Advances in Malaria Diagnostic Methods in Resource-Limited Settings: A Systematic Review
Abstract
1. Introduction
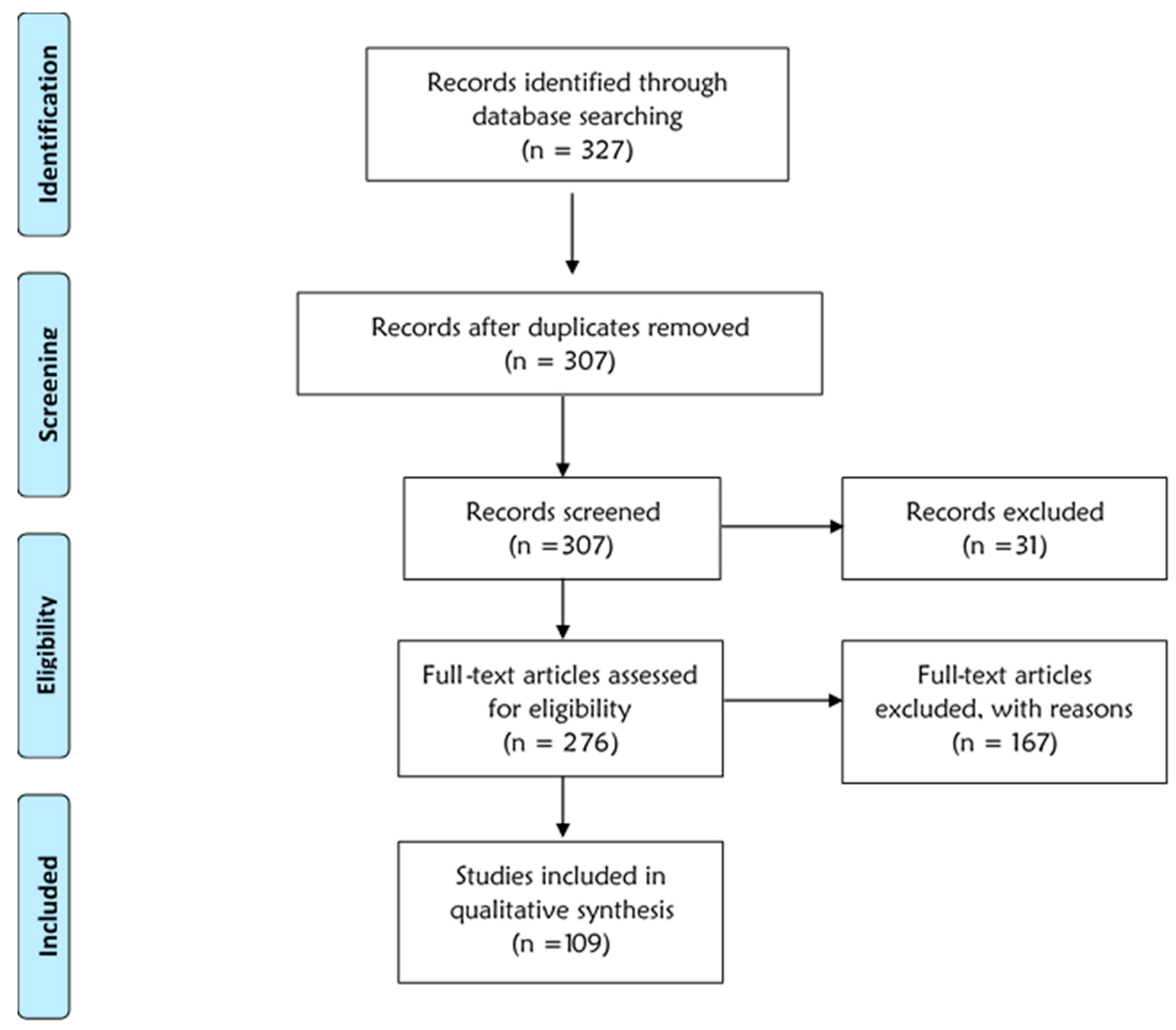
2. Materials and Methods
3. Results
3.1. Traditional Methods Used for Malaria Detection
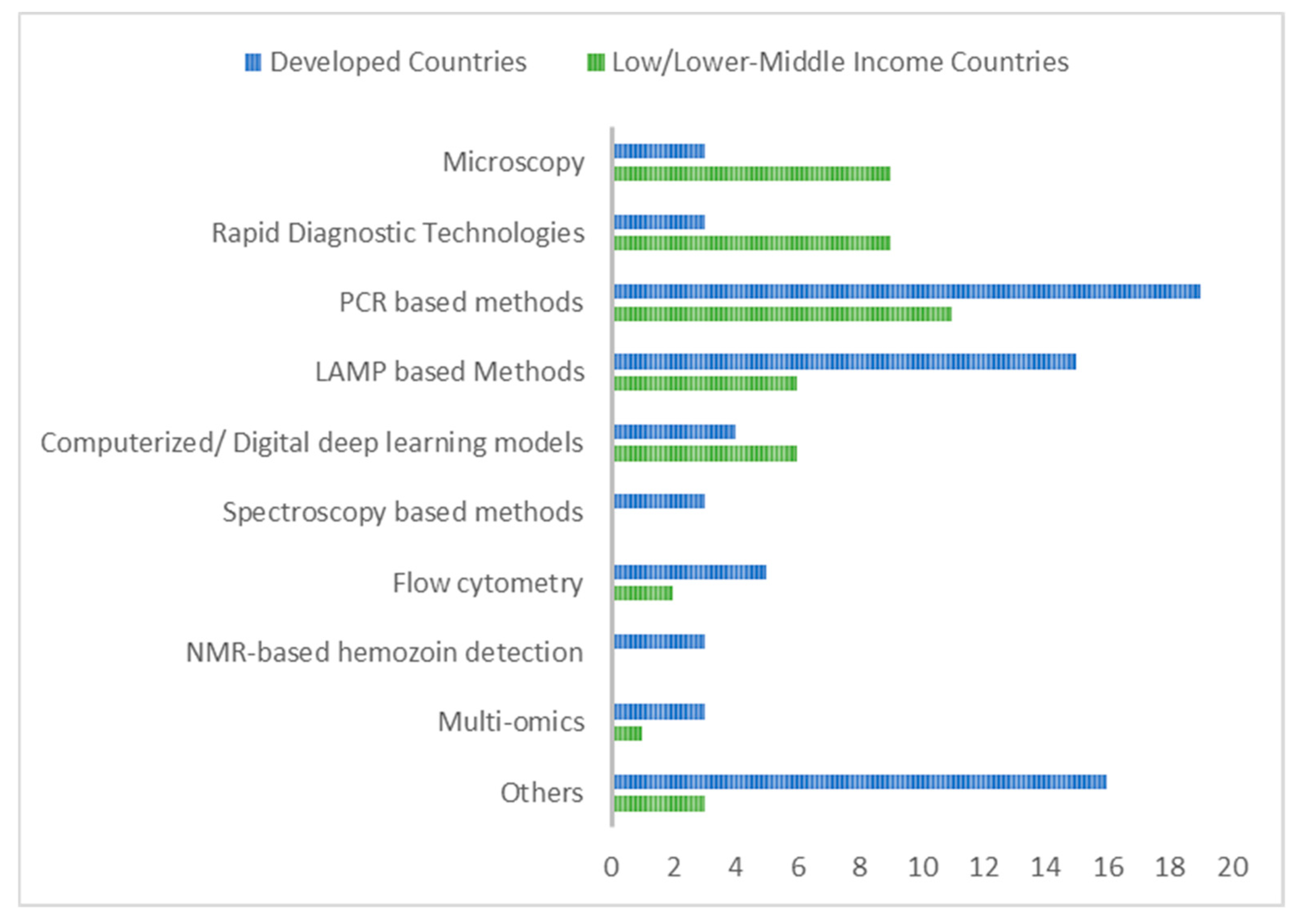
3.2. Modern Methods Used for Malaria Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Malaria Report. 2023. Available online: https://www.who.int/publications/i/item/9789240086173 (accessed on 19 July 2024).
