hartemink, N.A.; Davis, S.A.; Reiter, P.; Hubálek, Z.; Heesterbeek, J.A.P. Importance of Bird-to-Bird Transmission for the Establishment of West Nile Virus. Vector-Borne Zoonotic Dis. 2007, 7, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Fulton, C.D.M.; Beasley, D.W.C.; Bente, D.A.; Dineley, K.T. Long-Term, West Nile Virus-Induced Neurological Changes: A Comparison of Patients and Rodent Models. Brain Behav. Immun. Health 2020, 7, 100105. [Google Scholar] [CrossRef]
- Liu, W.; Tang, D.; Xu, X.-X.; Liu, Y.-J.; Jiu, Y. How Physical Factors Coordinate Virus Infection: A Perspective From Mechanobiology. Front. Bioeng. Biotechnol. 2021, 9, 764516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, Y.; Ling, Y.; Lu, G.; Liu, F.; Yi, Z.; Jia, X.; Wu, M.; Shi, B.; Xu, S.; et al. Viral and Host Factors Related to the Clinical Outcome of COVID-19. Nature 2020, 583, 437–440. [Google Scholar] [CrossRef]
- Hsu, T.-H.; Spindler, K.R. Identifying Host Factors That Regulate Viral Infection. PLoS Pathog. 2012, 8, e1002772. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Thi Hue Kien, D.; Tuan, T.V.; Quyen, N.T.H.; Tran, C.N.B.; Vo Thi, L.; Thi, D.L.; Nguyen, H.L.; Farrar, J.J.; Holmes, E.C.; et al. Host and Viral Features of Human Dengue Cases Shape the Population of Infected and Infectious Aedes Aegypti Mosquitoes. Proc. Natl. Acad. Sci. USA 2013, 110, 9072–9077. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, R.; Zhang, B.; Zhao, T.; Wang, P.; Liang, G.; Cheng, G. Blood Meal Acquisition Enhances Arbovirus Replication in Mosquitoes through Activation of the GABAergic System. Nat. Commun. 2017, 8, 1262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tong, L.; Nie, K.; Wiwatanaratanabutr, I.; Sun, P.; Li, Q.; Yu, X.; Wu, P.; Wu, T.; Yu, C.; et al. Host Serum Iron Modulates Dengue Virus Acquisition by Mosquitoes. Nat. Microbiol. 2019, 4, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Wagar, Z.L.; Tree, M.O.; Mpoy, M.C.; Conway, M.J. Low Density Lipopolyprotein Inhibits Flavivirus Acquisition in Aedes aegypti. Insect Mol. Biol. 2017, 26, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Neglected Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef]
- Sumathy, K.; Kulkarni, B.; Gondu, R.K.; Ponnuru, S.K.; Bonguram, N.; Eligeti, R.; Gadiyaram, S.; Praturi, U.; Chougule, B.; Karunakaran, L.; et al. Protective Efficacy of Zika Vaccine in AG129 Mouse Model. Sci. Rep. 2017, 7, 46375. [Google Scholar] [CrossRef]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Atkinson, B.; Hall, G.; Watson, R.J.; Bosworth, A.; Bonney, L.C.; Kitchen, S.; Hewson, R. A Susceptible Mouse Model for Zika Virus Infection. PLoS Neglected Trop. Dis. 2016, 10, e0004658. [Google Scholar] [CrossRef]
- Meier, K.C.; Gardner, C.L.; Khoretonenko, M.V.; Klimstra, W.B.; Ryman, K.D. A Mouse Model for Studying Viscerotropic Disease Caused by Yellow Fever Virus Infection. PLoS Pathog. 2009, 5, e1000614. [Google Scholar] [CrossRef]
- Metz, S.W.; Martina, B.E.; Van Den Doel, P.; Geertsema, C.; Osterhaus, A.D.; Vlak, J.M.; Pijlman, G.P. Chikungunya Virus-like Particles Are More Immunogenic in a Lethal AG129 Mouse Model Compared to Glycoprotein E1 or E2 Subunits. Vaccine 2013, 31, 6092–6096. [Google Scholar] [CrossRef]
- Campos, R.K.; Preciado-Llanes, L.; Azar, S.R.; Kim, Y.C.; Brandon, O.; López-Camacho, C.; Reyes-Sandoval, A.; Rossi, S.L. Adenoviral-Vectored Mayaro and Chikungunya Virus Vaccine Candidates Afford Partial Cross-Protection From Lethal Challenge in A129 Mouse Model. Front. Immunol. 2020, 11, 591885. [Google Scholar] [CrossRef]
- Boylan, B.T.; Moreira, F.R.; Carlson, T.W.; Bernard, K.A. Mosquito Cell-Derived West Nile Virus Replicon Particles Mimic Arbovirus Inoculum and Have Reduced Spread in Mice. PLoS Neglected Trop. Dis. 2017, 11, e0005394. [Google Scholar] [CrossRef]
