Unusual Localization of AIDS-Related Kaposi’s Sarcoma in a Heterosexual Male during the COVID-19 Pandemic: A Case Report
Abstract
1. Introduction
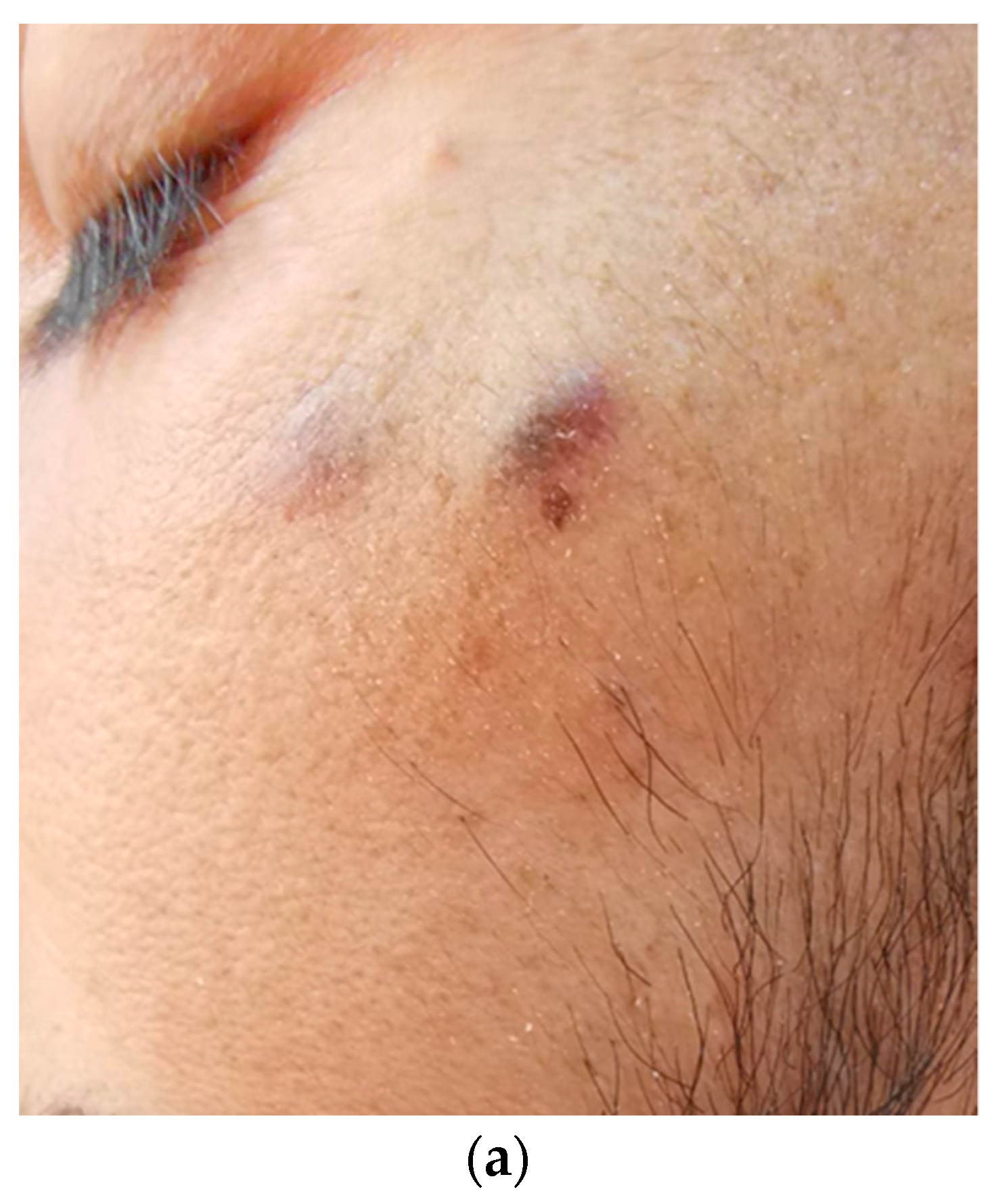
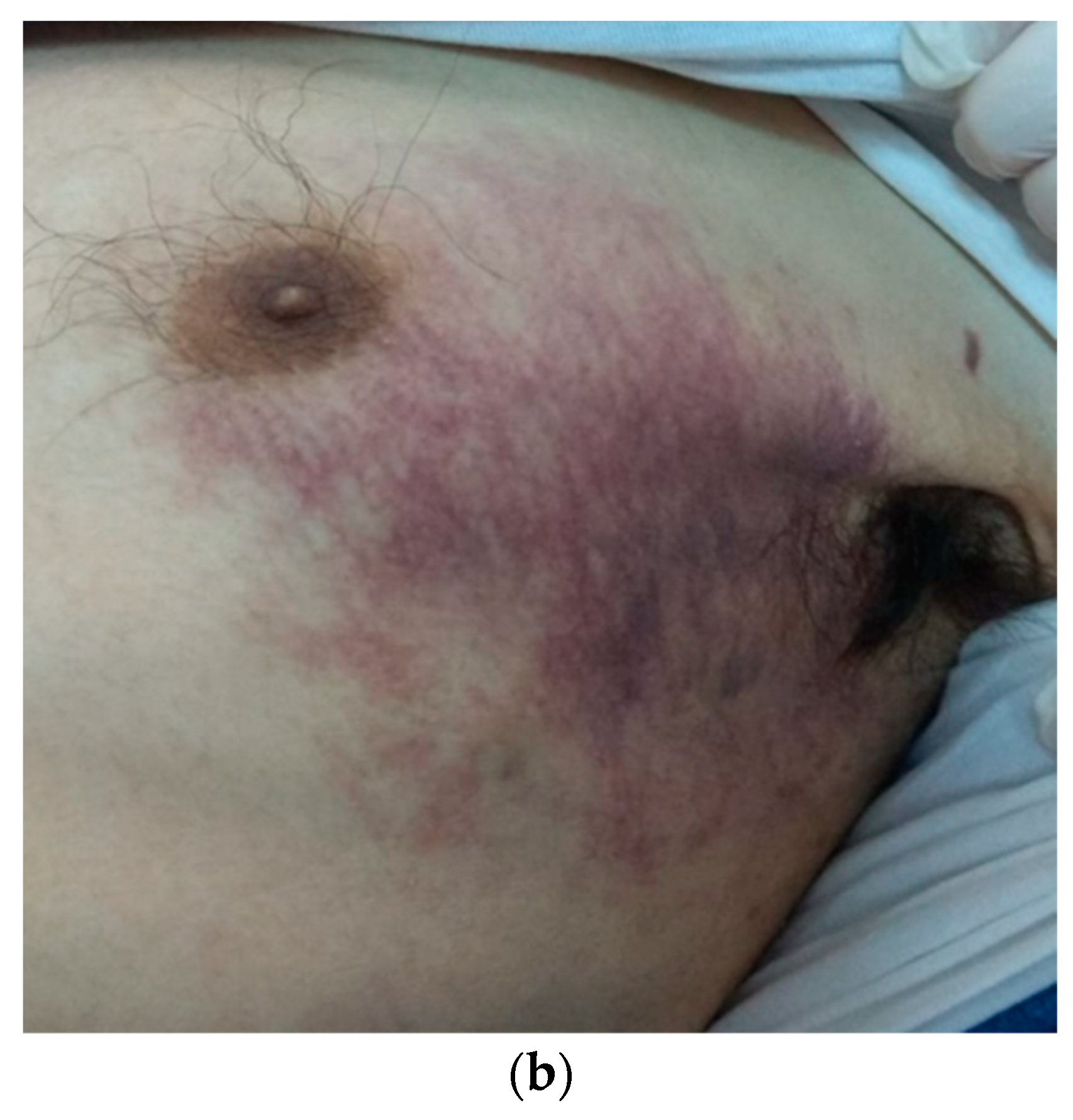
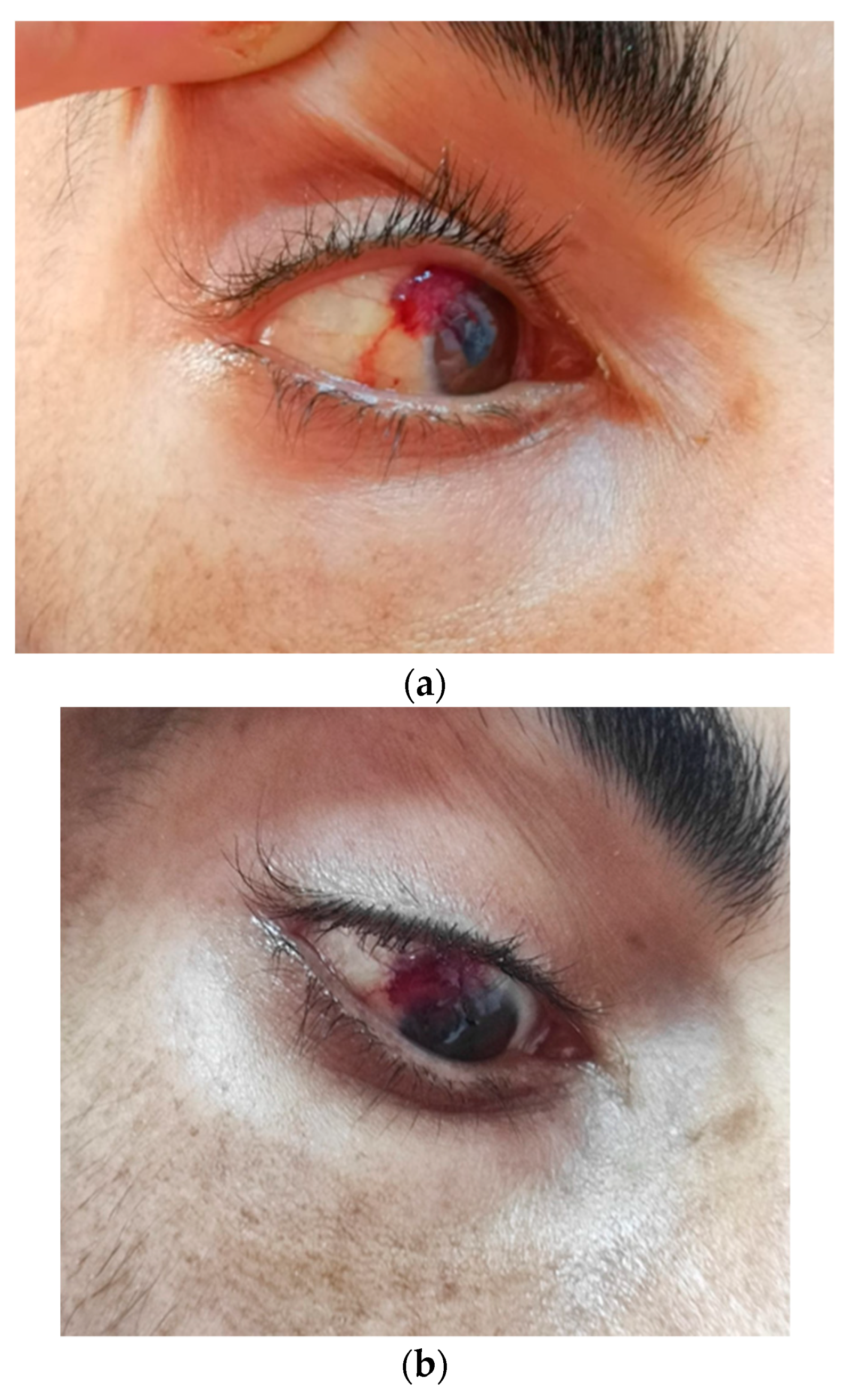
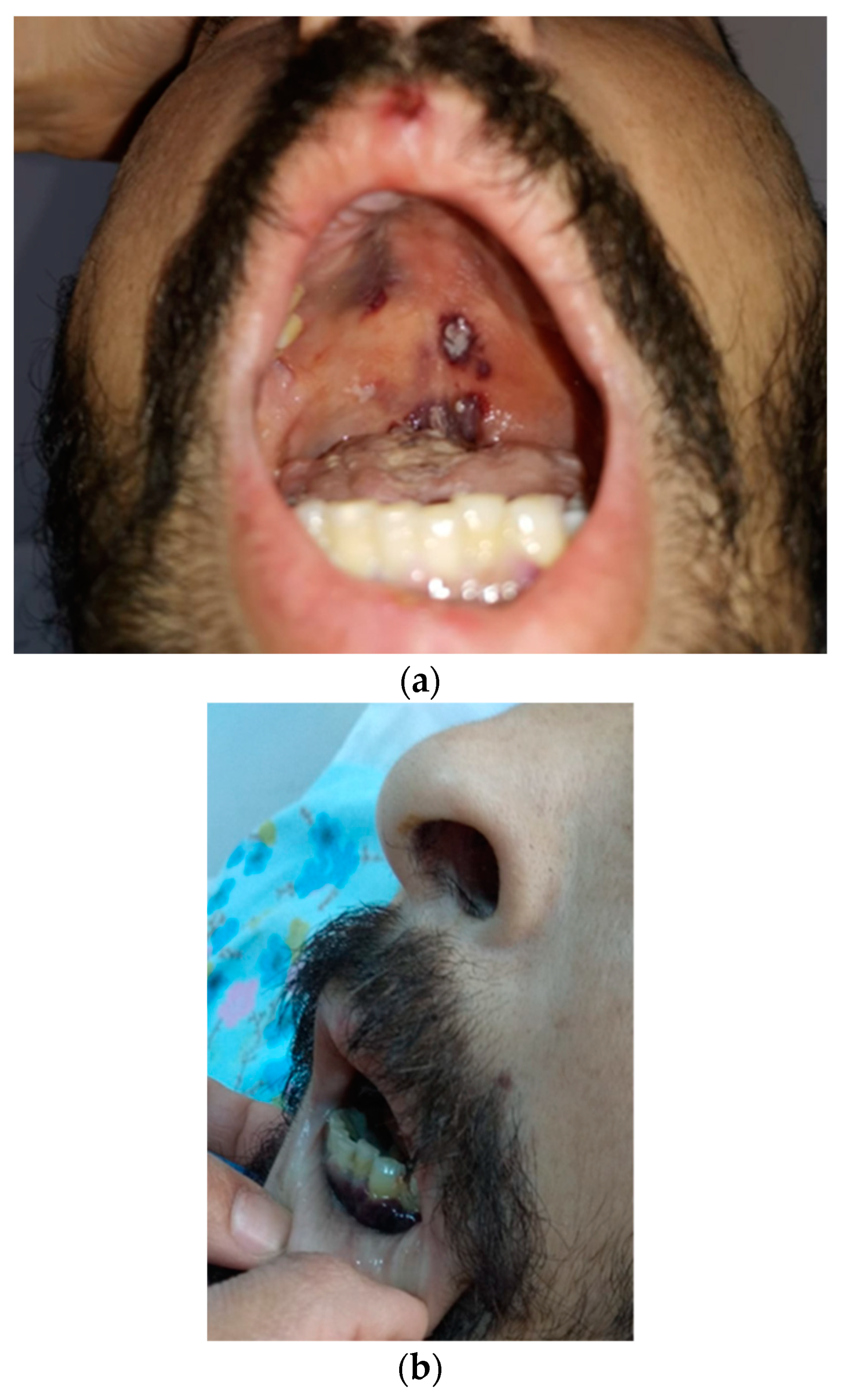
2. Case Report
3. Discussion
3.1. Demographic Features
3.2. Epidemiologic Features of HIV Infection
3.3. KS Ocular Involvement
3.4. Co-Infection Features and Hypotheses of the Pathogenic Role of KS
3.4.1. Human Herpes Virus-8
3.4.2. Staphylococcus aureus
3.4.3. SARS-CoV-2
3.4.4. Genetic Factors of Susceptibility to Infections
3.4.5. Possible Co-Existence of Multicentric Castleman Disease and Kaposi’s Sarcoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. AIDS-Related Kaposi’s Sarcoma in a 30 yo Patient with HIV, Infected since Early Childhood

| 12 mo before KS | 6 mo before KS | 3 mo before KS | 1 mo before KS | Time of KS Diagnostic | |
|---|---|---|---|---|---|
| Leukocytes/mm3 | 3800 | 1100 | 1300 | 1700 | 4300 |
| Lymphocytes/mm3 | 1210 | 450 | 1660 | 870 | 1380 |
| LCD4/mm3 | - | 2 | - | 7 | - |
| Thrombocytes/mm3 | 416,000 | 154,000 | 202,000 | 234,000 | 71,000 |
| Hemoglobin g/dL | 12.2 | 10.8 | 10 | 10.3 | 6 |
| ERS mm/h | 40 | 30 | 44 | 58 | 46 |
| Fibrinogen mg/dL | 444 | - | 5.64 | 564 | 353 |
| Glucose mg/dL | 79.5 | 79.6 | 97.5 | 102 | 105.5 |
| Creatinine mg/dL | 0.85 | 0.86 | 0.92 | 0.78 | 0.95 |
| ALT UI/L | 20.1 | 23.3 | 23.9 | 62.5 | 19.7 |
| AST UI/L | 15.2 | 21.8 | 21.9 | 48.8 | 14.8 |
| Albumin g/dL | 4.74 | 4.18 | - | - | 1.34 |
| D-Dimers g/L | - | 776 | 2608.2 | 2207 | 1875 |
| Feritin ng/mL | - | - | 1183 | 7786 | 1107 |
References
- Pantanowitz, L.; Dezube, B.J. Kaposi sarcoma in unusual locations. BMC Cancer 2008, 8, 190. [Google Scholar] [CrossRef]
- Gonçalves, P.H.; Uldrick, T.S.; Yarchoan, R. HIV-associated Kaposi sarcoma and related diseases. AIDS 2017, 31, 1903–1916. [Google Scholar] [CrossRef]