- Tetteh-Quarcoo, P.B.; Dayie, N.T.; Adutwum-Ofosu, K.K.; Ahenkorah, J.; Afutu, E.; Amponsah, S.K.; Abdul-Rahman, M.; Kretchy, J.-P.; Ocloo, J.Y.; Nii-Trebi, N.I. Unravelling the perspectives of day and night traders in selected markets within a sub-saharan african city with a malaria knowledge, attitude and practice survey. Int. J. Environ. Res. Public Health 2021, 18, 3468. [Google Scholar] [CrossRef]
- Zheng, Z.; Cheng, Z. Advances in molecular diagnosis of malaria. Adv. Clin. Chem. 2017, 80, 155–192. [Google Scholar]
- malERA Consultative Group on Diagnoses and Diagnostics. A research agenda for malaria eradication: Diagnoses and diagnostics. PLoS Med. 2011, 8, e1000396. [Google Scholar]
- Yalley, A.K.; Ahiatrogah, S.; Kafintu-Kwashie, A.A.; Amegatcher, G.; Prah, D.; Botwe, A.K.; Adusei-Poku, M.A.; Obodai, E.; Nii-Trebi, N.I. A systematic review on suitability of molecular techniques for diagnosis and research into infectious diseases of concern in resource-limited settings. Curr. Issues Mol. Biol. 2022, 44, 4367–4385. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Malaria Program. 2024. Available online: https://www.who.int/teams/global-malaria-programme/case-management/diagnosis/microscopy (accessed on 10 June 2024).
- Krampa, F.D.; Aniweh, Y.; Awandare, G.A.; Kanyong, P. Recent progress in the development of diagnostic tests for malaria. Diagnostics 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bharti, P.K.; Devi, N.C.; Ahmed, N.; Sharma, A. Plasmodium malariae Detected by Microscopy in the International Bordering Area of Mizoram, a Northeastern State of India. Diagnostics 2022, 12, 2015. [Google Scholar] [CrossRef]
- Edwards, H. Tales from the bench: Laboratory diagnosis of malaria. Trop. Dr. 2011, 41, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Worldbank. World Bank Country and Lending Groups. 2024. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 13 May 2024).
- Azikiwe, C.C.; Ifezulike, C.; Siminialayi, I.; Amazu, L.U.; Enye, J.; Nwakwunite, O. A comparative laboratory diagnosis of malaria: Microscopy versus rapid diagnostic test kits. Asian Pac. J. Trop. Biomed. 2012, 2, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Hira, P.R.; Al-Ali, F.; Khalid, N.; Sher, A. Modified Giemsa staining for rapid diagnosis of malaria infection. Med. Princ. Pract. 2003, 12, 156–159. [Google Scholar] [CrossRef]
- Nadeem, M.F.; Khattak, A.A.; Yaqoob, A.; Awan, U.A.; Zeeshan, N. Assessment of Microscopic detection of Malaria with Nested Polymerase Chain Reaction in War-torn Federally Administered Tribal Areas of Pakistan. Acta Parasitol. 2021, 66, 1186–1192. [Google Scholar] [CrossRef]
- Awosolu, O.B.; Yahaya, Z.S.; Farah Haziqah, M.T. Efficacy of Plasmodium falciparum histidine-rich protein 2 (Pfhrp 2) rapid diagnostic test (RDT) and microscopy in the detection of falciparum malaria among symptomatic patients in Akure, Nigeria. Trop. Biomed. 2022, 39, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Badiane, A.; Thwing, J.; Williamson, J.; Rogier, E.; Diallo, M.A.; Ndiaye, D. Sensitivity and specificity for malaria classification of febrile persons by rapid diagnostic test, microscopy, parasite DNA, histidine-rich protein 2, and IgG: Dakar, Senegal 2015. Int. J. Infect. Dis. 2022, 121, 92–97. [Google Scholar] [CrossRef]
- Fontecha, G.; Escobar, D.; Ortiz, B.; Pinto, A.; Serrano, D.; Valdivia, H.O. Field Evaluation of a Hemozoin-Based Malaria Diagnostic Device in Puerto Lempira, Honduras. Diagnostics 2022, 12, 1206. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Soni, P.; Kumar, L.; Singh, M.P.; Verma, A.K.; Sharma, A.; Das, A.; Bharti, P.K. Comparison of polymerase chain reaction, microscopy, and rapid diagnostic test in malaria detection in a high burden state (Odisha) of India. Pathog. Glob. Health 2021, 115, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ugah, U.I.; Alo, M.N.; Owolabi, J.O.; Okata-Nwali, O.D.G.; Ekejindu, I.M.; Ibeh, N.; Elom, M.O. Evaluation of the utility value of three diagnostic methods in the detection of malaria parasites in endemic area. Malar. J. 2017, 16, 189. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Navidbakhsh, M.; Haghi, A.M.; Faghihi, S. A morphology-based method for the diagnosis of red blood cells parasitized by Plasmodium malariae and Plasmodium ovale. Scand. J. Infect. Dis. 2014, 46, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Sharma, R.; Deb, M. Usefulness of a centrifuged buffy coat smear examination for diagnosis of malaria. Indian J. Med. Microbiol. 2015, 33, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, D.F.; Deason, N.A.; Davidson, J.; Makuru, V.; Xiao, H.; Niedbalski, J.; Kern, M.; Russell, T.L.; Burkot, T.R.; Collins, F.H.; et al. Human malaria diagnosis using a single-step direct-PCR based on the Plasmodium cytochrome oxidase III gene. Malar. J. 2016, 15, 128. [Google Scholar] [CrossRef]
- Schneider, R.; Lamien-Meda, A.; Auer, H.; Wiedermann-Schmidt, U.; Chiodini, P.L.; Walochnik, J. Validation of a novel FRET real-time PCR assay for simultaneous quantitative detection and discrimination of human Plasmodium parasites. PLoS ONE 2021, 16, e0252887. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Ghoshal, U. Utility of nested polymerase chain reaction over the microscopy and immuno-chromatographic test in the detection of Plasmodium species and their clinical spectrum. Parasitol. Res. 2016, 115, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.R.C.d.; Gomes, L.T.; Fontes, C.J.F.; Tauil, P.L.; Pang, L.W.; Duarte, E.C. Sensitivity of nested-PCR for Plasmodium detection in pooled whole blood samples and its usefulness to blood donor screening in endemic areas. Transfus. Apher. Sci. 2014, 50, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, Z.; Wang, Y.; Xing, H.; Parker, D.M.; Yang, Z.; Baum, E.; Li, W.; Sattabongkot, J.; Sirichaisinthop, J.; et al. Nested PCR detection of malaria directly using blood filter paper samples from epidemiological surveys. Malar. J. 2014, 13, 175. [Google Scholar] [CrossRef][Green Version]
- Pomari, E.; Silva, R.; Moro, L.; Marca, G.L.; Perandin, F.; Verra, F.; Bisoffi, Z.; Piubelli, C. Droplet digital PCR for the detection of Plasmodium falciparum DNA in whole blood and serum: A comparative analysis with other molecular methods. Pathogens 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Srisutham, S.; Saralamba, N.; Malleret, B.; Rénia, L.; Dondorp, A.M.; Imwong, M. Four human Plasmodium species quantification using droplet digital PCR. PLoS ONE 2017, 12, e0175771. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Morris, U.; Aydin-Schmidt, B.; Msellem, M.I.; Shakely, D.; Petzold, M.; Björkman, A.; Mårtensson, A. SYBR green real-time PCR-RFLP assay targeting the Plasmodium cytochrome B gene—A highly sensitive molecular tool for malaria parasite detection and species determination. PLoS ONE 2015, 10, e0120210. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Luque, A.; Parejo, J.C.; Clavijo-Chamorro, M.Z.; López-Espuela, F.; Munyaruguru, F.; Lorenzo, S.B.; Monroy, I.; Gómez-Nieto, L.C. Method for malaria diagnosis based on extractions of samples using non-invasive techniques: An opportunity for the nursing clinical practice. Int. J. Environ. Res. Public Health 2020, 17, 5551. [Google Scholar] [CrossRef]
- Chua, K.H.; Lee, P.C.; Chai, H.C. Development of insulated isothermal PCR for rapid on-site malaria detection. Malar. J. 2016, 15, 134. [Google Scholar] [CrossRef]
