In Vitro and Ex Vivo Synergistic Effect of Pyrvinium Pamoate Combined with Miltefosine and Paromomycin against Leishmania
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Parasites
2.3. Experimental Animals and Ethical Statement
2.4. Experimental Infections and Set up of Primary Cultures
2.5. Axenic and Intramacrophagic Amastigotes Viability Assays
2.6. Preparation of Murine Intestinal Organoids
2.7. Cytotoxicity and Gut Tolerability of Drugs and Drug Combinations
2.8. Statistical Analysis
3. Results
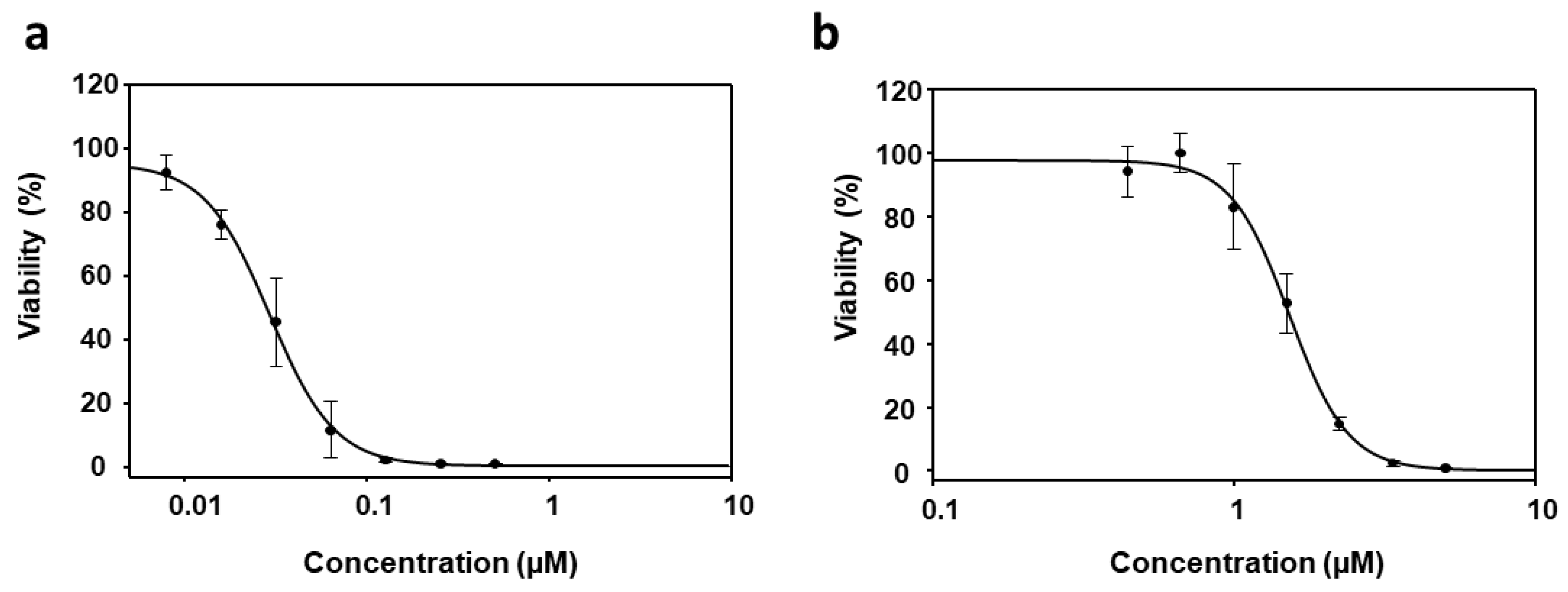