- Allan, A.E.; Shoji, T.; Li, N.; Burlage, A.; Davis, B.; Bhawan, J. Two cases of Kaposi’s sarcoma mimicking Stewart-Treves syndrome found to be human herpesvirus-8 positive. Am. J. Dermatopathol. 2001, 23, 431–436. [Google Scholar] [CrossRef]
- Vangipuram, R.; Tyring, S.K. Epidemiology of Kaposi sarcoma: Review and description of the nonepidemic variant. Int. J. Dermatol. 2018, 58, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Tounouga, D.N.; Kouotou, E.A.; Nansseu, J.R.; Zoung-Kanyi Bissek, A.C. Epidemiological and Clinical Patterns of Kaposi Sarcoma: A 16-Year Retrospective Cross-Sectional Study from Yaoundé, Cameroon. Dermatology 2018, 234, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Whitby, D.; Howard, M.R.; Tenant-Flowers, M.; Brink, N.S.; Copas, A.; Boshoff, C.; Hatzioannou, T.; Suggett, F.E.; Aldam, D.M.; Denton, A.S. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet 1995, 346, 799–802. [Google Scholar] [CrossRef]
- Vega, F.; Miranda, R.N.; Medeiros, L.J. KSHV/HHV8-positive large B-cell lymphomas and associated diseases: A heterogeneous group of lymphoproliferative processes with significant clinicopathological overlap. Mod. Pathol. 2020, 33, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Pantry, S.N.; Medveczky, P.G. Epigenetic regulation of Kaposi’s sarcoma-associated herpesvirus replication. Semin. Cancer Biol. 2009, 19, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Libson, K.; Himed, S.; Dunlop, H.; Nusbaum, K.B.; Korman, A.M.; Kaffenberger, B.H.; Trinidad, J. A description of Kaposi sarcoma risk factors and outcomes in HIV-positive and HIV-negative patients at a tertiary care medical center from 2005 to 2020. Arch. Dermatol. Res. 2023, 315, 2159–2162. [Google Scholar] [CrossRef]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 1029–1041. [Google Scholar] [CrossRef]