- Kim, J.; Lim, D.H.; Mihn, D.-C.; Nam, J.; Jang, W.S.; Lim, C.S. Clinical usefulness of labchip real-time PCR using lab-on-a-chip technology for diagnosing malaria. Korean J. Parasitol. 2021, 59, 77. [Google Scholar] [CrossRef]
- Barbosa, L.R.; da Silva, E.L.; de Almeida, A.C.; Salazar, Y.E.; Siqueira, A.M.; Alecrim, M.d.G.C.; Vieira, J.L.F.; Bassat, Q.; de Lacerda, M.V.; Monteiro, W.M. An Ultra-Sensitive Technique: Using Pv-mtCOX1 qPCR to Detect Early Recurrences of Plasmodium vivax in Patients in the Brazilian Amazon. Pathogens 2020, 10, 19. [Google Scholar] [CrossRef]
- Obaldía, N.; Barahona, I.; Lasso, J.; Avila, M.; Quijada, M.; Nuñez, M.; Marti, M. Comparison of PvLAP5 and Pvs25 qRT-PCR assays for the detection of Plasmodium vivax gametocytes in field samples preserved at ambient temperature from remote malaria endemic regions of Panama. PLoS Neglected Trop. Dis. 2022, 16, e0010327. [Google Scholar] [CrossRef] [PubMed]
- Bouzayene, A.; Zaffaroullah, R.; Bailly, J.; Ciceron, L.; Sarrasin, V.; Cojean, S.; Argy, N.; Houzé, S.; Joste, V.; Angoulvant, A.; et al. Evaluation of two commercial kits and two laboratory-developed qPCR assays compared to LAMP for molecular diagnosis of malaria. Malar. J. 2022, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Sazed, S.A.; Kibria, M.G.; Alam, M.S. An optimized real-time qpcr method for the effective detection of human malaria infections. Diagnostics 2021, 11, 736. [Google Scholar] [CrossRef]
- Grignard, L.; Nolder, D.; Sepúlveda, N.; Berhane, A.; Mihreteab, S.; Kaaya, R.; Phelan, J.; Moser, K.; van Schalkwyk, D.A.; Campino, S.; et al. A novel multiplex qPCR assay for detection of Plasmodium falciparum with histidine-rich protein 2 and 3 (pfhrp2 and pfhrp3) deletions in polyclonal infections. EBioMedicine 2020, 55, 102757. [Google Scholar] [CrossRef]
- Aimeé, K.K.; Lengu, T.B.; Nsibu, C.N.; Umesumbu, S.E.; Ngoyi, D.M.; Chen, T. Molecular detection and species identification of Plasmodium spp. infection in adults in the Democratic Republic of Congo: A populationbased study. PLoS ONE 2020, 15, e0242713. [Google Scholar] [CrossRef]
- Canier, L.; Khim, N.; Kim, S.; Eam, R.; Khean, C.; Loch, K.; Ken, M.; Pannus, P.; Bosman, P.; Stassijns, J.; et al. Malaria PCR detection in Cambodian low-transmission settings: Dried blood spots versus venous blood samples. Am. Soc. Trop. Med. Hyg. 2015, 92, 573–577. [Google Scholar] [CrossRef]
- Phuong, M.; Lau, R.; Ralevski, F.; Boggild, A.K. Sequence-based optimization of a quantitative real-time PCR assay for detection of Plasmodium ovale and Plasmodium malariae. J. Clin. Microbiol. 2014, 52, 1068–1073. [Google Scholar] [CrossRef]
- Leski, T.A.; Taitt, C.R.; Swaray, A.G.; Bangura, U.; Reynolds, N.D.; Holtz, A.; Yasuda, C.; Lahai, J.; Lamin, J.M.; Baio, V.; et al. Use of real-time multiplex PCR, malaria rapid diagnostic test and microscopy to investigate the prevalence of Plasmodium species among febrile hospital patients in Sierra Leone. Malar. J. 2020, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Murillo, E.; Muskus, C.; Agudelo, L.A.; Vélez, I.D.; Ruiz-Lopez, F. A new high-resolution melting analysis for the detection and identification of Plasmodium in human and Anopheles vectors of malaria. Sci. Rep. 2019, 9, 1674. [Google Scholar] [CrossRef]
- Amaral, L.C.; Robortella, D.R.; Guimarães, L.F.F.; Limongi, J.E.; Fontes, C.J.F.; Pereira, D.B.; De Brito, C.F.A.; Kano, F.S.; De Sousa, T.N.; Carvalho, L.H. Ribosomal and non-ribosomal PCR targets for the detection of low-density and mixed malaria infections. Malar. J. 2019, 18, 154. [Google Scholar] [CrossRef]
- Frickmann, H.; Wegner, C.; Ruben, S.; Behrens, C.; Kollenda, H.; Hinz, R.; Rojak, S.; Schwarz, N.G.; Hagen, R.M.; Tannich, E. Evaluation of the multiplex real-time PCR assays RealStar malaria S&T PCR kit 1.0 and FTD malaria differentiation for the differentiation of Plasmodium species in clinical samples. Travel Med. Infect. Dis. 2019, 31, 101442. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Chong, E.T.J.; Anderios, F.; Lim, Y.A.L.; Chew, C.H.; Chua, K.H. Molecular detection of human Plasmodium species in Sabah using PlasmoNex™ multiplex PCR and hydrolysis probes real-time PCR. Malar. J. 2015, 14, 28. [Google Scholar] [CrossRef]
- Hayashida, K.; Kajino, K.; Simukoko, H.; Simuunza, M.; Ndebe, J.; Chota, A.; Namangala, B.; Sugimoto, C. Direct detection of falciparum and non-falciparum malaria DNA from a drop of blood with high sensitivity by the dried-LAMP system. Parasites Vectors 2017, 10, 26. [Google Scholar] [CrossRef]
- Colbert, A.J.; Co, K.; Lima-Cooper, G.; Lee, D.H.; Clayton, K.N.; Wereley, S.T.; John, C.C.; Linnes, J.C.; Kinzer-Ursem, T.L. Towards the use of a smartphone imaging-based tool for point-of-care detection of asymptomatic low-density malaria parasitaemia. Malar. J. 2021, 20, 380. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Tarumoto, N.; Misawa, K.; Runtuwene, L.R.; Sakai, J.; Hayashida, K.; Eshita, Y.; Maeda, R.; Tuda, J.; Murakami, T.; et al. A novel diagnostic method for malaria using loop-mediated isothermal amplification (LAMP) and MinION™ nanopore sequencer. BMC Infect. Dis. 2017, 17, 621. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Upmanyu, K.; Gupta, R.; Sruthy, K.S.; Matlani, M.; Savargaonkar, D.; Singh, R. Development of two-tube loop-mediated isothermal amplification assay for differential diagnosis of Plasmodium falciparum and Plasmodium vivax and its comparison with loopamp™ malaria. Diagnostics 2021, 11, 1689. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Lim, D.H.; Choe, Y.; Jee, H.; Moon, K.C.; Kim, C.; Choi, M.; Park, I.S.; Lim, C.S. Development of a multiplex loop-mediated isothermal amplification assay for diagnosis of Plasmodium spp., Plasmodium falciparum and Plasmodium vivax. Diagnostics 2021, 11, 1950. [Google Scholar] [CrossRef]
- Mohon, A.N.; Getie, S.; Jahan, N.; Alam, M.S.; Pillai, D.R. Ultrasensitive loop mediated isothermal amplification (US-LAMP) to detect malaria for elimination. Malar. J. 2019, 18, 350. [Google Scholar] [CrossRef]
- Viana, G.M.R.; Silva-Flannery, L.; Barbosa, D.R.L.; Lucchi, N.; do Valle, S.C.N.; Farias, S.; Barbalho, N.; Marchesini, P.; Rossi, J.C.N.; Udhayakumar, V.; et al. Field evaluation of a real time loop-mediated isothermal amplification assay (RealAmp) for malaria diagnosis in Cruzeiro do Sul, Acre, Brazil. PLoS ONE 2018, 13, e0200492. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, J.; Martin Ramírez, A.; González, I.J.; Ding, X.C.; Perez Tanoira, R.; Rojo-Marcos, G.; Gómez-Herruz, P.; Rubio, J.M. LAMP kit for diagnosis of non-falciparum malaria in Plasmodium ovale infected patients. Malar. J. 2017, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.L.; Lai, M.Y.; Fong, M.Y.; Jelip, J.; Mahmud, R. Loop-mediated isothermal amplification assay for identification of five human Plasmodium species in Malaysia. Am. J. Trop. Med. Hyg. 2016, 94, 336–339. [Google Scholar] [CrossRef]
- Patel, J.C.; Lucchi, N.W.; Srivastava, P.; Lin, J.T.; Sug-Aram, R.; Aruncharus, S.; Bharti, P.K.; Shukla, M.M.; Congpuong, K.; Satimai, W.; et al. Field evaluation of a real-time fluorescence loop-mediated isothermal amplification assay, realamp, for the diagnosis of Malaria in Thailand and India. J. Infect. Dis. 2014, 210, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Moonga, L.C.; Hayashida, K.; Kawai, N.; Nakao, R.; Sugimoto, C.; Namangala, B.; Yamagishi, J. Development of a multiplex loop-mediated isothermal amplification (LAMP) method for simultaneous detection of spotted fever group rickettsiae and malaria parasites by dipstick DNA chromatography. Diagnostics 2020, 10, 897. [Google Scholar] [CrossRef]