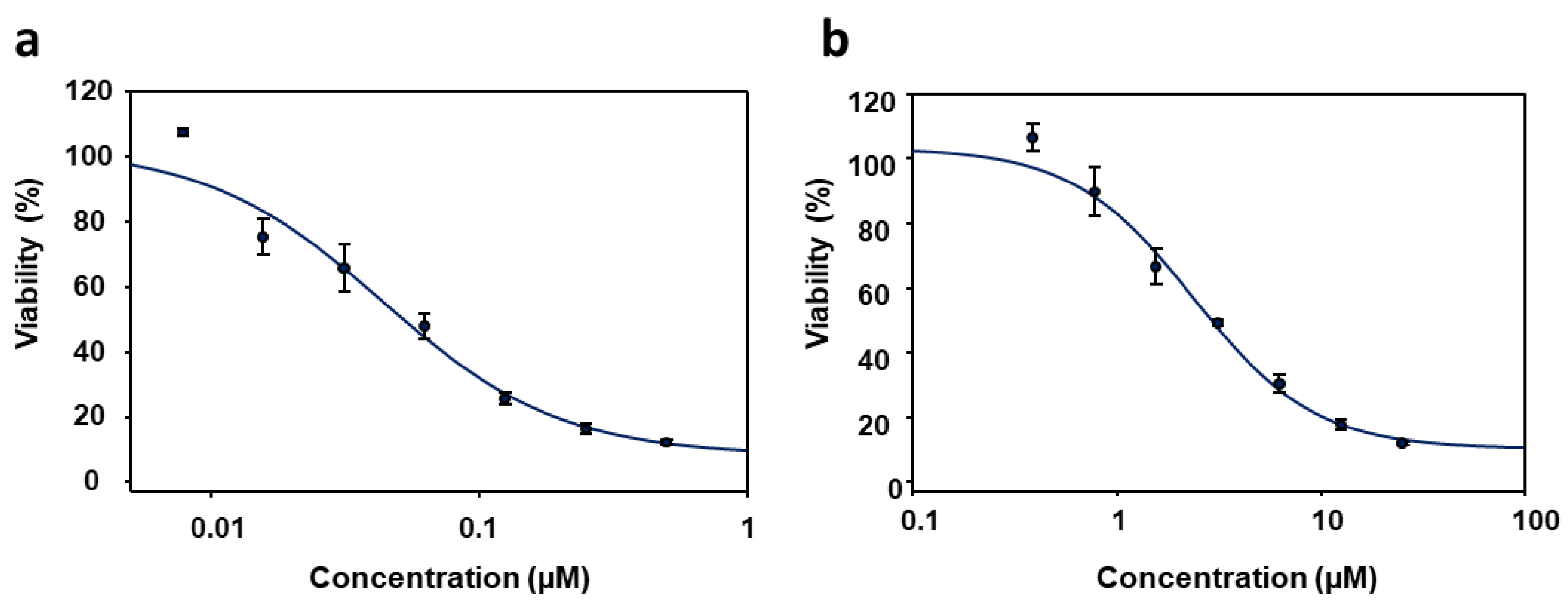
3.1. Effect of PyP Combined with MTF in Axenic Amastigotes
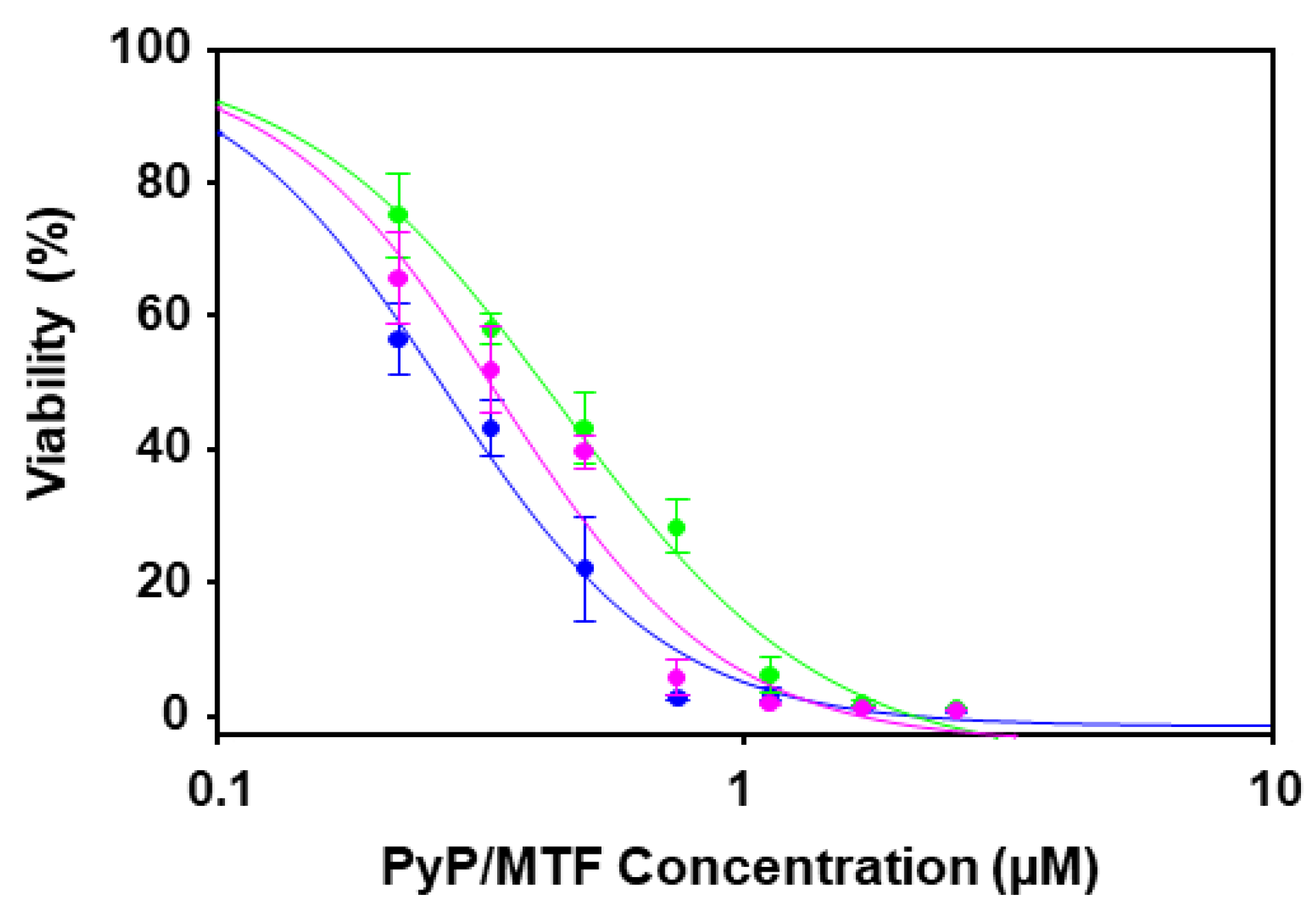
3.2. Effect of PyP Combined with MTF in Intramacrophagic Amastigotes
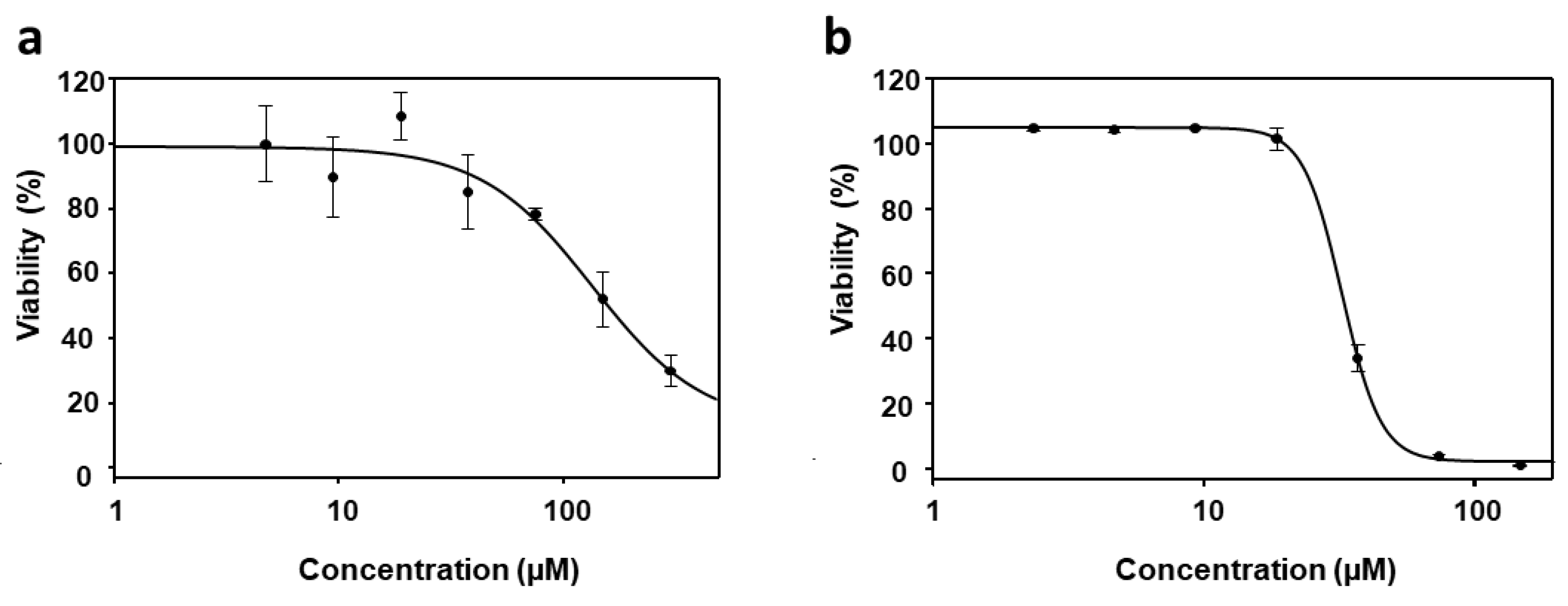
3.3. Cytotoxicity of PyP Combined with MTF in Spleen Cells and Intestinal Organoids
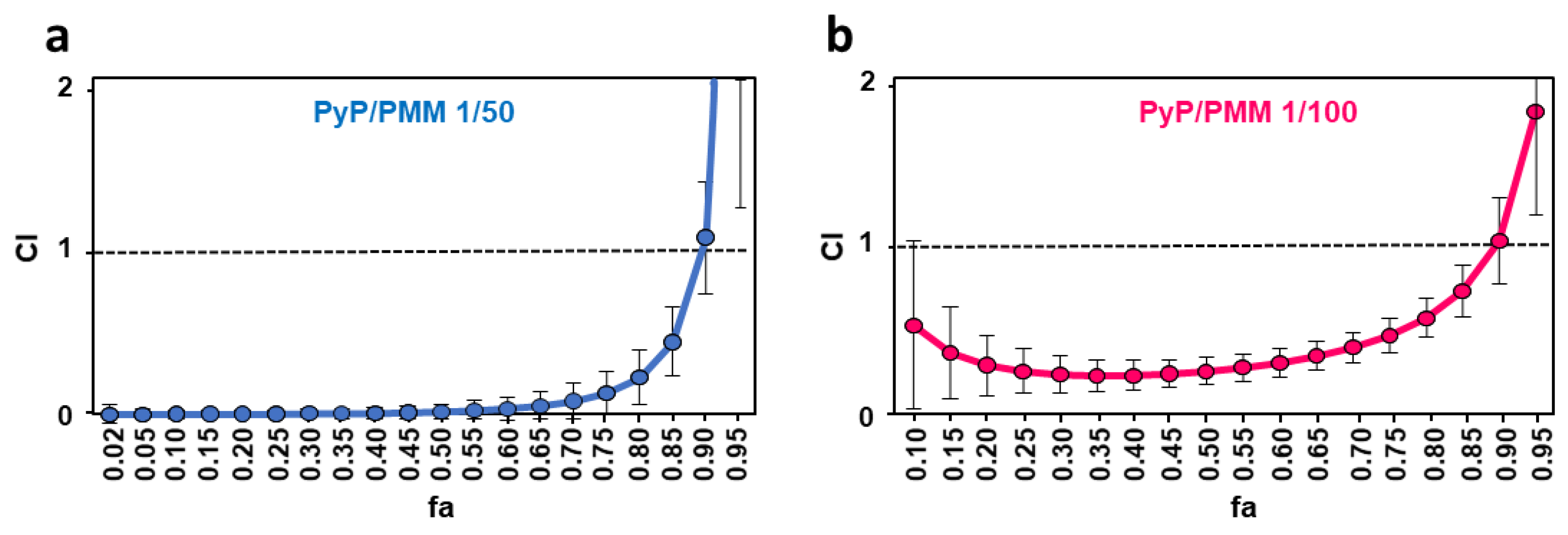
3.4. Effect of PyP Combined with PMM in Intramacrophagic Amastigotes
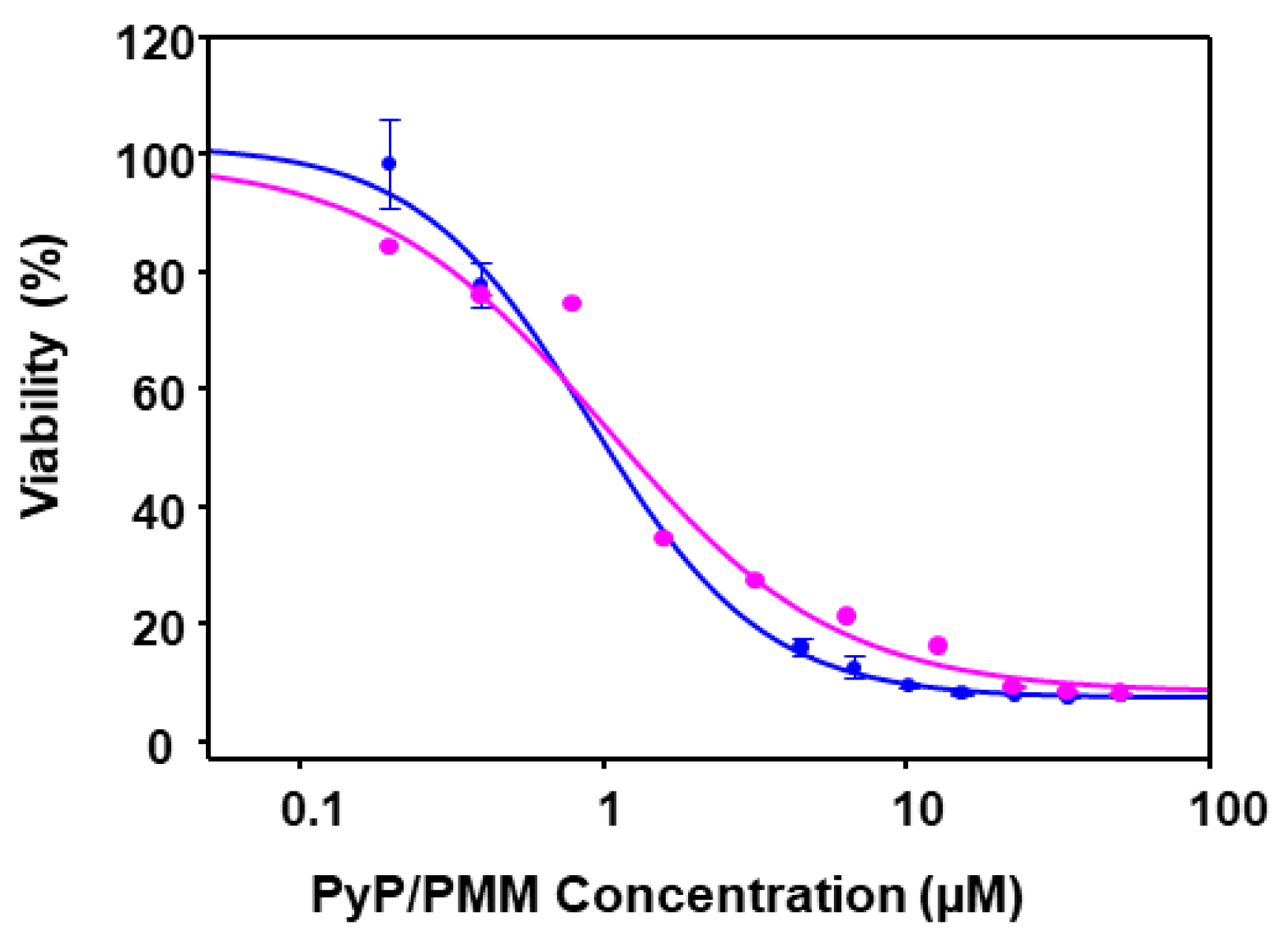
| CI Values at the Following Effect Levels | |||||||