- Esser, S.; Schöfer, H.; Hoffmann, C.; Claßen, J.; Kreuter, A.; Leiter, U.; Oette, M.; Becker, J.C.; Ziemer, M.; Mosthaf, F.; et al. S1 Guidelines for the Kaposi Sarcoma. J. Dtsch. Dermatol. Ges. 2022, 20, 892–904. [Google Scholar] [CrossRef]
- Luo, Q.; Satcher Johnson, A.; Hall, H.I.; Cahoon, E.K.; Shiels, M. Kaposi Sarcoma Rates Among Persons Living with Human Immunodeficiency Virus in the United States: 2008–2016. Clin. Infect. Dis. 2021, 73, e2226–e2233. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, Q.; Zuo, J.; Minhas, V.; Wood, C.; Zhang, T. The world-wide incidence of Kaposi’s sarcoma in the HIV/AIDS era. HIV Med. 2018, 19, 355–364. [Google Scholar] [CrossRef]
- Arbune, M.; Padurariu-Covit, M.-D.; Rebegea, L.F.; Lupasteanu, G.; Arbune, A.A.; Stefanescu, V.; Tatu, A.L. AIDS Related Kaposi’s Sarcoma: A 20-Year Experience in a Clinic from the South-East of Romania. J. Clin. Med. 2021, 10, 5346. [Google Scholar] [CrossRef]
- Padurariu-Covit, M.; Lupasteanu, G.; Arbune, M. Pancytopenia and Kaposi sarcoma indicators of HIV infection diagnosis: A case report. Rom. J. Infect. Dis. 2021, 24, 156–160. [Google Scholar] [CrossRef]
- Preda, M.; Manolescu, L.C.S. Romania, a Harbour of HIV-1 Subtype F1: Where Are We after 33 Years of HIV-1 Infection? Viruses 2022, 14, 2081. [Google Scholar] [CrossRef] [PubMed]
- Jianu, C.; Bolboacă, S.D.; Topan, A.V.; Filipescu, I.; Jianu, M.E.; Itu-Mureşan, C.A. View of Human Immunodeficiency Virus Infections in the North-West Region of Romania. Medicina 2019, 55, 765. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.; Loussert-Ajaka, I.; Collin, G.; Letourneur, F.; Duca, M.; Saragosti, S. HIV type 1 subtype F sequences in Romanian children and adults. AIDS Res. Hum. Retroviruses 1997, 13, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Damania, B.; Krown, S.E.; Martin, J.; Bower, M.; Whitby, B. Kaposi sarcoma. Nat. Rev. Dis. Primers 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Update: AIDS--United States, 2000. MMWR Morb. Mortal. Wkly. Rep. 2022, 51, 592–595. [Google Scholar]