- Aydin-Schmidt, B.; Morris, U.; Ding, X.C.; Jovel, I.; Msellem, M.I.; Bergman, D.; Islam, A.; Ali, A.S.; Polley, S.; Gonzalez, I.J.; et al. Field evaluation of a high throughput loop mediated isothermal amplification test for the detection of asymptomatic Plasmodium infections in Zanzibar. PLoS ONE 2017, 12, e0169037. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, N.W.; Gaye, M.; Diallo, M.A.; Goldman, I.F.; Ljolje, D.; Deme, A.B.; Badiane, A.; Ndiaye, Y.D.; Barnwell, J.W.; Udhayakumar, V.; et al. Evaluation of the Illumigene Malaria LAMP: A Robust Molecular Diagnostic Tool for Malaria Parasites. Sci. Rep. 2016, 6, 36808. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, S.; Ahmed, M.Z.; Bhardwaj, N.; Singh, J.; Kumari, S.; Savargaonkar, D.; Anvikar, A.R.; Das, J. Advanced multiplex loop mediated isothermal amplification (mLAMP) combined with lateral flow detection (LFD) for rapid detection of two prevalent malaria species in india and melting curve analysis. Diagnostics 2022, 12, 32. [Google Scholar] [CrossRef]
- Aninagyei, E.; Boakye, A.A.; Tettey, C.O.; Ntiri, K.A.; Ofori, S.O.; Tetteh, C.D.; Aphour, T.T.; Rufai, T. Utilization of 18s ribosomal RNA LAMP for detecting Plasmodium falciparum in microscopy and rapid diagnostic test negative patients. PLoS ONE 2022, 17, e0275052. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Y.; Ooi, C.H.; Jaimin, J.J.; Lau, Y.L. Evaluation of WarmStart colorimetric loop-mediated isothermal amplification assay for diagnosis of Malaria. Am. J. Trop. Med. Hyg. 2020, 102, 1370–1372. [Google Scholar] [CrossRef]
- Barazorda, K.A.; Salas, C.J.; Braga, G.; Ricopa, L.; Ampuero, J.S.; Siles, C.; Sanchez, J.F.; Montano, S.; Lizewski, S.E.; Joya, C.A.; et al. Validation study of Boil & Spin Malachite Green Loop Mediated Isothermal Amplification (B&S MG-LAMP) versus microscopy for malaria detection in the Peruvian Amazon. PLoS ONE 2021, 16, e0258722. [Google Scholar] [CrossRef]
- Vincent, J.P.; Komaki-Yasuda, K.; Iwagami, M.; Kawai, S.; Kano, S. Combination of PURE-DNA extraction and LAMP-DNA amplification methods for accurate malaria diagnosis on dried blood spots 11 Medical and Health Sciences 1108 Medical Microbiology. Malar. J. 2018, 17, 373. [Google Scholar] [CrossRef]
- Cordray, M.S.; Richards-Kortum, R.R. A paper and plastic device for the combined isothermal amplification and lateral flow detection of Plasmodium DNA. Malar. J. 2015, 14, 472. [Google Scholar] [CrossRef] [PubMed]
- Aninagyei, E.; Abraham, J.; Atiiga, P.; Antwi, S.D.; Bamfo, S.; Acheampong, D.O. Evaluating the potential of using urine and saliva specimens for malaria diagnosis in suspected patients in Ghana. Malar. J. 2020, 19, 349. [Google Scholar] [CrossRef]
- Turnbull, L.B.; Ayodo, G.; Knight, V.; John, C.C.; McHenry, M.S.; Tran, T.M. Evaluation of an ultrasensitive HRP2–based rapid diagnostic test for detection of asymptomatic Plasmodium falciparum parasitaemia among children in western Kenya. Malar. J. 2022, 21, 337. [Google Scholar] [CrossRef]
- Briand, V.; Cottrell, G.; Tuike Ndam, N.; Martiáñez-Vendrell, X.; Vianou, B.; Mama, A.; Kouwaye, B.; Houzé, S.; Bailly, J.; Gbaguidi, E.; et al. Prevalence and clinical impact of malaria infections detected with a highly sensitive HRP2 rapid diagnostic test in Beninese pregnant women. Malar. J. 2020, 19, 188. [Google Scholar] [CrossRef]
- Wardhani, P.; Butarbutar, T.V.; Adiatmaja, C.O.; Betaubun, A.M.; Hamidah, N.; Aryati. Performance comparison of two malaria rapid diagnostic test with real time polymerase chain reaction and gold standard of microscopy detection method. Infect. Dis. Rep. 2020, 12, 8731. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.A.; Ahmed, S.; Khan, S.A. Detection of asymptomatic carriers of malaria in Kohat district of Pakistan. Malar. J. 2018, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Maltha, J.; Guiraud, I.; Lompo, P.; Kaboré, B.; Gillet, P.; Van Geet, C.; Tinto, H.; Jacobs, J. Accuracy of PfHRP2 versus Pf-pLDH antigen detection by malaria rapid diagnostic tests in hospitalized children in a seasonal hyperendemic malaria transmission area in Burkina Faso. Malar. J. 2014, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Fedele, P.L.; Wheeler, M.; Lemoh, C.; Chunilal, S. Immunochromatographic antigen testing alone is sufficient to identify asymptomatic refugees at risk of severe malaria presenting to a single health service in Victoria. Pathology 2014, 46, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cao, X.E.; Finkelstein, J.L.; Cárdenas, W.B.; Erickson, D.; Mehta, S. A two-colour multiplexed lateral flow immunoassay system to differentially detect human malaria species on a single test line. Malar. J. 2019, 18, 313. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.; Ajuji, M.; Yahya, I.U. Deepfmd: Computational analysis for malaria detection in blood-smear images using deep-learning features. Appl. Syst. Innov. 2021, 4, 82. [Google Scholar] [CrossRef]
- Kassim, Y.M.; Palaniappan, K.; Yang, F.; Poostchi, M.; Palaniappan, N.; Maude, R.J.; Antani, S.; Jaeger, S. Clustering-Based Dual Deep Learning Architecture for Detecting Red Blood Cells in Malaria Diagnostic Smears. IEEE J. Biomed. Health Inform. 2021, 25, 1735–1746. [Google Scholar] [CrossRef]
- Sriporn, K.; Tsai, C.F.; Tsai, C.E.; Wang, P. Analyzing malaria disease using effective deep learning approach. Diagnostics 2020, 10, 744. [Google Scholar] [CrossRef]
- Nakasi, R.; Mwebaze, E.; Zawedde, A. Mobile-aware deep learning algorithms for malaria parasites and white blood cells localization in thick blood smears. Algorithms 2021, 14, 17. [Google Scholar] [CrossRef]
- Yang, F.; Poostchi, M.; Yu, H.; Zhou, Z.; Silamut, K.; Yu, J.; Maude, R.J.; Jaeger, S.; Antani, S. Deep Learning for Smartphone-Based Malaria Parasite Detection in Thick Blood Smears. IEEE J. Biomed. Health Inform. 2020, 24, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Manescu, P.; Shaw, M.J.; Elmi, M.; Neary-Zajiczek, L.; Claveau, R.; Pawar, V.; Kokkinos, I.; Oyinloye, G.; Bendkowski, C.; Oladejo, O.A.; et al. Expert-level automated malaria diagnosis on routine blood films with deep neural networks. Am. J. Hematol. 2020, 95, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Nahiduzzaman, M.; Goni, M.O.F.; Sayeed, A.; Anower, M.S.; Ahsan, M.; Haider, J. Explainable Transformer-Based Deep Learning Model for the Detection of Malaria Parasites from Blood Cell Images. Sensors 2022, 22, 4358. [Google Scholar] [CrossRef]
- Abdurahman, F.; Fante, K.A.; Aliy, M. Malaria parasite detection in thick blood smear microscopic images using modified YOLOV3 and YOLOV4 models. BMC Bioinform. 2021, 22, 112. [Google Scholar] [CrossRef]
- Chibuta, S.; Acar, A.C. Real-time Malaria Parasite Screening in Thick Blood Smears for Low-Resource Setting. J. Digit. Imaging 2020, 33, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Khalid, A.; de Vos, A.L.; Feng, Y.; Rohrbach, P.; Hasan, T. Malaria Detection Accelerated: Combing a High-Throughput NanoZoomer Platform with a ParasiteMacro Algorithm. Pathogens 2022, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Kongklad, G.; Chitaree, R.; Taechalertpaisarn, T.; Panvisavas, N.; Nuntawong, N. Discriminant Analysis PCA-LDA Assisted Surface-Enhanced Raman Spectroscopy for Direct Identification of Malaria-Infected Red Blood Cells. Methods Protoc. 2022, 5, 49. [Google Scholar] [CrossRef]