|---|---|---|---|---|---|---|---|
| Drug/s | Dm | m | r | 25% | 50% | 75% | 90% |
| PMM | 8.96 | 0.36 | 0.96 | *N/A | *N/A | *N/A | *N/A |
| PyP | 0.02 | 0.34 | 0.88 | *N/A | *N/A | *N/A | *N/A |
| PyP/PMM 1/50 | 0.02 | 0.34 | 0.88 | 0.003 ± 0.02 | 0.02 ± 0.05 | 0.13 ± 0.13 | 1.05 ± 0.33 |
| PyP/PMM 1/100 | 0.51 | 0.57 | 0.98 | 0.25 ± 0.13 | 0.25 ± 0.08 | 0.46 ± 0.1 | 1.02 ± 0.25 |
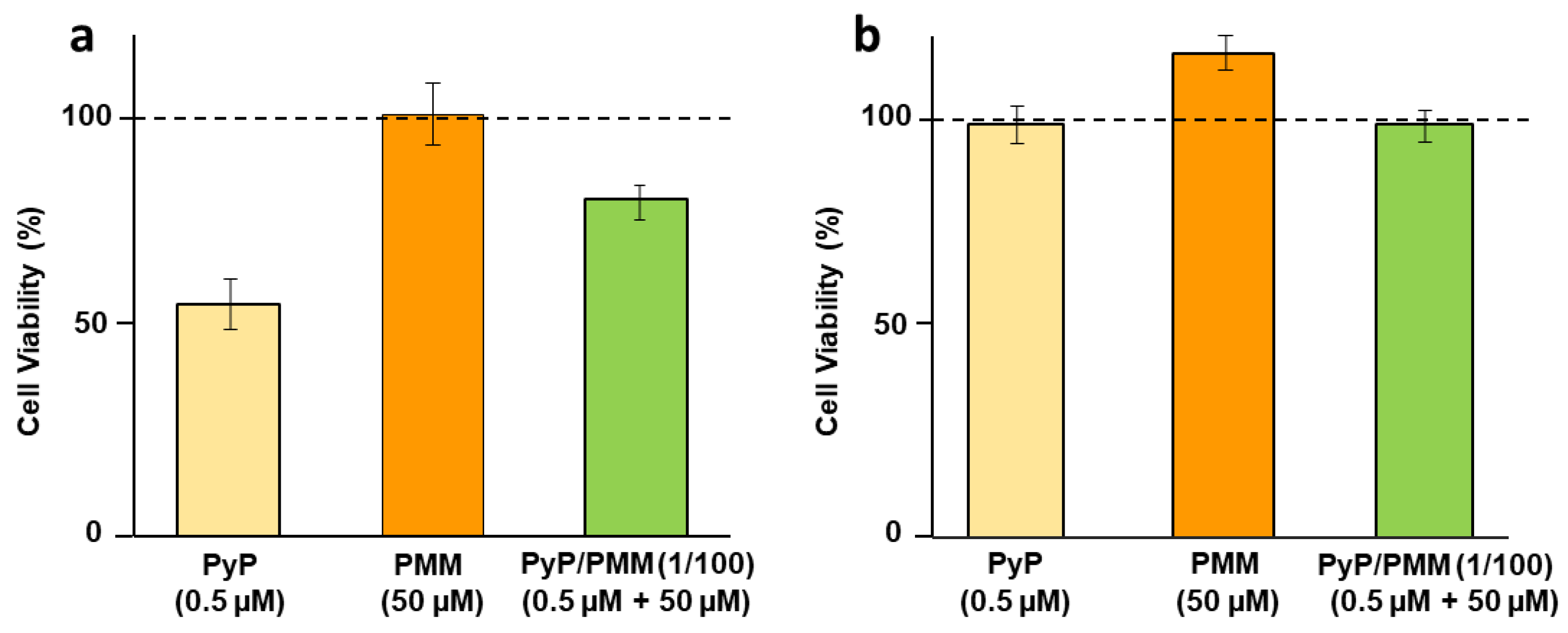
3.5. Cytotoxicity of PyP Combined with PMM in Spleen Cells and Intestinal Organoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef]
- van Griensven, J.; Diro, E. Visceral Leishmaniasis. Infect. Dis. Clin. N. Am. 2012, 26, 309–322. [Google Scholar] [CrossRef]
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Diro, E. Visceral leishmaniasis, recent advances in diagnostic and treatment regimens. Infect. Dis. Clin. N. Am. 2019, 33, 79–99. [Google Scholar] [CrossRef]
- WHO. Control of the Leishmaniasis. In Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases; World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/44412 (accessed on 21 June 2023).