- Ulloa-Padilla, J.P.; Ghassibi, M.P.; Dubovy, S.R.; Kerr, D.A. Clinicopathologic Correlation of Kaposi Sarcoma Involving the Ocular Adnexa: Immunophenotyping of Diagnostic and Therapeutic Targets. Ophthalmic Plast. Reconstr. Surg. 2020, 36, 185–190. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Ramos, J.C.; Cohen, A.K.; Alvarez, O.P.; Cohen, N.K.; Galor, A.; Karp, K.L. Spotlight on ocular Kaposi’s sarcoma: An update on the presentation, diagnosis, and management options. Expert Rev. Ophthalmol. 2021, 16, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D. Eye signs that alert the clinician to a diagnosis of AIDS. SADJ 2005, 60, 386–387. [Google Scholar] [PubMed]
- Rossetto, J.D.; Molles, S.; Gracitelli, C.P.B. Extensive bulbar conjunctival Kaposi’s sarcoma as initial symptom of human immunodeficiency virus. Arq. Bras. Oftalmol. 2019, 82, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Tatu, A.L.; Nadasdy, T.; Arbune, A.; Chioncel, V.; Bobeica, C.; Niculet, E.; Iancu, A.V.; Dumitru, C.; Popa, V.T.; Kluger, N.; et al. Interrelationship and Sequencing of Interleukins 4, 13, 31, and 33—An Integrated Systematic Review: Dermatological and Multidisciplinary Perspectives. J. Inflamm. Res. 2022, 15, 5163–5184. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, S.; Tosato, G. Viral interleukin-6: Role in Kaposi’s sarcoma-associated herpesvirus: Associated malignancies. J. Interferon Cytokine Res. 2011, 31, 791–801. [Google Scholar] [CrossRef]
- Ramdass, P.; Mullick, S.; Farber, H.F. Viral Skin Diseases. Prim. Care 2015, 42, 517–567. [Google Scholar] [CrossRef]
- Lopes, T.R.R.; Gonçales, J.P.; Silva Júnior, J.V.J.; Lorena, V.M.B.; Toscano, A.L.C.C.; Akamatsu, S.M.; Salles, A.C.; Tozetto-Mendoza, T.R.; Morais, V.M.S.; Coêlho, M.R.C.D. Association of IL-6, IL-10 and CXCL10 serum concentrations with visceral Kaposi’s sarcoma in people living with HIV/AIDS. Hum. Immunol. 2020, 81, 26–31. [Google Scholar] [CrossRef]