- Wang, W.; Dong, R.L.; Gu, D.; He, J.A.; Yi, P.; Kong, S.K.; Ho, H.P.; Loo, J.F.C.; Wang, W.; Wang, Q. Antibody-free rapid diagnosis of malaria in whole blood with surface-enhanced Raman Spectroscopy using Nanostructured Gold Substrate. Adv. Med. Sci. 2020, 65, 86–92. [Google Scholar] [CrossRef]
- Heraud, P.; Chatchawal, P.; Wongwattanakul, M.; Tippayawat, P.; Doerig, C.; Jearanaikoon, P.; Perez-Guaita, D.; Wood, B.R. Infrared spectroscopy coupled to cloud-based data management as a tool to diagnose malaria: A pilot study in a malaria-endemic country. Malar. J. 2019, 18, 348. [Google Scholar] [CrossRef] [PubMed]
- McBirney, S.E.; Chen, D.; Scholtz, A.; Ameri, H.; Armani, A.M. Rapid Diagnostic for Point-of-Care Malaria Screening. ACS Sens. 2018, 3, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.T.; Gandra, N.; Fales, A.M.; Taylor, S.M.; Vo-Dinh, T. Sensitive DNA detection and SNP discrimination using ultrabright SERS nanorattles and magnetic beads for malaria diagnostics. Biosens. Bioelectron. 2016, 81, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Jang, W.S.; Nam, J.; Mihn, D.C.; Lim, C.S. An automated microscopic Malaria parasite detection system using digital image analysis. Diagnostics 2021, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Nam, K.M.; Park, C.Y.; Kim, Y.S.; Song, H.J. Automatic detection of malaria parasite in blood images using two parameters. Technol. Health Care 2015, 24, S33–S39. [Google Scholar] [CrossRef]
- Linder, N.; Turkki, R.; Walliander, M.; Mårtensson, A.; Diwan, V.; Rahtu, E.; Pietikäinen, M.; Lundin, M.; Lundin, J. A malaria diagnostic tool based on computer vision screening and visualization of Plasmodium falciparum candidate areas in digitized blood smears. PLoS ONE 2014, 9, e104855. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Kaboré, B.; Reuling, I.J.; Bognini, J.; Van Der Heijden, W.; Diallo, S.; Lompo, P.; Kam, B.; Herssens, N.; Lanke, K.; et al. The XN-30 hematology analyzer for rapid sensitive detection of malaria: A diagnostic accuracy study. BMC Med. 2019, 17, 103. [Google Scholar] [CrossRef]
- Dumas, C.; Bienvenu, A.L.; Girard, S.; Picot, S.; Debize, G.; Durand, B. Automated Plasmodium detection by the Sysmex XN hematology analyzer. J. Clin. Pathol. 2018, 71, 594–599. [Google Scholar] [CrossRef]
- Racsa, L.D.; Gander, R.M.; Southern, P.M.; McElvania TeKippe, E.; Doern, C.; Luu, H.S. Detection of intracellular parasites by use of the CellaVision DM96 analyzer during routine screening of peripheral blood smears. J. Clin. Microbiol. 2015, 53, 167–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pillay, E.; Khodaiji, S.; Bezuidenhout, B.C.; Litshie, M.; Coetzer, T.L. Evaluation of automated malaria diagnosis using the Sysmex XN-30 analyser in a clinical setting. Malar. J. 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Yokota, K.; Kajimoto, K.; Matsumoto, M.; Tatsumi, A.; Yamamoto, K.; Hyodo, T.; Matsushita, K.; Minakawa, N.; Mita, T.; et al. Quantitative detection of Plasmodium falciparum using, luna-fl, a fluorescent cell counter. Microorganisms 2020, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Baptista, V.; Ferreira, G.M.; Lima, D.; Minas, G.; Veiga, M.I.; Catarino, S.O. Multilayer thin-film optical filters for reflectance-based malaria diagnostics. Micromachines 2021, 12, 890. [Google Scholar] [CrossRef]
- Orbán, Á.; Longley, R.J.; Sripoorote, P.; Maneechai, N.; Nguitragool, W.; Butykai, Á.; Mueller, I.; Sattabongkot, J.; Karl, S.; Kézsmárki, I. Sensitive detection of Plasmodium vivax malaria by the rotating-crystal magneto-optical method in Thailand. Sci. Rep. 2021, 11, 18547. [Google Scholar] [CrossRef] [PubMed]
- Pukáncsik, M.; Molnár, P.; Orbán, Á.; Butykai, Á.; Marton, L.; Kézsmárki, I.; Vértessy, B.G.; Kamil, M.; Abraham, A.; Aly, A.S.I. Highly sensitive and rapid characterization of the development of synchronized blood stage malaria parasites via magneto-optical hemozoin quantification. Biomolecules 2019, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Orbán, Á.; Butykai, Á.; Molnár, A.; Pröhle, Z.; Fülöp, G.; Zelles, T.; Forsyth, W.; Hill, D.; Müller, I.; Schofield, L.; et al. Evaluation of a novel magneto-optical method for the detection of malaria parasites. PLoS ONE 2014, 9, e96981. [Google Scholar] [CrossRef]
- Lukianova-Hleb, E.Y.; Campbell, K.M.; Constantinou, P.E.; Braam, J.; Olson, J.S.; Ware, R.E.; Sullivan, D.J.; Lapotko, D.O. Hemozoin-generated vapor nanobubbles for transdermal reagent- and needle-free detection of malaria. Proc. Natl. Acad. Sci. USA 2014, 111, 900–905. [Google Scholar] [CrossRef]
- Gandarilla, A.M.D.; Glória, J.C.; Barcelay, Y.R.; Mariuba, L.A.M.; Brito, W.R. Electrochemical immunosensor for detection of Plasmodium vivax lactate dehydrogenase. Mem. Inst. Oswaldo Cruz 2022, 117, e220085. [Google Scholar] [CrossRef] [PubMed]
- de la Serna, E.; Arias-Alpízar, K.; Borgheti-Cardoso, L.N.; Sanchez-Cano, A.; Sulleiro, E.; Zarzuela, F.; Bosch-Nicolau, P.; Salvador, F.; Molina, I.; Ramírez, M.; et al. Detection of Plasmodium falciparum malaria in 1 h using a simplified enzyme-linked immunosorbent assay. Anal. Chim. Acta 2021, 1152, 338254. [Google Scholar] [CrossRef] [PubMed]
- Hemben, A.; Ashley, J.; Tothill, I.E. Development of an Immunosensor for Pf HRP 2 as a biomarker for malaria detection. Biosensors 2017, 7, 28. [Google Scholar] [CrossRef]
- Jang, I.K.; Jiménez, A.; Rashid, A.; Barney, R.; Golden, A.; Ding, X.C.; Domingo, G.J.; Mayor, A. Comparison of two malaria multiplex immunoassays that enable quantification of malaria antigens. Malar. J. 2022, 21, 176. [Google Scholar] [CrossRef]
- Singh, N.K.; Jain, P.; Das, S.; Goswami, P. Dye coupled aptamer-captured enzyme catalyzed reaction for detection of pan malaria and p. Falciparum species in laboratory settings and instrument-free paper-based platform. Anal. Chem. 2019, 91, 4213–4221. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhao, S.; Liu, Y.; Shao, L.; Ye, Y.; Jiang, N.; Yang, K. Rapid Visual Detection of Plasmodium Using Recombinase-Aided Amplification With Lateral Flow Dipstick Assay. Front. Cell. Infect. Microbiol. 2022, 12, 922146. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Subramanian, G.; Duan, J.; Gao, S.; Bai, L.; Chandramohanadas, R.; Ai, Y. A portable image-based cytometer for rapid malaria detection and quantification. PLoS ONE 2017, 12, e0179161. [Google Scholar] [CrossRef]
- Liu, Q.; Nam, J.; Kim, S.; Lim, C.T.; Park, M.K.; Shin, Y. Two-stage sample-to-answer system based on nucleic acid amplification approach for detection of malaria parasites. Biosens. Bioelectron. 2016, 82, 1–8. [Google Scholar] [CrossRef]
- Shah, J.; Mark, O.; Weltman, H.; Barcelo, N.; Lo, W.; Wronska, D.; Kakkilaya, S.; Rao, A.; Bhat, S.T.; Sinha, R.; et al. Fluorescence In Situ hybridization (FISH) assays for diagnosing malaria in endemic areas. PLoS ONE 2015, 10, e0136726. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Poruri, A.; Mark, O.; Khadilka, U.; Mohring, F.; Moon, R.W.; Ramasamy, R. A dual colour fluorescence in situ hybridization (FISH) assay for identifying the zoonotic malaria parasite Plasmodium knowlesi with a potential application for the specific diagnosis of knowlesi malaria in peripheral-level laboratories of Southeast Asia. Parasites Vectors 2017, 10, 342. [Google Scholar] [CrossRef]