- Goyal, V.; Das, V.N.R.; Singh, S.N.; Singh, R.S.; Pandey, K.; Verma, N.; Hightower, A.; Rijal, S.; Das, P.; Alvar, J.; et al. Long-term incidence of relapse and post-kala-azar dermal leishmaniasis after three different visceral leishmaniasis treatment regimens in Bihar, India. PLoS Negl. Trop. Dis. 2020, 14, e0008429. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A.; Rai, M.; Prajapati, V.K.; Singh, A.K.; Ostyn, B.; Boelaert, M.; Dujardin, J.C.; Chakravarty, J. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin. Infec. Dis. 2012, 55, 543–550. [Google Scholar] [CrossRef]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef]
- Dorlo, T.P.; Rijal, S.; Ostyn, B.; de Vries, P.J.; Singh, R.; Bhattarai, N.; Uranw, S.; Dujardin, J.C.; Boelaert, M.; Beijnen, J.H.; et al. Failure of miltefosine in visceral leishmaniasis is associated with low drug exposure. J. Infect. Dis. 2014, 210, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hailu, A.; Musa, A.; Wasunna, M.; Balasegaram, M.; Yifru, S.; Mengistu, G.; Hurissa, Z.; Hailu, W.; Weldegebreal, T.; Tesfaye, S.; et al. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: A multicentre, open-label, randomized trial. PLoS Negl. Trop. Dis. 2010, 4, e709. [Google Scholar] [CrossRef]
- Reguera, R.; Pérez-Pertejo, Y.; Gutiérrez-Corbo, C.; Domínguez-Asenjo, B.; Ordóñez, C.; García-Estrada, C.; Martínez-Valladares, M.; Balaña-Fouce, R. Current and promising novel drug candidates against visceral leishmaniasis. Pure Appl. Chem. 2019, 91, 1385–1404. [Google Scholar] [CrossRef]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef]
- Musa, A.; Khalil, E.; Hailu, A.; Olobo, J.; Balasegaram, M.; Omollo, R.; Edwards, T.; Rashid, J.; Mbui, J.; Musa, B.; et al. Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: A randomised controlled trial. PLoS Negl. Trop. Dis. 2012, 6, e1674. [Google Scholar]
- Balaña-Fouce, R.; Pérez-Pertejo, M.Y.; Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Reguera, R.M. Walking a tightrope: Drug discovery in visceral leishmaniasis. Drug Discov. Today 2019, 24, 1209–1216. [Google Scholar] [CrossRef]
- Musa, A.M.; Mbui, J.; Mohammed, R.; Olobo, J.; Ritmeijer, K.; Alcoba, G.; Muthoni Ouattara, G.; Egondi, T.; Nakanwagi, P.; Omollo, T.; et al. Paromomycin and miltefosine combination as an alternative to treat patients with visceral leishmaniasis in eastern Africa: A randomized, controlled, multicountry trial. Clin. Infect. Dis. 2023, 76, e1177–e1185. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Balasegaram, M.; Meheus, F.; Alvar, J.; Lynen, L.; Boelaert, M. Combination therapy for visceral leishmaniasis. Lancet Infec. Dis. 2010, 10, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Chen, X.; Shi, L.; Liu, J.O.; Sullivan, D.J., Jr. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat. Chem. Biol. 2006, 2, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Lougheed, K.E.; Taylor, D.L.; Osborne, S.A.; Bryans, J.S.; Buxton, R.S. New anti-tuberculosis agents amongst known drugs. Tuberculosis 2009, 89, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Abdollahi, E.; Ghasemi, F.; Caraglia, M.; Sahebkar, A. The novel role of pyrvinium in cancer therapy. J. Cell. Physiol. 2018, 233, 2871–2881. [Google Scholar] [CrossRef]
- Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Balaña-Fouce, R.; Reguera, R.M. Ex. vivo phenotypic screening of two small repurposing drug collections identifies nifuratel as a potential new treatment against visceral and cutaneous leishmaniasis. ACS Infect. Dis. 2021, 7, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.A.; Barrow, W.B.; Heffelfinger, J.C.; Kinkel, A.W.; Smith, T.C.; Turner, J.L. Pyrvinium pamoate. Clin. Pharmacol. Ther. 1974, 16, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.C.; Kinkel, A.W.; Gryczko, C.M.; Goulet, J.R. Absorption of pyrvinium pamoate. Clin. Pharmacol. Ther. 1976, 19, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.O.; Bolton, E.C.; Huang, Y.; Feau, C.; Guy, R.K.; Yamamoto, K.R.; Hann, B.; Diamond, M.I. Non-competitive androgen receptor inhibition in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 7233–7238. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.W.; McCarthy, G.A.; Nerwal, T.; Nevler, A.; DuHadaway, J.B.; McCoy, M.D.; Jiang, W.; Brown, S.Z.; Goetz, A.; Jain, A.; et al. The FDA-approved anthelmintic pyrvinium pamoate inhibits pancreatic cancer cells in nutrient-depleted conditions by targeting the mitochondria. Mol. Cancer Ther. 2021, 20, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Álvarez, E.; Stamatakis, K.; Punzón, C.; Álvarez-Velilla, R.; Tejería, A.; Escudero-Martínez, J.M.; Pérez-Pertejo, Y.; Fresno, M.; Balaña-Fouce, R.; Reguera, R.M. Infrared fluorescent imaging as a potent tool for in vitro, ex vivo and in vivo models of visceral leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003666. [Google Scholar] [CrossRef] [PubMed]
- Filonov, G.S.; Piatkevich, K.D.; Ting, L.M.; Zhang, J.; Kim, K.; Verkhusha, V.V. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat. Biotechnol. 2012, 29, 757–761. [Google Scholar] [CrossRef]
- Reguera, R.; Fouce, R.B.; Cubria, J.C.; Bujidos, M.L.A.; Ordonez, D. Putrescine uptake inhibition by aromatic diamidines in Leishmania. infantum promastigotes. Biochem. Pharmacol. 1994, 47, 1859–1866. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Montano, N.; Amador, L.A.; Álvarez-Velilla, R.; Balaña-Fouce, R. Novel very long-chain α-methoxylated Δ5.9 fatty acids from the sponge Asteropus niger are effective inhibitors of topoisomerases IB. Lipids 2016, 51, 245–256. [Google Scholar] [CrossRef]
- De Muylder, G.; Ang, K.K.; Chen, S.; Arkin, M.R.; Engel, J.C.; McKerrow, J.H. A screen against Leishmania intracellular amastigotes: Comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl. Trop. Dis. 2011, 5, e1253. [Google Scholar] [CrossRef]
- Nandikolla, A.; Srinivasarao, S.; Karan Kumar, B.; Murugesan, S.; Aggarwal, H.; Balaña-Fouce, R.; Melcón-Fernández, E.; Pérez-Pertejo, Y.; Chandra Sekhar, K.V.G. Novel phenanthridine amide analogs as potential anti-leishmanial agents: In vitro and in silico insights. Bioorg. Chem. 2021, 117, 105414. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Niu, Q.; Mo, X.; Qui, M.; Ma, T.; Kuo, C.J.; Fu, H. Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening. J. Mol. Cell Biol. 2020, 12, 630–643. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical Basis, Experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Sundar, S.; More, D.K.; Singh, M.K.; Singh, V.P.; Sharma, S.; Makharia, A.; Kumar, P.C.; Murray, H.W. Failure of pentavalent antimony in visceral leishmaniasis in India: Report from the center of the Indian epidemic. Clin. Infect. Dis. 2000, 31, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Lira, R.; Sundar, S.; Makharia, A.; Kenney, R.; Gam, A.; Saraiva, E.; Sacks, D. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 1999, 180, 564–567. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e000605. [Google Scholar] [CrossRef]
- Deep, D.K.; Singh, R.; Bhandari, V.; Verma, A.; Sharma, V.; Wajid, S.; Sundar, S.; Ramesh, V.; Dujardin, J.C.; Salotra, P. Increased miltefosine tolerance in clinical isolates of Leishmania donovani is associated with reduced drug accumulation, increased infectivity and resistance to oxidative stress. PLoS Negl. Trop. Dis. 2017, 11, e0005641. [Google Scholar] [CrossRef]
- Maarouf, M.; Adeline, M.T.; Solignac, M.; Vautrin, D.; Robert-Gero, M. Development and characterization of paromomycin-resistant Leishmania. donovani promastigotes. Parasite 1998, 5, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.M.; Balaña Fouce, R.; Cubría, J.C.; Alvarez Bujidos, M.L.; Ordóñez, D. Fluorinated analogues of L-ornithine are powerful inhibitors of ornithine decarboxylase and cell growth of Leishmania infantum promastigotes. Life Sci. 1994, 56, 223–230. [Google Scholar] [CrossRef]
