Snakebites in Cameroon by Species Whose Effects Are Poorly Described
Abstract
1. Introduction
2. Materials and Methods
3. Results
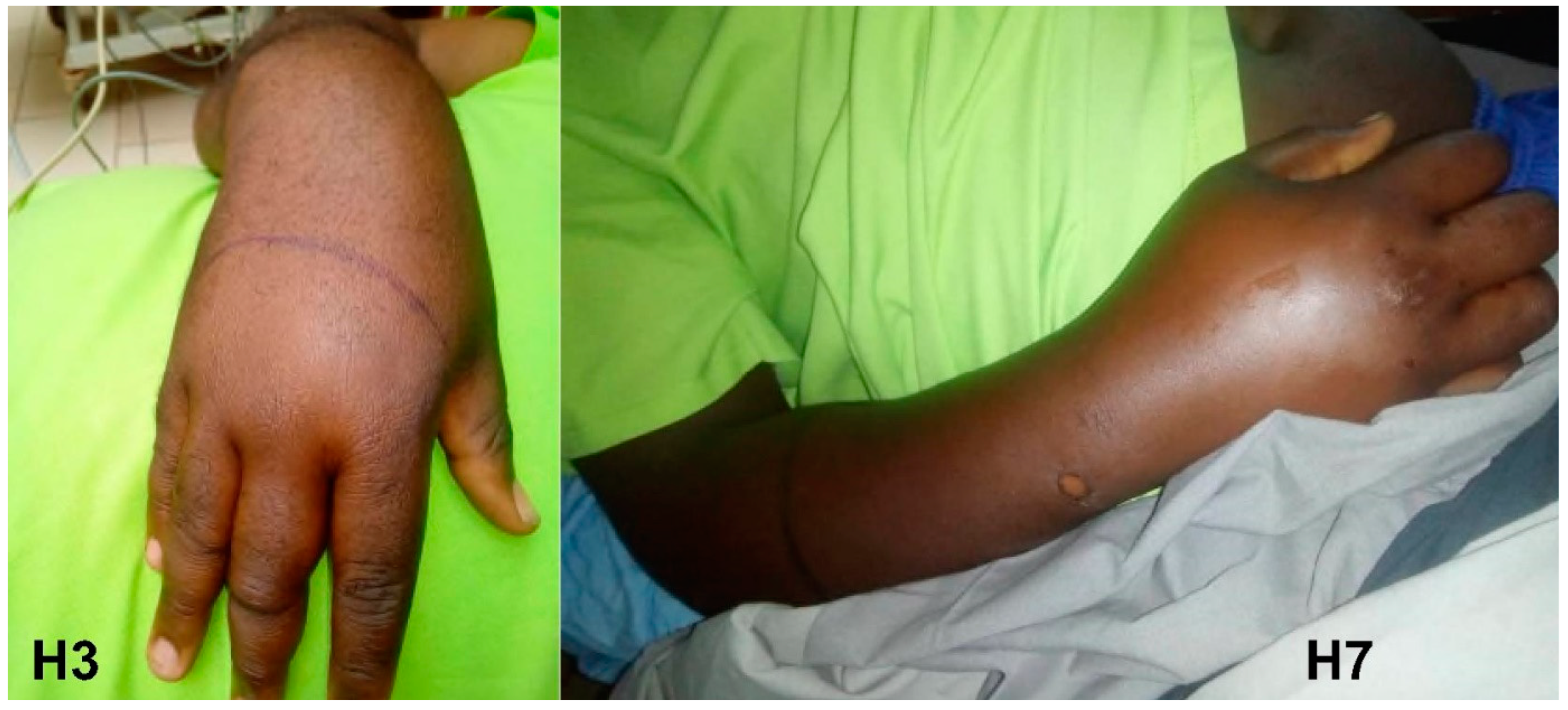
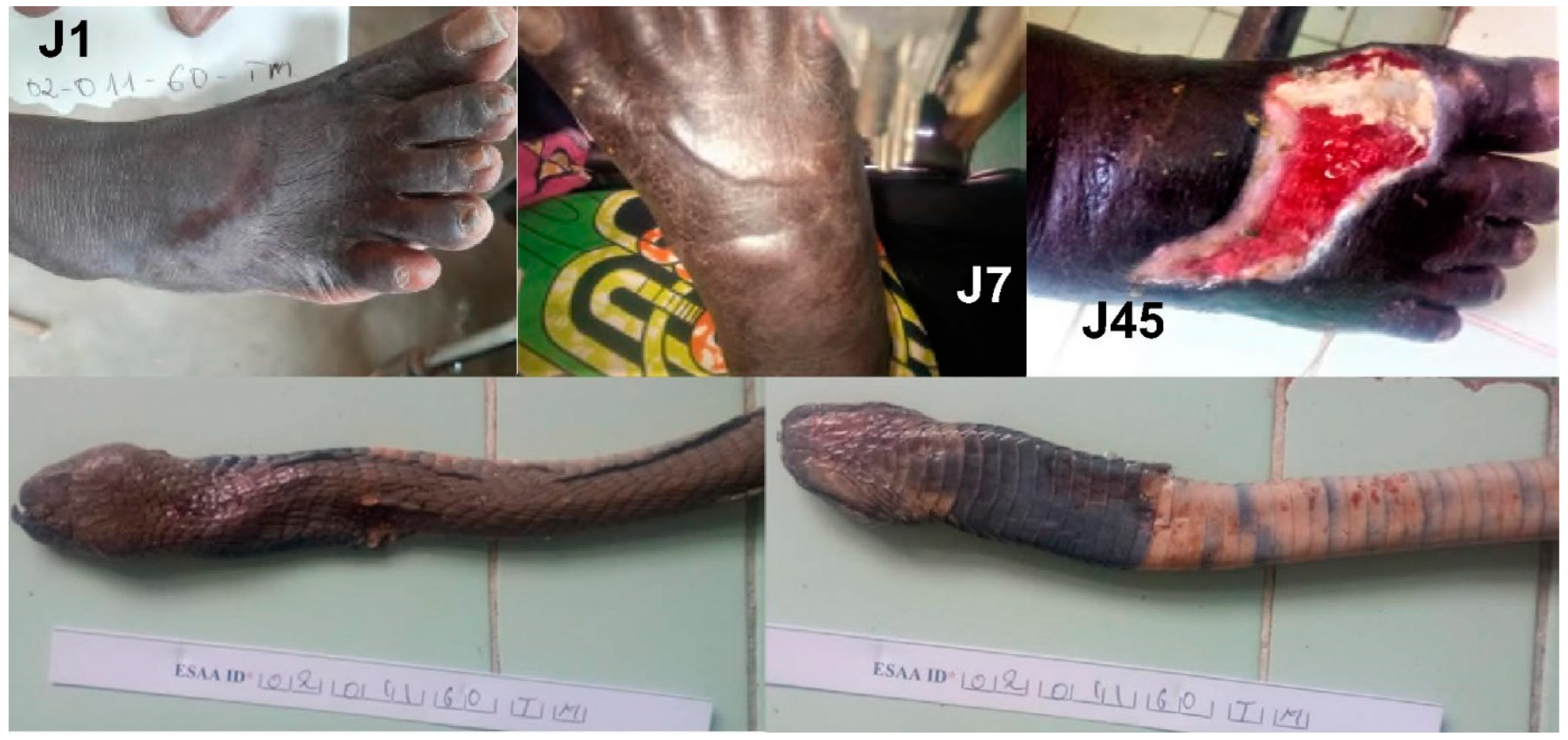
3.1. Atheris squamigera (Table 2)
| # | Gender (Age) | Time to Hosp. (h) | Edema | WBCT | Bleeding | Neuro | Necrosis | # Vials AV | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| AS1 | M (10) | 