- Peng, W.K.; Kong, T.F.; Ng, C.S.; Chen, L.; Huang, Y.; Bhagat, A.A.S.; Nguyen, N.-T.; Preiser, P.R.; Han, J. Micromagnetic resonance relaxometry for rapid label-free malaria diagnosis. Nat. Med. 2014, 20, 1069–1073. [Google Scholar] [CrossRef]
- Thamarath, S.S.; Xiong, A.; Lin, P.-H.; Preiser, P.R.; Han, J. Enhancing the sensitivity of micro magnetic resonance relaxometry detection of low parasitemia Plasmodium falciparum in human blood. Sci. Rep. 2019, 9, 2555. [Google Scholar] [CrossRef]
- Fook Kong, T.; Ye, W.; Peng, W.K.; Wei Hou, H.; Marcos; Preiser, P.R.; Nguyen, N.-T.; Han, J. Enhancing malaria diagnosis through microfluidic cell enrichment and magnetic resonance relaxometry detection. Sci. Rep. 2015, 5, 11425. [Google Scholar] [CrossRef]
- Tomescu, O.A.; Mattanovich, D.; Thallinger, G.G. Integrative omics analysis. A study based on Plasmodium falciparum mRNA and protein data. BMC Syst. Biol. 2014, 8, S4. [Google Scholar] [CrossRef][Green Version]
- Awasthi, G.; Tyagi, S.; Kumar, V.; Patel, S.K.; Rojh, D.; Sakrappanavar, V.; Kochar, S.K.; Talukdar, A.; Samanta, B.; Das, A. A proteogenomic analysis of haptoglobin in malaria. PROTEOMICS–Clin. Appl. 2018, 12, 1700077. [Google Scholar] [CrossRef]
- Lindner, S.E.; Swearingen, K.E.; Shears, M.J.; Walker, M.P.; Vrana, E.N.; Hart, K.J.; Minns, A.M.; Sinnis, P.; Moritz, R.L.; Kappe, S.H. Transcriptomics and proteomics reveal two waves of translational repression during the maturation of malaria parasite sporozoites. Nat. Commun. 2019, 10, 4964. [Google Scholar] [CrossRef] [PubMed]
- Gardinassi, L.G.; Arévalo-Herrera, M.; Herrera, S.; Cordy, R.J.; Tran, V.; Smith, M.R.; Johnson, M.S.; Chacko, B.; Liu, K.H.; Darley-Usmar, V.M. Integrative metabolomics and transcriptomics signatures of clinical tolerance to Plasmodium vivax reveal activation of innate cell immunity and T cell signaling. Redox Biol. 2018, 17, 158–170. [Google Scholar] [CrossRef]
- Commonwealth. The Commonwealth Malaria Report. 2022. Available online: https://reliefweb.int/report/world/commonwealth-malaria-report-2022 (accessed on 15 June 2024).
- Yan, S.L.R.; Wakasuqui, F.; Wrenger, C. Point-of-care tests for malaria: Speeding up the diagnostics at the bedside and challenges in malaria cases detection. Diagn. Microbiol. Infect. Dis. 2020, 98, 115122. [Google Scholar]
- Veiga, M.I.; Peng, W.K. Rapid phenotyping towards personalized malaria medicine. Malar. J. 2020, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-Z.; Zhang, C.; Joy, D.A. Host-malaria parasite interactions and impacts on mutual evolution. Front. Cell. Infect. Microbiol. 2020, 10, 587933. [Google Scholar] [CrossRef]
- Laishram, D.D.; Sutton, P.L.; Nanda, N.; Sharma, V.L.; Sobti, R.C.; Carlton, J.M.; Joshi, H. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar. J. 2012, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Leski, T.A.; Taitt, C.R.; Colston, S.M.; Bangura, U.; Holtz, A.; Yasuda, C.Y.; Reynolds, N.D.; Lahai, J.; Lamin, J.M.; Baio, V. Prevalence of malaria resistance-associated mutations in Plasmodium falciparum circulating in 2017–2018, Bo, Sierra Leone. Front. Microbiol. 2022, 13, 1059695. [Google Scholar] [CrossRef] [PubMed]
- Bull, P.C.; Berriman, M.; Kyes, S.; Quail, M.A.; Hall, N.; Kortok, M.M.; Marsh, K.; Newbold, C.I. Plasmodium falciparum variant surface antigen expression patterns during malaria. PLoS Pathog. 2005, 1, e26. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Peng, W.K.; Srivastava, S. Multi-omics advancements towards Plasmodium vivax malaria diagnosis. Diagnostics 2021, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
| LOW AND LOWER-MIDDLE INCOME COUNTRIES | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 70% GLOBAL MALARIA BURDEN | |||||||||
| LOW-INCOME COUNTRIES | Afghanistan | Burundi | Central African Republic | Chad | Eritrea | Ethiopia | Gambia | Burkina Faso | Congo, Dem. Rep |
| Guinea-Bissau | Korea, Dem. People’s Rep | Liberia | Madagascar | Malawi | Rwanda | Sierra Leone | Mali | Mozambique | |
| Somalia | South Sudan | Sudan | Syrian Arab Republic | Togo | Yemen, Rep | Niger | Uganda | ||
| LOWER-MIDDLE INCOME COUNTRIES | Angola | Algeria | Bangladesh | Benin | Bhutan | Bolivia | Cabo Verde | Cameroon | Ghana |
| Cambodia | Comoros | Congo, Rep. | Côte d’Ivoire | Djibouti | Egypt, Arab Rep. | Eswatini | India | Nigeria | |
| Guinea | Haiti | Honduras | Jordan | Iran, Islamic Rep | Kenya | Kiribati | Tanzania | ||
| Kyrgyz Republic | Lao PDR | Lebanon | Lesotho | Mauritania | Micronesia, Fed. Sts. | Mongolia | |||
| Morocco | Myanmar | Nepal | Nicaragua | Pakistan | Papua New Guinea | Philippines | |||
| Samoa | São Tomé and Principe | Senegal | Solomon Islands | Sri Lanka | Tajikistan | Timor-Leste | |||
| Tunisia | Ukraine | Uzbekistan | Vanuatu | Vietnam | Zambia | Zimbabwe | |||
| Traditional Methods | Specimen Used | Summary of Procedure | Invasive/Non-Invasive | Advantages | Disadvantages | Refer-ences |
|---|---|---|---|---|---|---|
| Thin film microscopy | Blood | Thin blood smears are prepared and stained using Giemsa stain. Thin smears are examined with a 100× oil immersion objective. | Invasive | Reliable in the identification of four human plasmodium species and their various stages | Limited by quality of blood smears as well as availability of skilled microscopists. Lack of sensitivity where non-falciparum or mixed infections exist. | [8,13,14,15,16,17,18] |
| Thick film microscopy | Blood | Thick blood smears are prepared and stained using Giemsa stain. Thin smears are examined with a 100× oil immersion objective. | Invasive | Reliable in the detection of four human plasmodium species | Limited by quality of blood smears as well as availability of skilled microscopists. | [8,13,14,15,16,17,18] |
| Morphology-based diagnosis | Blood | Optical images from Giemsa-stained infected blood are measured using Olysia and Scanning Probe Image Processor software based on morphology of red blood cells. | Invasive | Faster prediction of malaria cases | Expertise needed | [19] |
| Centrifuged buffy coat smear examination (CBCS) | Blood | Centrifugation of buffy coat is done prior to Giemsa staining and microscopic examination | Invasive | Specificity is similar to conventional method but sensitivity a bit better than conventional method | Limited by availability of skilled microscopists | [20] |
| Modern Methods | Specimen Used | Description | Invasive/Non-Invasive | Point of Care/Molecular/Other | Advantages | Disadvantages | Developed Countries | References |
|---|---|---|---|---|---|---|---|---|
| Direct conventional PCR | Blood | With plasmodium cytochrome oxidase III gene (COX-III) as target, direct conventional PCR is conducted on bloodspot samples. Results are visualized on a gel. | Invasive | Molecular | High Sensitivity; faster than nested; does not require DNA isolation | Requires much expertise and expensive | USA | [21] |
| Nested Polymerase Chain Reaction (PCR) | Blood | Using different primer pairs to run 2 sequential amplification reactions. Plasmodium genomic DNA extracted from dried blood spots | Invasive | Molecular | High sensitivity and specificity | Time consuming, expensive, requires much expertise | Thailand, USA, Brazil, United Kingdom, Austria | [13,16,18,21,22,23,24,25] |
