Ecology and Infection Status of Sand Flies in Rural and Urban Cutaneous Leishmaniasis Endemic Areas in Northwest Ethiopia
Abstract
1. Background
2. Methods
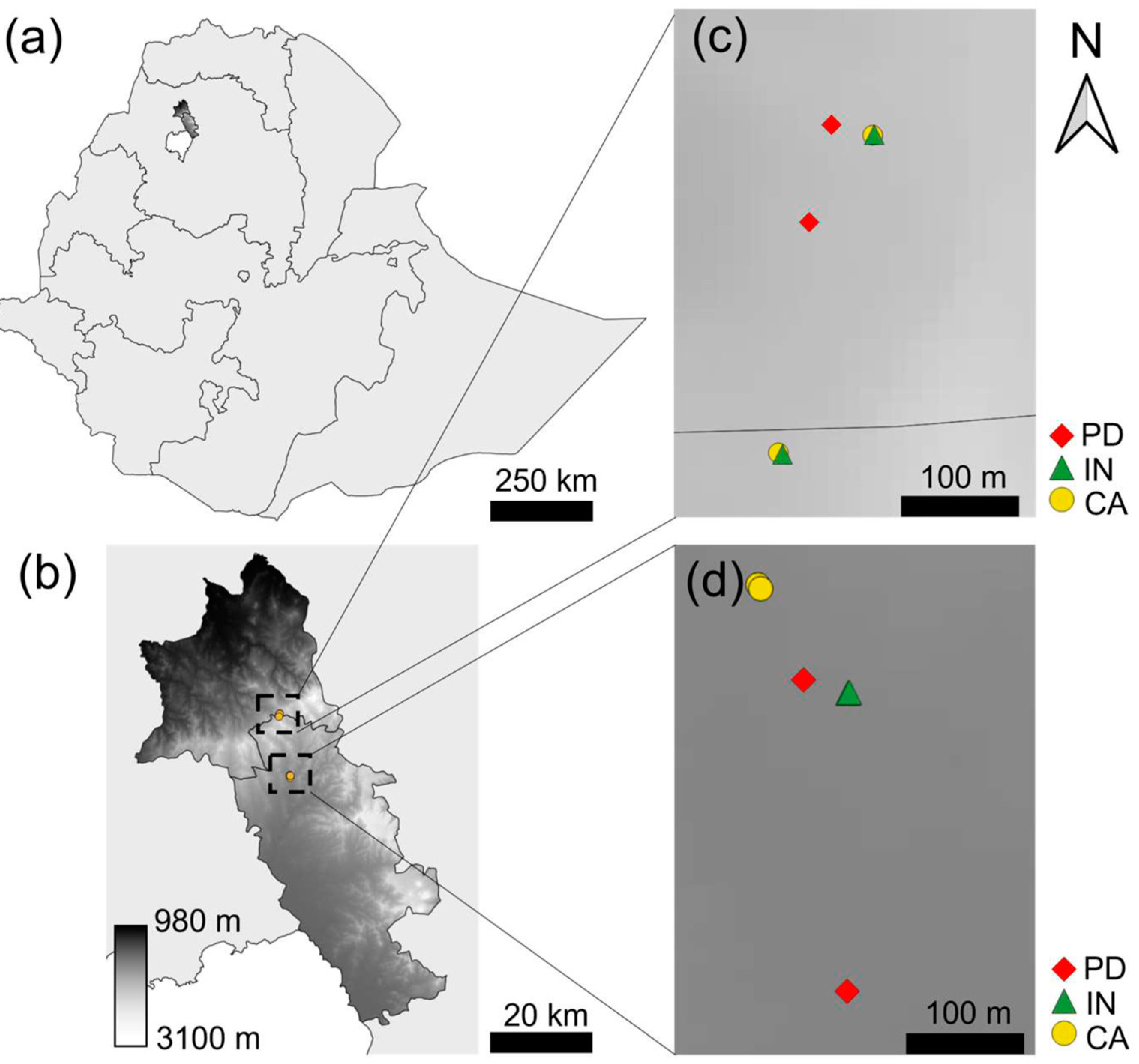
2.1. Description of Study Sites
2.2. Sand Fly Collections
2.3. Sandfly Dissection and Morphological Identification
2.4. Leishmania Detection in Sandflies
2.5. Data Analysis
3. Results
3.1. Species Typing
3.2. Spatio-Temporal Sandfly Abundance
3.3. Spatio-Temporal Variation in Sandfly Physiological Status
3.4. Leishmania Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory Data Repository; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Seid, A.; Gadisa, E.; Tsegaw, T.; Abera, A.; Teshome, A.; Mulugeta, A.; Herrero, M.; Argaw, D.; Jorge, A.; Kebede, A.; et al. Risk map for cutaneous leishmaniasis in Ethiopia based on environmental factors as revealed by geographical information systems and statistics. Geospat. Health 2014, 8, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Lemma, W.; Erenso, G.; Gadisa, E.; Balkew, M.; Gebre-Michael, T.; Hailu, A. A zoonotic focus of cutaneous leishmaniasis in Addis Ababa, Ethiopia. Parasites Vectors 2009, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Bugssa, G.; Hailu, A.; Demtsu, B. The Current Status of Cutaneous Leishmaniasis and the Pattern of Lesions in Ochollo Primary School Students, Ochollo, Southwestern Ethiopia. Sci. J. Clin. Med. 2014, 3, 111–116. [Google Scholar] [CrossRef]
- Ashford, R.W.; Bray, M.A.; Hutchinson, M.P.; Bray, R.S. The epidemiology of cutaneous leishmaniasis in Ethiopia. R. Soc. Trop. Med. Hyg. 1973, 67, 568–601. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.S.; Ashford, R.W.; Bray, M.A. The parasite causing cutaneous leishmaniasis in Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 1973, 67, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Bsrat, A.; Berhe, N.; Balkew, M.; Yohannes, M.; Teklu, T.; Gadisa, E. Epidemiological study of cutaneous leishmaniasis in Saesie Tsaeda-emba district, eastern Tigray, northern Ethiopia. Parasites Vectors 2015, 8, 149. [Google Scholar] [CrossRef]
- Amare, G.; Mekonnen, G.; Kassa, M.; Addisu, A.; Kendie, D.; Tegegne, B.; Abera, A.; Tadesse, D.; Getahun, S.; Wondmagegn, Y.; et al. First report of cutaneous leishmaniasis caused by Leishmania donovani in Ethiopia. Parasites Vectors 2023, 16, 457. [Google Scholar] [CrossRef]
- Ashford, W. Leishmaniasis Reservoirs and Their Significance in Control. Clin. Dermatol. 1996, 14, 523–532. [Google Scholar] [CrossRef]
- Mutinga, M.J.; Odhiambo, T.R. Cutaneous leishmaniasis in kenya—2. Studies on vector potential of phlebotomus-pedifer (diptera, phlebotomidae) in kenya. Insect Sci. Appl. 1986, 7, 171–174. [Google Scholar] [CrossRef]