- Yun, O.; Priotto, G.; Tong, J.; Flevaud, L.; Chappuis, F. NECT is next: Implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e720. [Google Scholar] [CrossRef] [PubMed]
- Melcón-Fernández, E.; Galli, G.; García-Estrada, C.; Balaña-Fouce, R.; Reguera, R.M.; Pérez-Pertejo, Y. Miltefosine and nifuratel combination: A promising therapy for the treatment of Leishmania donovani visceral leishmaniasis. Int. J. Mol. Sci. 2023, 24, 1635. [Google Scholar] [CrossRef] [PubMed]
- Downey, A.S.; Chong, C.R.; Graczyk, T.K.; Sullivan, D.J. Efficacy of pyrvinium pamoate against Cryptosporidium. parvum infection in vitro and in a neonatal mouse model. Antimicrob. Agents Chemother. 2008, 52, 3106–3112. [Google Scholar] [CrossRef]
- Beck, J.W.; Saavedra, D.; Antell, G.J.; Tejeiro, B. The treatment of pinworm infections in humans (enterobiasis) with pyrvinium chloride and pyrvinium pamoate. Am. J. Trop. Med. Hyg. 1959, 8, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Teguh, S.C.; Klonis, N.; Duffy, S.; Lucantoni, L.; Avery, V.M.; Hutton, C.A.; Baell, J.B.; Tilley, L. Novel conjugated quinoline-indoles compromise Plasmodium falciparum mitochondrial function and show promising antimalarial activity. J. Med. Chem. 2013, 56, 6200–6215. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, V.R.; Karale, U.B.; Govindarajalu, G.; Adhikari, N.; Krishna, E.V.; Krishna, V.S.; Misra, S.; Sriram, D.; Sijwali, P.S.; Rode, H.B. Synthesis and efficacy of pyrvinium-inspired analogs against tuberculosis and malaria pathogens. Bioorganic Med. Chem. Lett. 2020, 30, 127037. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.W.; Nevler, A. Pyrvinium pamoate: Past, present, and future as an anti-cancer drug. Biomedicines 2022, 10, 3249. [Google Scholar] [CrossRef]
- Barratt, G.; Saint-Pierre-Chazalet, M.; Loiseau, P.M. Cellular transport and lipid interactions of miltefosine. Curr. Drug Metab. 2009, 10, 247–255. [Google Scholar] [CrossRef]
- Luque-Ortega, J.R.; Rivas, L. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1327–1332. [Google Scholar] [CrossRef]
- Paris, C.; Loiseau, P.M.; Bories, C.; Breard, J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother 2004, 48, 852–859. [Google Scholar] [CrossRef]
- Iacano, A.J.; Lewis, H.; Hazen, J.E.; Andro, H.; Smith, J.D.; Gulshan, K. Miltefosine increases macrophage cholesterol release and inhibits NLRP3-inflammasome assembly and IL-1β release. Sci. Rep. 2019, 9, 11128. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, M.; de Kouchkovsky, Y.; Brown, S.; Petit, P.X.; Robert-Gero, M. In vivo interference of paromomycin with mitochondrial activity of Leishmania. Exp. Cell Res. 1997, 232, 339–348. [Google Scholar] [CrossRef]
- Maarouf, M.; Lawrence, F.; Croft, S.L.; Robert-Gero, M. Ribosomes of Leishmania are a target for the aminoglycosides. Parasitol. Res. 1995, 81, 421–425. [Google Scholar] [CrossRef]
- Shalev, M.; Rozenberg, H.; Smolkin, B.; Nasereddin, A.; Kopelyanskiy, D.; Belakhov, V.; Schrepfer, T.; Schacht, J.; Jaffe, C.L.; Adir, N.; et al. Structural basis for selective targeting of leishmanial ribosomes: Aminoglycoside derivatives as promising therapeutics. Nucleic Acids Res. 2015, 43, 8601–8613. [Google Scholar] [CrossRef]
- Shalev-Benami, M.; Zhang, Y.; Rozenberg, H.; Nobe, Y.; Taoka, M.; Matzov, D.; Zimmerman, E.; Bashan, A.; Isobe, T.; Jaffe, C.L.; et al. Atomic resolution snapshot of Leishmania ribosome inhibition by the aminoglycoside paromomycin. Nat. Commun. 2017, 8, 1589. [Google Scholar] [CrossRef]
- Das, S.; Rani, M.; Pandey, K.; Sahoo, G.C.; Rabidas, V.N.; Singh, D.; Das, P. Combination of paromomycin and miltefosine promotes TLR4-dependent induction of antileishmanial immune response in vitro. J. Antimicrob. Chemother. 2012, 67, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Tomitsuka, E.; Kita, K.; Esumi, H. The NADH-fumarate reductase system, a novel mitochondrial energy metabolism, is a new target for anticancer therapy in tumor microenvironments. Ann. N. Y. Acad. Sci. 2010, 1201, 44–49. [Google Scholar] [CrossRef] [PubMed]