| Droplet Digital PCR (ddPCR) | Blood, Serum | DNA extracted from blood and serum samples are analyzed using the ddPCR method, which is based on water–oil emulsion droplet technology | Invasive | Molecular | High sensitivity using blood samples | Low sensitivity using serum samples; expensive | Italy, Thailand | [26,27] |
| Photo- Induced Electron transfer PCR (PET-PCR) | Blood | Total DNA is extracted from dried blood spots and PCR performed using photo-induced electron transfer fluorogenic primers | Invasive | Molecular | High sen-sitivity for parasite identification and characterization. | Requires much expertise and is expensive | USA | [15] |
| Fluoresen-ce reson-ance energy transfer (FRET) real time PCR | Blood | Real-time PCR utilizing FRET whereby fluorophores are brought in close proximity after hybridization is performed on DNA extracted from lyophilized blood samples targeting the 18S rRNA gene | Invasive | Molecular | High sensit-ivity, and allows for simultaneous quantitative and species-specific detection | This specific protocol could not differentiate between P. vivax and P. knowlesi; expensive | United Kingdom, Austria | [22] |
| SYBR Green Real-Time PCR-RFLP Assay | Blood | Real-time PCR using sybr green dye that binds to all double-stranded DNA followed by restriction fragment polymorphism to differentiate species | Invasive | Molecular | High sensitivity | Meltcurve required in PCR since Sybr green alone can be non-specific; expensive | Sweden | [28] |
| Hair qPCR | Head hairs | Hairs without roots are taken from patients and qPCR assay conducted | Non-invasive | molecular | Requires no special trans-port/storage conditions for samples | Sensitivity lower than when blood samples are used | Spain | [29] |
| Insulated Isothermal PCR (iiPCR) | Blood | PCR is performed in a portable device using an assay based on the Rayleigh–Bénard convection method | Invasive | Molecular/point of care | Portable, easy and fast operation; direct interpretation | Not as sensitive as qPCR | Malaysia | [30] |
| Lab Chip Real Time PCR (LRP) | Blood | DNA is extracted from collected blood samples and a portable LRP device is used to detect malarial parasites based on lab-on-chip technology | Invasive | Molecular/point of care | High sensitivity and specificity. Fast and cost effective | Risk of false negatives higher than traditional real-time PCR | Korea | [31] |
| Pv-mt Cox PCR | Blood | DNA is extracted from collected blood samples and qPCR with mitochondrial gene target is carried out | Invasive | Molecular | More sensitive in the detection of P. vivax | Expensive | Brazil | [32] |
| PvLAP5 and Pvs25qRT-PCR assays | Blood | Extracted RNA is subjected to quantitative reverse transcription PCR | Invasive | Molecular | Suitable assay for the determination of human transmission reservoir | Expensive | Panama | [33] |
| Other Quantita-tive PCR (qPCR) | Blood | Real-time PCR performed using primers targeting different regions and SYBR green or probe-based technology on DNA extracted from whole blood | Invasive | Molecular | High sensitivity and rapid | Extreme caution needed to prevent contamination; expensive | France, Canada, USA Columbia Germany, Brazil, China, Malaysia | [34,35,36,37,38,39,40,41,42,43,44] |
| Dry LAMP system (CZC-LAMP) | Blood | Blood samples are analyzed directly without extraction using the assay made up of dried reagents | Invasive | Point of care/molecular | High sensitivity and specificity; no need for prior extraction | Not widely available | [45] | |
| Particle Diffusometry (PD)-LAMP | Blood | PD, which senses images, is combined with LAMP on a smartphone-enabled device to detect low levels of parasitemia | Invasive | Point of care/molecular | Sensitivitities higher than RDTs and similar to qPCR | Sensitivity slightly lower than nested PCR | USA | [46] |
| LAMP and MinION™ nanopore sequencer | Blood | Primers targeting the 18S–rRNA gene of all five human Plasmodium species are generated and samples subjected to LAMP. Min-ION™ nanopore sequencer is used on amplicons to identify Plasmodium spp. | Invasive | Molecular | Highly sensitive, and simple | Expensive | Japan | [47] |
| Other Loop-mediated isothermal amplification (LAMP), | Blood | Extracted DNA is subjected to loop-mediated isothermal amplification with a variety of detection methods | Invasive | Point of care/molecular | Simple, low cost; can be used in resource-limited settings and point-of-care settings | Some cannot quantify par-asite density; some are insensitive towards low parasitemia and mixed infections | France, Korea, Thailand Italy, Brazil Spain, Mala-ysia, Japan, Peru, USA | [26,34,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] |
| Modern Methods | Specimen Used | Description | Invasive/Non-Invasive | Point of Care/Molecular/Other | Advantages | Disadvantages | Developed Countries | References |
|---|---|---|---|---|---|---|---|---|
| Malaria SD Bioline RDT kit | Urine, Saliva, Blood | Using immuno-chromatography to detect PfhRP2 and PLDH following manufacturer’s instructions | Non-invasive/Invasive | Point of care | Effective for non-invasive detection of malaria; low cost | Low sensitivity | [64] | |
| Other (RDTs) | Blood | Immunochromatography/ according to manufacturer’s instructions | Invasive | Point of care | Suitable for point of care in hard-to-access areas; low cost | Low sensit-ivity for some kits; poor identification of non-falciparum infections for some brands | Indonesia Australia, USA | [14,15,17,18,65,66,67,68,69,70,71] |
| Computeri-zed/digital deep mach-ine learnin-g approach | Blood | Machine learning models are used to detect malaria parasites in blood smears. Some can be integrated into smartphone detection apps | Invasive | Other | Accurate/ reliable | For some, results are affected by quality of smears | USA, Taiwan, China, Turkey | [72,73,74,75,76,77,78,79,80,81] |
| Spectros-copy | Blood | Blood samples are analyzed using spectroscopy | Invasive | Other | Highly effective for identifying infected cell | Only qualitative results obtained | Thailand, China, Australia | [82,83,84] |
| Portable Optical Diagnostic System (PODS) | Blood | Works by differential optical spectroscopy. The change in optical power before and after a magnet is applied, is monitored in order to determine β-hematin concentration in whole blood | Invasive | Point of care | Portable; low cost; useful for low resource settings; high sensitivity | Not widely available | USA | [85] |
| Ultra bright SERS nanorattles | Blood | DNA detection method that uses sandwich hybridization of magnetic bead, target sequence, and ultrabright SERS nanorattle are employed | Invasive | Molecular/point of care | Sensitive; can be automated and added to portable devi-ces for POC diagnosis; can identify SNPs hence, discri-minate betw-een wild-type and mutant parasites | Not widely available | USA | [86] |
| Automated Microscopy/Digital Analysis | Blood | Comprises a fluorescent dye staining or Giemsa staining and an automated microscopy platform and digital analysis | Invasive | Other | Rapid diagn-osis and par-asite density monitoring. High sens- itivity, linear-ity, and precision | Not widely available | Korea, Finland, Sweden | [87,88,89] |