- Sabbagh, P.; Riahi, S.M.; Gamble, H.R.; Rostami, A. The global and regional prevalence, burden, and risk factors for methicillin-resistant Staphylococcus aureus colonization in HIV-infected people: A systematic review and meta-analysis. Am. J. Infect. Control 2019, 47, 323–333. [Google Scholar] [CrossRef]
- Dai, L.; Qiao, J.; Yin, J.; Goldstein, A.; Lin, H.Y.; Post, S.R.; Qin, Z. Kaposi Sarcoma-Associated Herpesvirus and Staphylococcus aureus Coinfection in Oral Cavities of HIV-Positive Patients: A Unique Niche for Oncogenic Virus Lytic Reactivation. J. Infect. Dis. 2020, 221, 1331–1341. [Google Scholar] [CrossRef]
- Jauslin, A.S.; Simon, N.R.; Giudici, N.L.; Rueegg, M.; Zimmermann, T.; Diebold, M.; Tschudin-Sutter, S.; Twerenbold, R.; Nickel, C.H.; Bingisser, R. Mimics and chameleons of COVID-19: Patient presentation and accuracy of triage during the first wave. Swiss Med. Wkly. 2021, 151, w30103. [Google Scholar] [CrossRef]
- Magri, F.; Giordano, S.; Latini, A.; Muscianese, M. New-onset cutaneous kaposi’s sarcoma following SARS-CoV-2 infection. J. Cosmet. Dermatol. 2021, 20, 3747–3750. [Google Scholar] [CrossRef]
- Lambarey, H.; Blumenthal, M.J.; Chetram, A.; Joyimbana, W.; Jennings, L.; Orrell, C.; Schafer, G. Reactivation of Kaposi’s sarcoma-associated herpesvirus (KSHV) by SARS-CoV-2 in non-hospitalised HIV-infected patients. eBioMedicine 2024, 100, 104986. [Google Scholar] [CrossRef]
- Peng, X.; Ouyang, J.; Isnard, S.; Lin, J.; Fombuena, B.; Zhu, B.; Routy, J.P. Sharing CD4+ T Cell Loss: When COVID-19 and HIV Collide on Immune System. Front. Immunol. 2020, 11, 596631. [Google Scholar] [CrossRef]
- Tajarernmuang, P.; Fiset, P.O.; Routy, J.P.; Beaudoin, S. Intractable pleural effusion in Kaposi sarcoma following antiretroviral therapy in a Caucasian female infected with HIV. BMJ Case Rep. 2020, 13, e233335. [Google Scholar] [CrossRef]