- Svobodová, M.; Volf, P.; Votýpka, J. Experimental transmission of Leishmania tropica to hyraxes (Procavia capensis) by the bite of Phlebotomus arabicus. Microbes Infect. 2006, 8, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Pareyn, M.; Van Den Bosch, E.; Girma, N.; Van Houtte, N.; Van Dongen, S.; Van Der Auwera, G.; Massebo, F.; Shibru, S.; Leirs, H. Ecology and seasonality of sandflies and potential reservoirs of cutaneous leishmaniasis in Ochollo, a hotspot in southern Ethiopia. PLoS Negl. Trop. Dis. 2019, 13, e0007667. [Google Scholar] [CrossRef] [PubMed]
- Pareyn, M.; Kochora, A.; Van Rooy, L.; Eligo, N.; Vanden Broecke, B.; Girma, N.; Merdekios, B.; Wegayehu, T.; Maes, L.; Caljon, G.; et al. Feeding behavior and activity of Phlebotomus pedifer and potential reservoir hosts of Leishmania aethiopica in southwestern Ethiopia. PLoS Negl. Trop. Dis. 2020, 14, e0007947. [Google Scholar] [CrossRef] [PubMed]
- Van Breugel, P.; Lillesø, J.-P.B.; Kindt, R.; Bingham, M.; Demissew, S.; Dudley, C.; Friis, I.; Gachathi, F.; Kalema, J.; Mbago, F.; et al. Potential Natural Vegetation of Eastern Africa (Ethiopia, Kenya, Malawi, Rwanda, Tanzania, Uganda and Zambia); Forest & Landscape Denmark University of Copenhagen: Hørsholm, Denmark, 2011. [Google Scholar]
- Shaira, H.; Naik, P.R.; Pracheth, R.; Nirgude, A.S.; Nandy, S.; Hiba, M.M.; Karthika, S. Epidemiological profile and mapping geographical distribution of road traffic accidents reported to a tertiary care hospital, Mangaluru using quantum geographic information system (QGIS). J. Fam. Med. Prim. Care 2020, 9, 3652. [Google Scholar]
- Lewis, D. A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae). Bull. Br. Mus. 1982, 45, 121–209. [Google Scholar]
- Lewis, D.J.; Minter, D.M.; Ashford, R.W. The subgenus Larroussius of Phlebotomus (Diptera, Psychodidae) in the Ethiopian region. Bull. Entomol. Res. 1974, 64, 435–442. [Google Scholar] [CrossRef]
- Killick-Kendrick, R.; Tang, Y.; Killick-Kendrick, M.; Johnson, R.N.; Ngumbi, P.; Sang, D.; Lawyer, P. Phlebotomine sandflies of Kenya (Diptera: Psychodidae). III. The identification and distribution of species of the subgenus Larroussius. Ann. Trop. Med. Parasitol. 1994, 88, 183–196. [Google Scholar] [CrossRef]
- Abonnenc, E.; Minter, D.M. Keys for the identification of the sandflies of the Ethiopian region. Cah ORSTOM Entomol. Med. 1965, 5, 25–63. [Google Scholar]
- Kirk, K.; Lewis, D.J. Taxonomy of the Ethiopian sandflies (Phlebotomus)—II. Keys for the identification of the Ethiopian species. Ann. Trop. Med. Parasitol. 1946, 40, 117–129. [Google Scholar] [CrossRef]