| Flow cytometry | Blood | Parasites are detected and quantified in blood by use of analyzers utilizing flow cytometry technology | Invasive | Molecular | Rapid and high sensiti-vity; useful for mass screening | May not be able to distinguish plasmodium species | Netherlands, France, USA, South Africa, Japan | [90,91,92,93,94] |
| Thin-Film Optical Filters | Blood | A thin film optical device is used based on optical reflectance spectrophotometry, for the parasite detection through haemozoin quantification | Invasive | Point of care | High sensitivity | High transmittance regions outside target wavelength | Portugal | [95] |
| Rotating cr- ystal magn-eto optical detection (RMOD) method | Blood | RMOD works by detection of the periodic modulation of light transmission. This is induced by hemozoin crystals which co-rotates with a rotating magnetic field | Invasive | Other | Higher sensitivity and accuracy than light microscopy | Sensitivity is poorer than PCR methods | Thailand, Hungary | [96,97,98] |
| Hemozin-Based Malaria diagnostic device (GazelleTM) | Blood | Using magneto-optical technology, the device detects hemozoin produced by Plasmodium | Invasive | Other | Sensitivities comparable to light micr-oscopy; faster than micros-copy; portab-le; can run on battery power | Unable to distinguish between species | [16] | |
| Hemozoin-generated vapor nanobubbles | Blood vessel (transdermal) | Hemozoin generates a transient vapor nanobubble around hemozoin in response to a short and safe laser pulse. The acoustic signals of these nanobubbles that are malaria specific enable detection | Non-invasive | Point of care | Non-invasive; rapid | Not widely available | USA | [99] |
| Electroche-mical immunosensor | Blood | Egg yolk IgY antibodies against Plasmodium vivax lactate dehydrogenase antigen are immobilized on a gold electrode surface followed by differential pulse voltammetry and contact angle measurements are made. | Invasive | Point of care | High Sensitivity for malaria caused by P. vivax | Only malaria caused by P. vivax can be detected | Brazil | [100] |
| Simplified ELISA)/PfHRP 2 ELISA | Blood | Modified ElISA was performed on blood samples. | Invasive | Point of care | High sensitivity, portable and low cost | Not widely available | Spain UK Denmark | [101,102] |
| Multiple-xed ELISA based assay | Blood | Multiplexed ELISA-based (either planar-based array or magnetic bead-based platforms) technologies are used for malaria detection | Invasive | Molecular | Can detect malaria spe-cies mutants; have high throughput potential | Not widely available | USA | [103] |
| Dye-Cou-pledApt-amer-Capt-ured Enzy-me-Cataly-zed assay | Blood | Aptamer- and enzyme-based method is used to detect malaria infection in blood. Method can be used on instrument or instrument free platform | Invasive | Molecular/point of care | Low cost; useful for resource-limited and point-of-care settings. | Not widely available | [104] | |
| Recombinase-Aided Amplificat-ion with Lateral Flow Dip-stick Assay (RAA-LFD) | Blood | A combination of recombinase-aided amplification lasting for 15 min at 37 degrees and lateral flow dipstick is used to detect plasmodium species in blood | Invasive | Molecular/point of care | Highly sensitive, specific, low cost, convenient for on-site screening and low resource settings. | Not widely available | China | [105] |
| Portable image-based Cytometer | Blood | P. falciparum-infected blood cells are identified and counted from Giemsa-stained smears using the image based portable cytometer. | Invasive | Other | Simple to operate; low cost | Not widely available | Singapore | [106] |
| Two-stage sample-to-answer sy-stem based on nucleic acid ampl-ification approach | Blood | It combines the dimethyl adipimidate (DMA)/thin film sample processing (DTS) technique and the Mach–Zehnder interferometer isothermal solid-phase DNA amplification (MZI-IDA) technique to detect infection in blood | Invasive | Molecular | High sensitivity, rapid | Not widely available | Singapore, Korea | [107] |
| Fluorescen-ce In Situ Hybridization (FISH) Assays | Blood | Detects and localizes specific malaria nucleic acid sequences by hybridizing with complementary sequences that are labeled with fluorescent probes | Invasive | Molecular | High sensitivity | Skilled expertise required. | USA | [108,109] |
| NMR-based hemozoin detection | Blood | Detection is based on the ability to recognize the paramagnetic susceptibility of malaria hemozoin crystals | Invasive | Molecular/point of care | High sensitivity and rapid | Not widely available | Australia, Singapore, USA | [110,111,112] |
| Multi-omics | Varies | Integrating data from different omic methods | Invasive/non-invasive | Other | Comprehen-sive underst-anding of the infection | Requires skilled experitise | Austria USA Columbia | [113,114,115,116] |
| Modern Method | Resource-Limited Countries | References |
|---|---|---|
| Malaria rapid test kit (SD Bioline RDT kit) using urine and saliva samples | Ghana | [64] |
| Other rapid diagnostic tests | Nigeria, Senegal, Kenya, Benin, Pakistan, Burkina Faso | [14,15,17,18,65,66,68,69] |
| Nested polymerase chain reaction (PCR) | Pakistan, Nigeria, Myanmar, Honduras, India | [13,16,18,23,25] |
| Hair qPCR | Rwanda | [29] |
| Other quantitative polymerase chain reaction (qPCR) | Bangladesh, Eritrea, Tanzania D.R. Congo, Sierra Leone, Cambodia | [35,36,37,38,40] |
| Dry LAMP system (CZC-LAMP | Zambia | [45] |
| Other loop-mediated isothermal amplification (LAMP), | India, Tanzania, Senegal, Ghana | [48,56,57,58,59] |
| Computerized/digital deep machine learning approach | Nigeria, Uganda, Bangladesh, Ethiopia, Zambia, | [59,75,77,78,79,80] |
| The rotating-crystal magneto-optical detection (RMOD) method | Papua New Guinea | [96] |
| Hemozin-based malaria diagnostic device (GazelleTM) | Honduras | [16] |
| Flow cytometry | Burkina Faso, India | [90,93] |
| Dye-coupled aptamer-captured enzyme-catalyzed assay | India | [104] |
| Multi-omics | India | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalley, A.K.; Ocran, J.; Cobbinah, J.E.; Obodai, E.; Yankson, I.K.; Kafintu-Kwashie, A.A.; Amegatcher, G.; Anim-Baidoo, I.; Nii-Trebi, N.I.; Prah, D.A. Advances in Malaria Diagnostic Methods in Resource-Limited Settings: A Systematic Review. Trop. Med. Infect. Dis. 2024, 9, 190. https://doi.org/10.3390/tropicalmed9090190
Yalley AK, Ocran J, Cobbinah JE, Obodai E, Yankson IK, Kafintu-Kwashie AA, Amegatcher G, Anim-Baidoo I, Nii-Trebi NI, Prah DA. Advances in Malaria Diagnostic Methods in Resource-Limited Settings: A Systematic Review. Tropical Medicine and Infectious Disease. 2024; 9(9):190. https://doi.org/10.3390/tropicalmed9090190
Chicago/Turabian StyleYalley, Akua K., Joyous Ocran, Jacob E. Cobbinah, Evangeline Obodai, Isaac K. Yankson, Anna A. Kafintu-Kwashie, Gloria Amegatcher, Isaac Anim-Baidoo, Nicholas I. Nii-Trebi, and Diana A. Prah. 2024. "Advances in Malaria Diagnostic Methods in Resource-Limited Settings: A Systematic Review" Tropical Medicine and Infectious Disease 9, no. 9: 190. https://doi.org/10.3390/tropicalmed9090190
APA StyleYalley, A. K., Ocran, J., Cobbinah, J. E., Obodai, E., Yankson, I. K., Kafintu-Kwashie, A. A., Amegatcher, G., Anim-Baidoo, I., Nii-Trebi, N. I., & Prah, D. A. (2024). Advances in Malaria Diagnostic Methods in Resource-Limited Settings: A Systematic Review. Tropical Medicine and Infectious Disease, 9(9), 190. https://doi.org/10.3390/tropicalmed9090190