- Mehri, F.; Rahbar, A.H.; Ghane, E.T.; Souri, B.; Esfahani, M. Changes in oxidative markers in COVID-19 patients. Arch. Med. Res. 2021, 52, 843–849. [Google Scholar] [CrossRef]
- Shafiee, A.; Teymouri Athar, M.M.; Amini, M.J.; Hajishah, H.; Siahvoshi, S.; Jalali, M.; Jahanbakshi, B.; Mozhgani, S.-H. Reactivation of herpesviruses during COVID-19: A systematic review and metaanalysis. Rev. Med. Virol. 2023, 33, e2437. [Google Scholar] [CrossRef]
- Devi, P.; Khan, A.; Chattopadhyay, P.; Mehta, P.; Sahni, S.; Sharma, S.; Pandey, R. Co-infections as Modulators of Disease Outcome: Minor Players or Major Players? Front. Microbiol. 2021, 12, 664386. [Google Scholar] [CrossRef]
- Nasrullah, A.; Patel, S.; Ud Din, M.T.; Javed, A.; Arshad, H.; Raja, A.; Dumont, T. A case of acquired immunodeficiency syndrome-related Kaposi sarcoma in a patient with COVID-19—A brief review of HIV-COVID Co-infection and its Therapeutic challenges! Respir. Med. Case Rep. 2021, 34, 101524. [Google Scholar] [CrossRef]
- Guimaraes, V.; Guimaraes, R.; Brandao, L.; da Silva, M.F.B.; Milanese, M.; Segat, L.; Castelletti, H.; Bruneska, D.; de Lima Filho, J.L.; De Freitas, A.C.; et al. Association between MBL2 gene functional polymorphisms and high-risk human papillomavirus infection in Brazilian women. Hum. Immunol. 2008, 69, 273–278. [Google Scholar] [CrossRef]
- Alves Pedroso, M.L.; Boldt, A.B.; Pereira-Ferrari, L.; Steffensen, R.; Strauss, E.; Jensenius, J.C.; Ioshii, S.O.; Messias-Reason, I. Mannan-binding lectin MBL2 gene polymorphism in chronic hepatitis C: Association with the severity of liver fibrosis and response to interferon therapy. Clin. Exp. Immunol. 2008, 152, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, G.; Ozkan, T.B.; Ozgur, T.; Budak, F.; Kilic, S.S.; Onay, H. Mannose-binding lectin gene polymorphism and chronic hepatitis B infection in children. Saudi J. Gastroenterol. 2015, 21, 84–89. [Google Scholar] [CrossRef]