- Eberhardt, E.; Van Den Kerkhof, M.; Bulté, D.; Mabille, D.; Van Bockstal, L.; Monnerat, S.; Alves, F.; Mbui, J.; Delputte, P.; Cos, P.; et al. Evaluation of a pan-Leishmania Spliced-Leader RNA detection method in human blood and experimentally infected syrian golden hamsters. J. Mol. Diagn. 2018, 20, 253–263. [Google Scholar] [CrossRef]
- Talmi-Frank, D.; Nasereddin, A.; Schnur, L.F.; Schönian, G.; Töz, S.Ö.; Jaffe, C.L.; Baneth, G. Detection and identification of old world leishmania by high resolution melt analysis. PLoS Negl. Trop. Dis. 2010, 4, 4–8. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- Yared, S.; Gebresilassie, A.; Akililu, E.; Deribe, K.; Balkew, M.; Warburg, A.; Hailu, A.; Gebre-michael, T. Diversity and altitudinal distribution of phlebotomine sand fl ies (Diptera: Psychodidae) in visceral leishmaniasis endemic areas of northwest Ethiopia. Acta Trop. 2017, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Anyosa, R.; Galvez-Petzoldt, C.; Garcia, P.J.; Carcamo, C.P. Housing characteristics and leishmaniasis: A systematic review. Am. J. Trop. Med. Hyg. 2018, 99, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Getawen, S.K.; Ashine, T.; Massebo, F.; Woldeyes, D.; Lindtjørn, B. Exploring the impact of house screening intervention on entomological indices and incidence of malaria in Arba Minch town, southwest Ethiopia: A randomized control trial. Acta Trop. 2018, 181, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Votýpka, J.; Pavlasova, M.; Volfova, V.; Volf, P. Rotation of male genitalia in various species of phlebotomine sandfly. Med. Vet. Entomol. 2015, 29, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Ashford, R. A possible reservoir for Leishmania tropica in Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 1969, 64, 936–937. [Google Scholar] [CrossRef] [PubMed]
- Alebie, G.; Worku, A.; Yohannes, S.; Urga, B.; Hailu, A.; Tadesse, D. Epidemiology of visceral leishmaniasis in Shebelle Zone of Somali Region, eastern Ethiopia. Parasites Vectors 2019, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Ashford, R. Sandflies (Diptera: Phlebotomidae) from Ethiopia: Taxinomic and Biological Notes. J. Med. Entomol. 1974, 11, 605–616. [Google Scholar] [CrossRef]
- Aklilu, E.; Gebresilassie, A.; Yared, S.; Kindu, M.; Tekie, H.; Balkew, M.; Warburg, A.; Hailu, A.; Gebre-Michael, T. Studies on sand fly fauna and ecological analysis of Phlebotomus orientalis in the highland and lowland foci of kala-azar in northwestern Ethiopia. PLoS ONE 2017, 12, e0175308. [Google Scholar] [CrossRef]