| Drug/s | CI Values at the Following Effect Levels | ||||||
|---|---|---|---|---|---|---|---|
| Dm | m | r | 25% | 50% | 75% | 90% | |
| MTF | 1.45 | 3.82 | 0.99 | *N/A | *N/A | *N/A | *N/A |
| PyP | 0.03 | 1.86 | 0.97 | *N/A | *N/A | *N/A | *N/A |
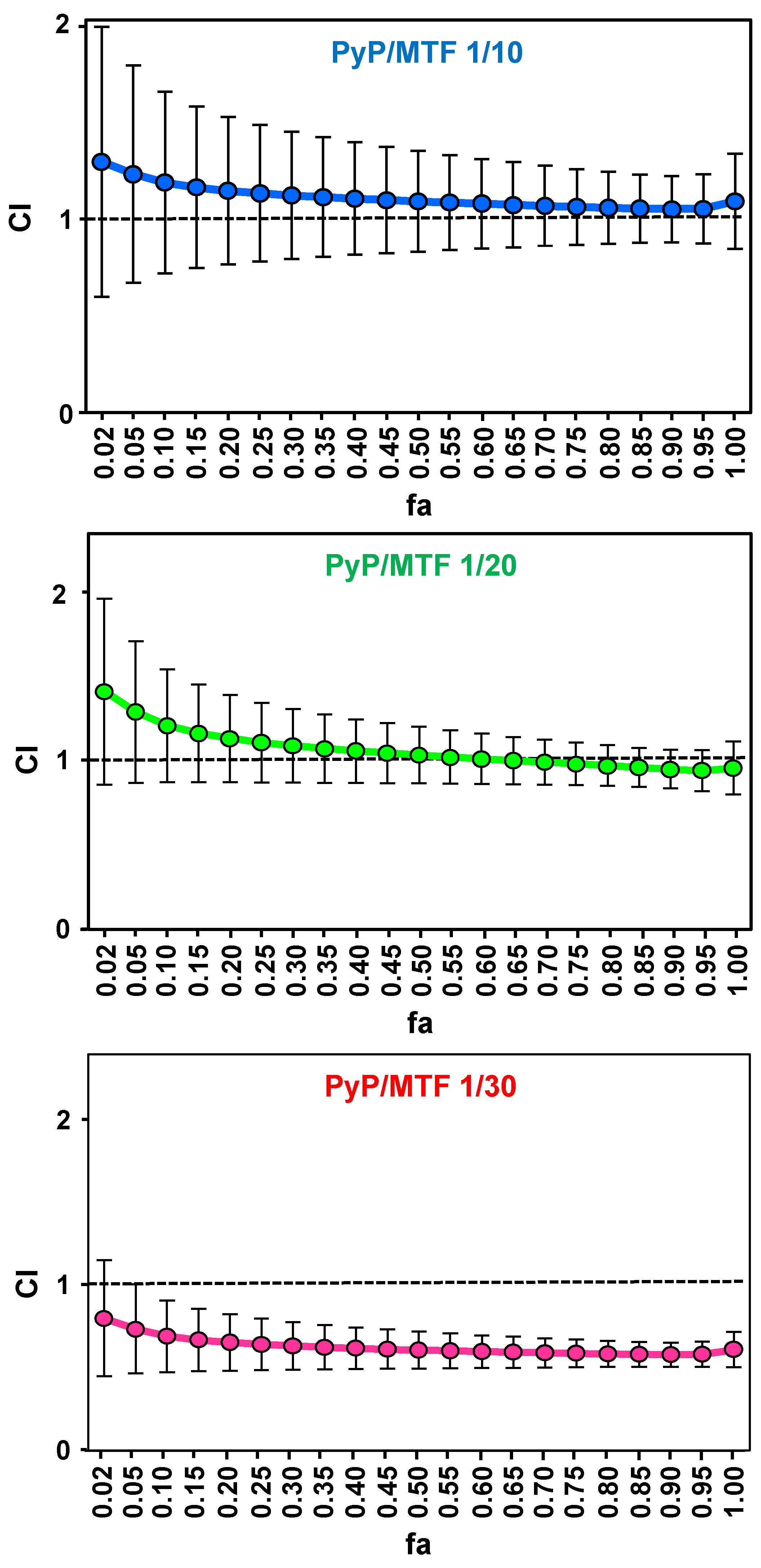
| PyP/MTF 1/10 | 0.24 | 2.15 | 0.96 | 1.13 ± 0.35 | 1.09 ± 0.26 | 1.06 ± 0.35 | 1.05 ± 0.17 |
| PyP/MTF 1/20 | 0.39 | 2.45 | 0.99 | 1.09 ± 0.23 | 1.02 ± 0.17 | 0.97 ± 0.13 | 0.94 ± 0.11 |
| PyP/MTF 1/30 | 0.31 | 2.50 | 0.97 | 0.64 ± 0.16 | 0.60 ± 0.11 | 0.58 ± 0.08 | 0.58 ± 0.07 |
| Drug Combination | DRI Values at the Following Effect Levels | |||||||
|---|---|---|---|---|---|---|---|---|
| 25% | 50% | 75% | 90% | |||||
| MTF | PyP | MTF | PyP | MTF | PyP | MTF | PyP | |
| PyP/MTF 1/10 | 7.51 | 1.00 | 6.00 | 1.08 | 4.79 | 1.17 | 3.83 | 1.27 |
| PyP/MTF 1/20 | 4.34 | 1.16 | 3.69 | 1.33 | 3.14 | 1.54 | 2.68 | 1.77 |
| PyP/MTF 1/30 | 5.48 | 2.19 | 4.71 | 2.55 | 4.05 | 2.97 | 3.48 | 3.46 |
| Drug/s | CI Values at the Following Effect Levels | ||||||
|---|---|---|---|---|---|---|---|
| Dm | m | r | 25% | 50% | 75% | 90% | |
| MTF | 3.67 | 2.72 | 0.81 | *N/A | *N/A | *N/A | *N/A |
| PyP | 0.09 | 1.74 | 0.86 | *N/A | *N/A | *N/A | *N/A |
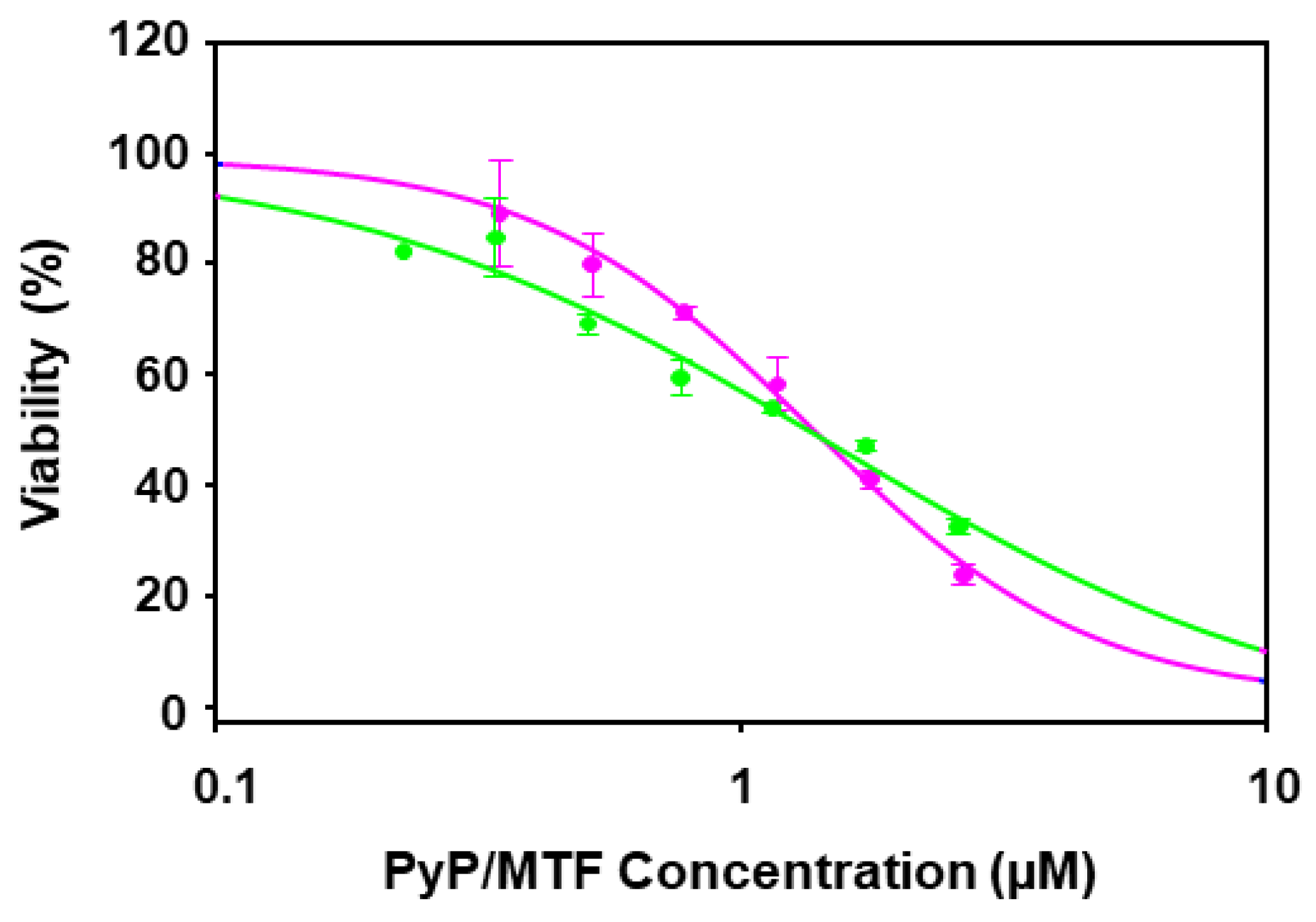
| PyP/MTF 1/20 | 1.28 | 2.66 | 0.88 | 1.27 ± 0.44 | 1.09 ± 0.40 | 0.95 ± 0.43 | 0.84 ± 0.49 |
| PyP/MTF 1/30 | 1.27 | 0.92 | 0.99 | 0.43 ± 0.11 | 0.83 ± 0.23 | 1.63 ± 0.57 | 3.23 ± 1.51 |
| Drug Combination | DRI Values at the Following Effect Levels | |||||||
|---|---|---|---|---|---|---|---|---|
| 25% | 50% | 75% | 90% | |||||
| MTF | PyP | MTF | PyP | MTF | PyP | MTF | PyP | |
| PyP/MTF 1/20 | 2.90 | 1.09 | 2.87 | 1.35 | 2.84 | 1.67 | 2.82 | 2.08 |
| PyP/MTF 1/30 | 6.43 | 3.61 | 2.90 | 2.04 | 1.31 | 1.15 | 0.59 | 0.65 |
| Drug Combination | DRI Values at the Following Effect Levels | |||||||
|---|---|---|---|---|---|---|---|---|
| 25% | 50% | 75% | 90% | |||||
| PMM | PyP | PMM | PyP | PMM | PyP | PMM | PyP | |
| PyP/PMM 1/50 | 586.97 | 575.55 | 463.90 | 68.12 | 366.64 | 8.06 | 287.77 | 0.95 |
| PyP/PMM 1/100 | 11.77 | 6.00 | 5.14 | 17.51 | 2.25 | 51.12 | 0.98 | 149.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melcón-Fernández, E.; Galli, G.; Balaña-Fouce, R.; García-Fernández, N.; Martínez-Valladares, M.; Reguera, R.M.; García-Estrada, C.; Pérez-Pertejo, Y. In Vitro and Ex Vivo Synergistic Effect of Pyrvinium Pamoate Combined with Miltefosine and Paromomycin against Leishmania. Trop. Med. Infect. Dis. 2024, 9, 30. https://doi.org/10.3390/tropicalmed9020030
Melcón-Fernández E, Galli G, Balaña-Fouce R, García-Fernández N, Martínez-Valladares M, Reguera RM, García-Estrada C, Pérez-Pertejo Y. In Vitro and Ex Vivo Synergistic Effect of Pyrvinium Pamoate Combined with Miltefosine and Paromomycin against Leishmania. Tropical Medicine and Infectious Disease. 2024; 9(2):30. https://doi.org/10.3390/tropicalmed9020030
Chicago/Turabian StyleMelcón-Fernández, Estela, Giulio Galli, Rafael Balaña-Fouce, Nerea García-Fernández, María Martínez-Valladares, Rosa M. Reguera, Carlos García-Estrada, and Yolanda Pérez-Pertejo. 2024. "In Vitro and Ex Vivo Synergistic Effect of Pyrvinium Pamoate Combined with Miltefosine and Paromomycin against Leishmania" Tropical Medicine and Infectious Disease 9, no. 2: 30. https://doi.org/10.3390/tropicalmed9020030
APA StyleMelcón-Fernández, E., Galli, G., Balaña-Fouce, R., García-Fernández, N., Martínez-Valladares, M., Reguera, R. M., García-Estrada, C., & Pérez-Pertejo, Y. (2024). In Vitro and Ex Vivo Synergistic Effect of Pyrvinium Pamoate Combined with Miltefosine and Paromomycin against Leishmania. Tropical Medicine and Infectious Disease, 9(2), 30. https://doi.org/10.3390/tropicalmed9020030