- Seppänen, M.; Lokki, M.L.; Lappalainen, M.; Hiltunen-Back, E.; Rovio, A.T.; Kares, S.; Hurme, M.; Aittoniemi, J. Mannose-binding lectin 2 gene polymorphism in recurrent herpes simplex virus 2 infection. Hum. Immunol. 2009, 70, 218–221. [Google Scholar] [CrossRef]
- Friborg, J.T.; Jarrett, R.F.; Koch, A.; Garred, P.; Freeland, J.M.; Andersen, A.; Melbye, M. Mannose-binding lectin genotypes and susceptibility to epstein-barr virus infection in infancy. Clin. Vaccine Immunol. 2010, 17, 1484–1487. [Google Scholar] [CrossRef]
- Song, B.; Sheng, X.; Justice, J.L.; Lum, K.K.; Metzger, P.J.; Cook, K.C.; Kostas, J.C.; Cristea, I.M. Intercellular communication within the virus microenvironment affects the susceptibility of cells to secondary viral infections. Sci. Adv. 2023, 9, eadg3433. [Google Scholar] [CrossRef]
- da Silva, G.K.; Guimarães, R.; Mattevi, V.S.; Lazzaretti, R.K.; Sprinz, E.; Kuhmmer, R.; Brandão, L.; Crovella, S.; Chies, A. The role of mannose-binding lectin gene polymorphisms in susceptibility to HIV-1 infection in Southern Brazilian patients. AIDS 2011, 25, 411–418. [Google Scholar] [CrossRef]
- Lebbe, C.; Garbe, C.; Stratigos, A.J.; Harwood, C.; Peris, K.; Marmol, V.D.; Malvehy, J.; Zalaudek, I.; Hoeller, C.; Dummer, R.; et al. Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Eur. J. Cancer 2019, 114, 117–127. [Google Scholar] [CrossRef]
- Yanes, R.R.; Malijan, G.M.B.; Escora-Garcia, L.K.; Ricafrente, S.A.M.; Salazar, M.J.; Suzuki, S.; Smith, C.; Ariyoshi, K.; Solante, R.M.; Edrada, E.M.; et al. Detection of SARS-CoV-2 and HHV-8 from a large pericardial effusion in an HIV-positive patient with COVID-19 and clinically diagnosed Kaposi sarcoma: A case report. Trop. Med. Health 2022, 50, 72. [Google Scholar] [CrossRef]
- Davari, S.; Taunk, P.S.; Madaline, T. COVID-19 complications in a newly diagnosed HIV patient: A case of multiple herpesvirus reactivation and HLH post-ART initiation. Am. J. Case Rep. 2023, 24, e939847. [Google Scholar] [CrossRef]