- Gebre-Michael, T.; Balkew, M.; Gudeta, N.; Reta, M. Preliminary entomological observations in a highland area of Amhara region, northern Ethiopia, with epidemic visceral leishmaniasis. Ann. Trop. Med. Parasitol. 2007, 101, 367–370. [Google Scholar] [CrossRef]
- Odiwuor, S.; De Docker, S.; Maes, I.; Dujardin, J.; Van der Auwera, G. Natural Leishmania donovani/Leishmania aethiopica hybrids identified from Ethiopia. Infect. Genet. Evol. 2011, 11, 2113–2118. [Google Scholar] [CrossRef]
- Hadermann, A.; Heeren, S.; Maes, I.; Dujardin, J.; Domagalska, M.A.; Van Den Broeck, F.; Mauricio, I. Genome diversity of Leishmania aethiopica. Front. Cell. Infect. Microbiol. 2023, 13, 406. [Google Scholar] [CrossRef]

| Gindmeteaye | Addis-Alem | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rural | Urban | |||||||||
| Female | Male | Total | Female | Male | Total | Female | Male | Total | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Phlebotomus | ||||||||||
| P. longipes | 203 | 266 | 466 | 227 | 203 | 429 | 430 | 469 | 899 | |
| (80.2) | (89.0) | (84.9) | (69.8) | (71.0) | (70.1) | (74.7) | (80.2) | (76.6) | ||
| P. orientalis | 2 | 4 | 6 | 0 | 0 | 0 | 2 | 4 | 6 | |
| (0.8) | (1.3) | (1.1) | (0.0) | (0.0) | (0.0) | (0.3) | (0.7) | (0.5) | ||
| Sergentomyia | ||||||||||
| S. adleri | 7 | 9 | 16 | 1 | 9 | 10 | 5 | 18 | 23 | |
| (2.8) | (3.0) | (2.9) | (0.3) | (3.1) | (1.6) | (0.9) | (3.1) | (2.0) | ||
| S. clydie | 17 | 17 | 34 | 7 | 34 | 41 | 24 | 51 | 77 | |
| (6.7) | (5.7) | (6.2) | (2.2) | (11.9) | (6.7) | (4.2) | (8.8) | (6.6) | ||
| S. schwezi | 24 | 3 | 27 | 90 | 40 | 132 | 113 | 43 | 156 | |
| (9.5) | (1.0) | (4.9) | (27.7) | (14.0) | (21.6) | (19.6) | (7.4) | (13.4) | ||
| Overall total | 253 | 299 | 549 | 325 | 286 | 612 | 576 | 585 | 1161 | |
| Dry | Wet | Both Seasons | ||
|---|---|---|---|---|
| Addis-Alem (urban) | ||||
| Indoor | 49 (28%) | 30 (60%) | 79 (35%) | |
| Peridomestic | 61 (34%) | 13 (26%) | 74 (32%) | |
| Rocky/cave | 68 (38%) | 7 (14%) | 75 (33%) | |
| Total | 178 (78%) | 50 (22%) | 228 | |
| Gindmeteaye (rural) | ||||
| Indoor | 24 (31%) | 79 (63%) | 103 (51%) | |
| Peridomestic | 27 (36%) | 37 (29%) | 64 (32%) | |
| Rocky/cave | 25 (33%) | 10 (8%) | 35 (17%) | |
| Total | 76 (38%) | 126 (62%) | 202 | |
| Villages combined | ||||
| Indoor | 73 (29%) | 109 (62%) | 182 (42%) | |
| Peridomestic | 88 (34%) | 50 (28%) | 138 (32%) | |
| Rocky/cave | 93 (37%) | 17 (10%) | 110 (26%) | |
| Total | 254 (59%) | 176 (41%) | 430 | |
| Dry | Wet | Both Seasons | ||
|---|---|---|---|---|
| Indoor | ||||
| Blood-fed | 15 (20%) | 29 (27%) | 44 (24%) | |