- Aubry, F.; Dabo, S.; Manet, C.; Filipović, I.; Rose, N.H.; Miot, E.F.; Martynow, D.; Baidaliuk, A.; Merkling, S.H.; Dickson, L.B.; et al. Enhanced Zika Virus Susceptibility of Globally Invasive Aedes Aegypti Populations. Science 2020, 370, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.S.; Higgs, S. The Enhancement of Arbovirus Transmission and Disease by Mosquito Saliva Is Associated with Modulation of the Host Immune Response. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 400–408. [Google Scholar] [CrossRef]
- Billingsley, P.F.; Baird, J.; Mitchell, J.A.; Drakeley, C. Immune Interactions between Mosquitoes and Their Hosts. Parasite Immunol. 2006, 28, 143–153. [Google Scholar] [CrossRef]
- Wanasen, N.; Nussenzveig, R.H.; Champagne, D.E.; Soong, L.; Higgs, S. Differential Modulation of Murine Host Immune Response by Salivary Gland Extracts from the Mosquitoes Aedes aegypti and Culex quinquefasciatus. Med. Vet Entomol. 2004, 18, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Higgs, L.; Beaty, P. Potentiation of Vesicular Stomatitis New Jersey Virus Infection in Mice by Mosquito Saliva. Parasite Immunol. 2000, 22, 461–467. [Google Scholar] [CrossRef]
- Baldon, L.V.R.; De Mendonça, S.F.; Ferreira, F.V.; Rezende, F.O.; Amadou, S.C.G.; Leite, T.H.J.F.; Rocha, M.N.; Marques, J.T.; Moreira, L.A.; Ferreira, A.G.A. AG129 Mice as a Comprehensive Model for the Experimental Assessment of Mosquito Vector Competence for Arboviruses. Pathogens 2022, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Richards, S.L.; Smartt, C.T. A Simple Method for Determining Arbovirus Transmission in Mosquitoes. J. Am. Mosq. Control. Assoc. 2010, 26, 108–111. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA Using Real-Time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Ruiz-Villalba, A.; Ruijter, J.M.; Van Den Hoff, M.J.B. Use and Misuse of Cq in qPCR Data Analysis and Reporting. Life 2021, 11, 496. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Rajamanonmani, R.; Nkenfou, C.; Clancy, P.; Yau, Y.H.; Shochat, S.G.; Sukupolvi-Petty, S.; Schul, W.; Diamond, M.S.; Vasudevan, S.G.; Lescar, J. On a Mouse Monoclonal Antibody That Neutralizes All Four Dengue Virus Serotypes. J. Gen. Virol. 2009, 90, 799–809. [Google Scholar] [CrossRef]
- Gloria-Soria, A.; Brackney, D.E.; Armstrong, P.M. Saliva Collection via Capillary Method May Underestimate Arboviral Transmission by Mosquitoes. Parasites Vectors 2022, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.N.; Carvalho, F.D.; De Mendonça, S.F.; Rocha, M.N.; Moreira, L.A. Vector Competence of Aedes Aegypti, Aedes Albopictus, and Culex Quinquefasciatus Mosquitoes for Mayaro Virus. PLoS Neglected Trop. Dis. 2020, 14, e0007518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldon, L.; de Mendonça, S.; Santos, E.; Marçal, B.; de Freitas, A.C.; Rezende, F.; Moreira, R.; Sousa, V.; Comini, S.; Lima, M.; et al. Suitable Mouse Model to Study Dynamics of West Nile Virus Infection in Culex quinquefasciatus Mosquitoes. Trop. Med. Infect. Dis. 2024, 9, 201. https://doi.org/10.3390/tropicalmed9090201
Baldon L, de Mendonça S, Santos E, Marçal B, de Freitas AC, Rezende F, Moreira R, Sousa V, Comini S, Lima M, et al. Suitable Mouse Model to Study Dynamics of West Nile Virus Infection in Culex quinquefasciatus Mosquitoes. Tropical Medicine and Infectious Disease. 2024; 9(9):201. https://doi.org/10.3390/tropicalmed9090201
Chicago/Turabian StyleBaldon, Lívia, Silvana de Mendonça, Ellen Santos, Bruno Marçal, Amanda Cupertino de Freitas, Fernanda Rezende, Rafaela Moreira, Viviane Sousa, Sara Comini, Mariana Lima, and et al. 2024. "Suitable Mouse Model to Study Dynamics of West Nile Virus Infection in Culex quinquefasciatus Mosquitoes" Tropical Medicine and Infectious Disease 9, no. 9: 201. https://doi.org/10.3390/tropicalmed9090201
APA StyleBaldon, L., de Mendonça, S., Santos, E., Marçal, B., de Freitas, A. C., Rezende, F., Moreira, R., Sousa, V., Comini, S., Lima, M., Ferreira, F., de Almeida, J. P., Silva, E., Amadou, S., Rocha, M., Leite, T., Todjro, Y., de Carvalho, C., Santos, V., ... Ferreira, A. (2024). Suitable Mouse Model to Study Dynamics of West Nile Virus Infection in Culex quinquefasciatus Mosquitoes. Tropical Medicine and Infectious Disease, 9(9), 201. https://doi.org/10.3390/tropicalmed9090201