- Lin, I.F.; Lin, J.N.; Tsai, T.H.; Hsu, C.T.; Wu, Y.Y.; Lai, C.H. Coexistence of disseminated Kaposi sarcoma and multicentric Castleman disease in an HIV-infected patient under viral suppression. Int. J. STD AIDS 2021, 32, 286–289. [Google Scholar] [CrossRef]
- Saadallah, M.A.H. Castleman’s disease: A rare case report and review of literature. Int. J. Surg. Case Rep. 2022, 95, 107282. [Google Scholar] [CrossRef]
- Karass, M.; Grossniklaus, E.; Seoud, T.; Jain, S.; Goldstein, D.A. Kaposi Sarcoma Inflammatory Cytokine Syndrome (KICS): A Rare but Potentially Treatable Condition. Oncologist 2017, 22, 623–625. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbune, M.; Padurariu-Covit, M.-D.; Tiutiuca, C.; Mihailov, R.; Niculet, E.; Arbune, A.-A.; Tatu, A.-L. Unusual Localization of AIDS-Related Kaposi’s Sarcoma in a Heterosexual Male during the COVID-19 Pandemic: A Case Report. Trop. Med. Infect. Dis. 2024, 9, 47. https://doi.org/10.3390/tropicalmed9020047
Arbune M, Padurariu-Covit M-D, Tiutiuca C, Mihailov R, Niculet E, Arbune A-A, Tatu A-L. Unusual Localization of AIDS-Related Kaposi’s Sarcoma in a Heterosexual Male during the COVID-19 Pandemic: A Case Report. Tropical Medicine and Infectious Disease. 2024; 9(2):47. https://doi.org/10.3390/tropicalmed9020047
Chicago/Turabian StyleArbune, Manuela, Monica-Daniela Padurariu-Covit, Carmen Tiutiuca, Raul Mihailov, Elena Niculet, Anca-Adriana Arbune, and Alin-Laurentiu Tatu. 2024. "Unusual Localization of AIDS-Related Kaposi’s Sarcoma in a Heterosexual Male during the COVID-19 Pandemic: A Case Report" Tropical Medicine and Infectious Disease 9, no. 2: 47. https://doi.org/10.3390/tropicalmed9020047
APA StyleArbune, M., Padurariu-Covit, M.-D., Tiutiuca, C., Mihailov, R., Niculet, E., Arbune, A.-A., & Tatu, A.-L. (2024). Unusual Localization of AIDS-Related Kaposi’s Sarcoma in a Heterosexual Male during the COVID-19 Pandemic: A Case Report. Tropical Medicine and Infectious Disease, 9(2), 47. https://doi.org/10.3390/tropicalmed9020047