| Gravid | 5 (7%) | 24 (22%) | 29 (16%) | |
| Non-fed/sugar-fed | 53 (73%) | 56 (51%) | 109 (60%) | |
| Total | 73 | 109 | 182 | |
| Peridomestic | ||||
| Blood-fed | 6 (7%) | 20 (40%) | 26 (19%) | |
| Gravid | 8 (9%) | 10 (20%) | 18 (13%) | |
| Non-fed/sugar-fed | 74 (84%) | 20 (40%) | 94 (68%) | |
| Total | 88 | 50 | 138 | |
| Caves/rocky areas | ||||
| Blood-fed | 12 (13%) | 6 (35%) | 18 (16%) | |
| Gravid | 8 (9%) | 4 (24%) | 12 (11%) | |
| Non-fed/sugar-fed | 73 (78%) | 7 (41%) | 80 (73%) | |
| Total | 93 | 17 | 110 | |
| All habitats combined | ||||
| Blood-fed | 33 (13%) | 55 (31%) | 88 (20%) | |
| Gravid | 21 (8%) | 38 (22%) | 59 (14%) | |
| Non-fed/sugar-fed | 200 (79%) | 83 (47%) | 283 (66%) | |
| Total | 254 | 176 | 430 | |
| Total | Infected | ||
|---|---|---|---|
| Trap site | |||
| Addis Alem (urban) | 228 | 14 (6%) | |
| Gindmeteaye (rural) | 202 | 4 (2%) | |
| Habitat | |||
| Caves | 110 | 8 (7%) | |
| Peridomestic | 138 | 5 (4%) | |
| Indoors | 182 | 5 (3%) | |
| Season | |||
| Dry | 254 | 15 (6%) | |
| Wet | 176 | 3 (2%) | |
| Blood feeding status | |||
| Non-fed | 283 | 10 (4%) | |
| Blood-fed | 88 | 5 (6%) | |
| Gravid | 59 | 3 (5%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jemberie, W.; Animut, A.; Dugassa, S.; Gebresilassie, A.; Melkamu, R.; Aklilu, E.; Aemero, M.; van Griensven, J.; Pareyn, M. Ecology and Infection Status of Sand Flies in Rural and Urban Cutaneous Leishmaniasis Endemic Areas in Northwest Ethiopia. Trop. Med. Infect. Dis. 2024, 9, 52. https://doi.org/10.3390/tropicalmed9030052
Jemberie W, Animut A, Dugassa S, Gebresilassie A, Melkamu R, Aklilu E, Aemero M, van Griensven J, Pareyn M. Ecology and Infection Status of Sand Flies in Rural and Urban Cutaneous Leishmaniasis Endemic Areas in Northwest Ethiopia. Tropical Medicine and Infectious Disease. 2024; 9(3):52. https://doi.org/10.3390/tropicalmed9030052
Chicago/Turabian StyleJemberie, Wondmeneh, Abebe Animut, Sisay Dugassa, Araya Gebresilassie, Roma Melkamu, Esayas Aklilu, Mulugeta Aemero, Johan van Griensven, and Myrthe Pareyn. 2024. "Ecology and Infection Status of Sand Flies in Rural and Urban Cutaneous Leishmaniasis Endemic Areas in Northwest Ethiopia" Tropical Medicine and Infectious Disease 9, no. 3: 52. https://doi.org/10.3390/tropicalmed9030052
APA StyleJemberie, W., Animut, A., Dugassa, S., Gebresilassie, A., Melkamu, R., Aklilu, E., Aemero, M., van Griensven, J., & Pareyn, M. (2024). Ecology and Infection Status of Sand Flies in Rural and Urban Cutaneous Leishmaniasis Endemic Areas in Northwest Ethiopia. Tropical Medicine and Infectious Disease, 9(3), 52. https://doi.org/10.3390/tropicalmed9030052