49:30 | Grade 2 | Grade 2 | Grade 4 | No | No | 12 | Recovered |
| AS2 | M (32) | 4 | Grade 1 | Normal | No | No | No | 2 | Recovered |
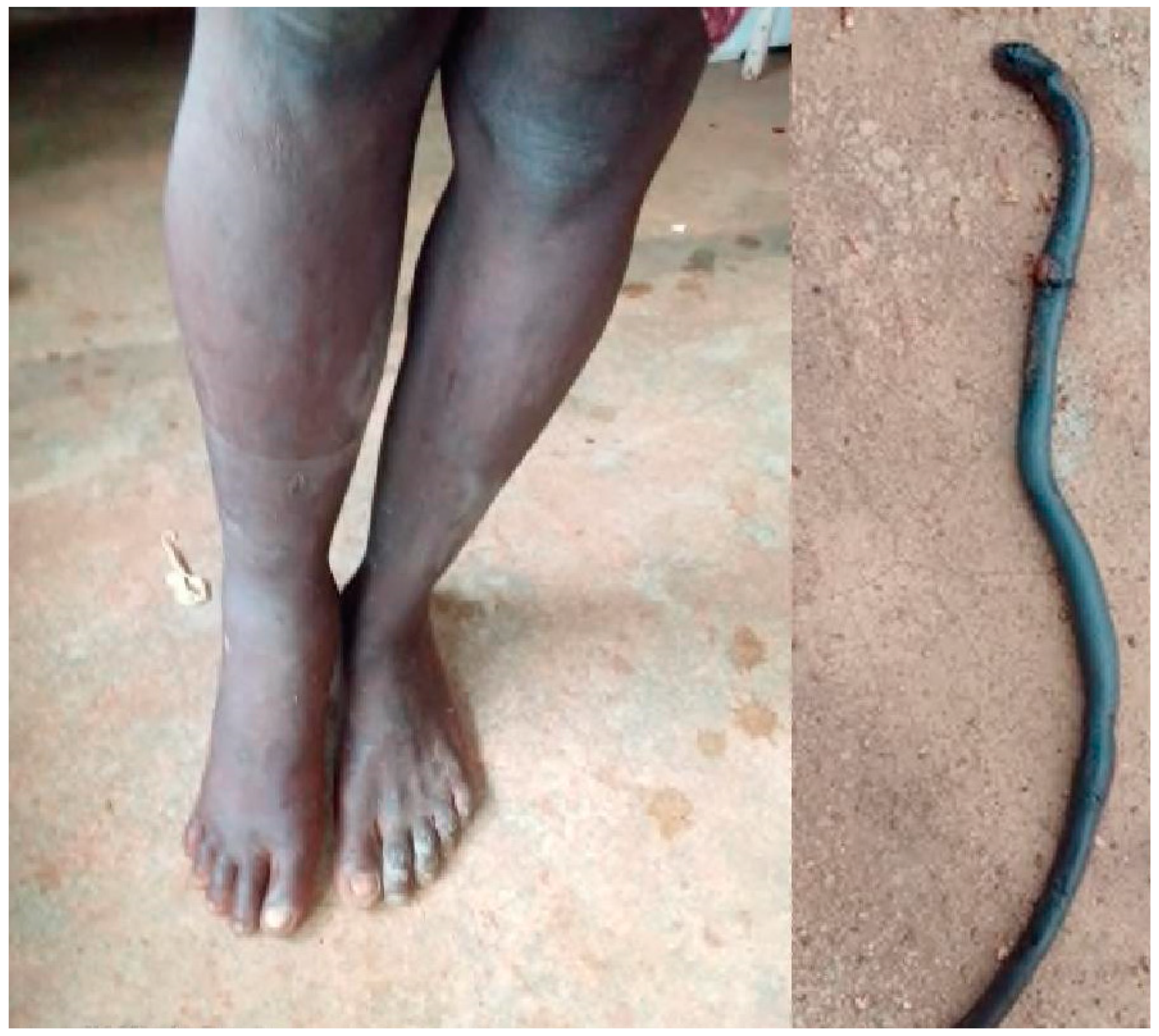
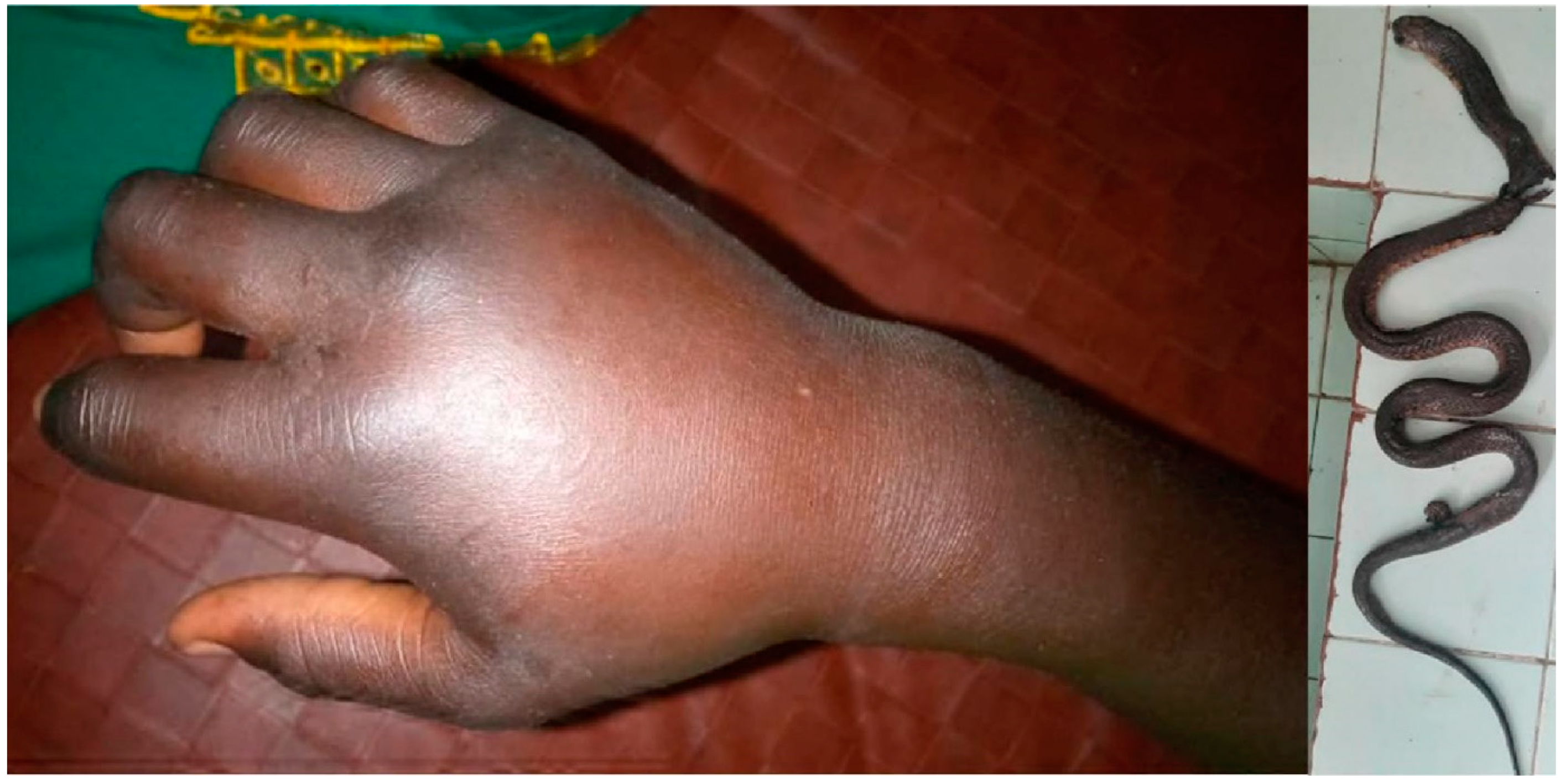
3.2. Atractaspis spp. (Table 3)
| # | Gender (Age) | Time to Hosp. (h) | Edema | WBCT | Bleeding | Neuro | Necrosis | # Vials AV | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| AT1 | F (32) | 1:30 | Grade 1 | Normal | No | No | No | 2 | Recovered |
| AT2 | F (8) | 4:20 | Grade 1 | Grade 2 | No | No | No | 2 | Recovered |
| AT3 | F (16) | 0:25 | Grade 2 | Normal | No | No | No | 2 | Recovered |
| AT4 | M (45) | 0:20 | Grade 1 | Grade 2 | Grade 2 | No | No | 2 | Recovered |
| AT5 | F (30) | 7:40 | Grade 2 | Normal | No | No | No | 2 | Recovered |
| AT6 | F (22) | 17 | Grade 2 | Normal | No | No | No | 2 | Recovered |
| AT7 | M (50) | 0:50 | Grade 2 | Normal | No | No | No | 2 | Recovered |
| AT8 | F (9) | 1:10 | Grade 2 | Normal | No | No | No | 2 | Recovered |
| AT9 | F (9) | 2 | Grade 1 | Normal | No | No | No | 2 | Recovered |
| AT10 | F (35) | 17:15 | No | Normal | No | No | No | 0 | Dry bite |
| AT11 | F (11) | 2:30 | Grade 2 | Normal | No | No | No | 2 | Recovered |
| AT12 | M (12) | 2:20 | No | Normal | No | No | No | 0 | Dry bite |
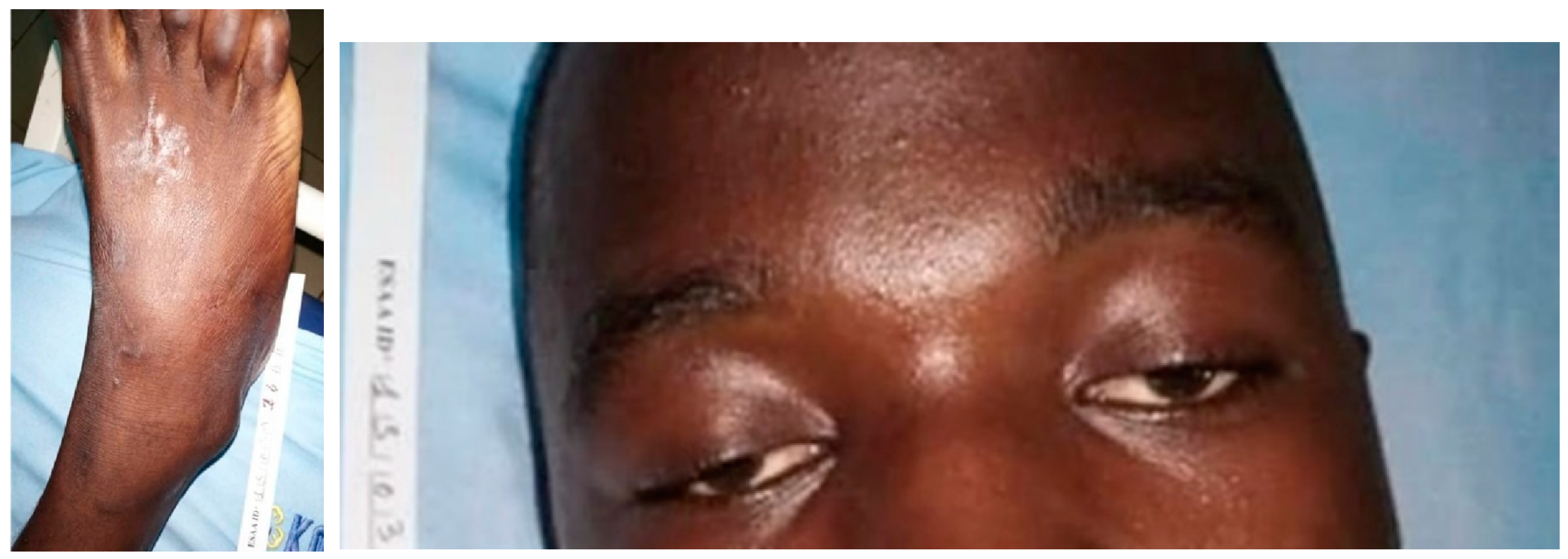
3.3. Bitis arietans (Table 4)
| # | Gender (Age) | Time to Hosp. (h) | Edema | WBCT | Bleeding | Neuro | Necrosis | # Vials AV | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| BA1 | F (24) | 0:10 | Grade 1 | Normal | No | No | No | 2 | Recovered |
| BA2 | M (31) | 17:15 | Grade 2 | Normal | No | No | No | 2 | Recovered |
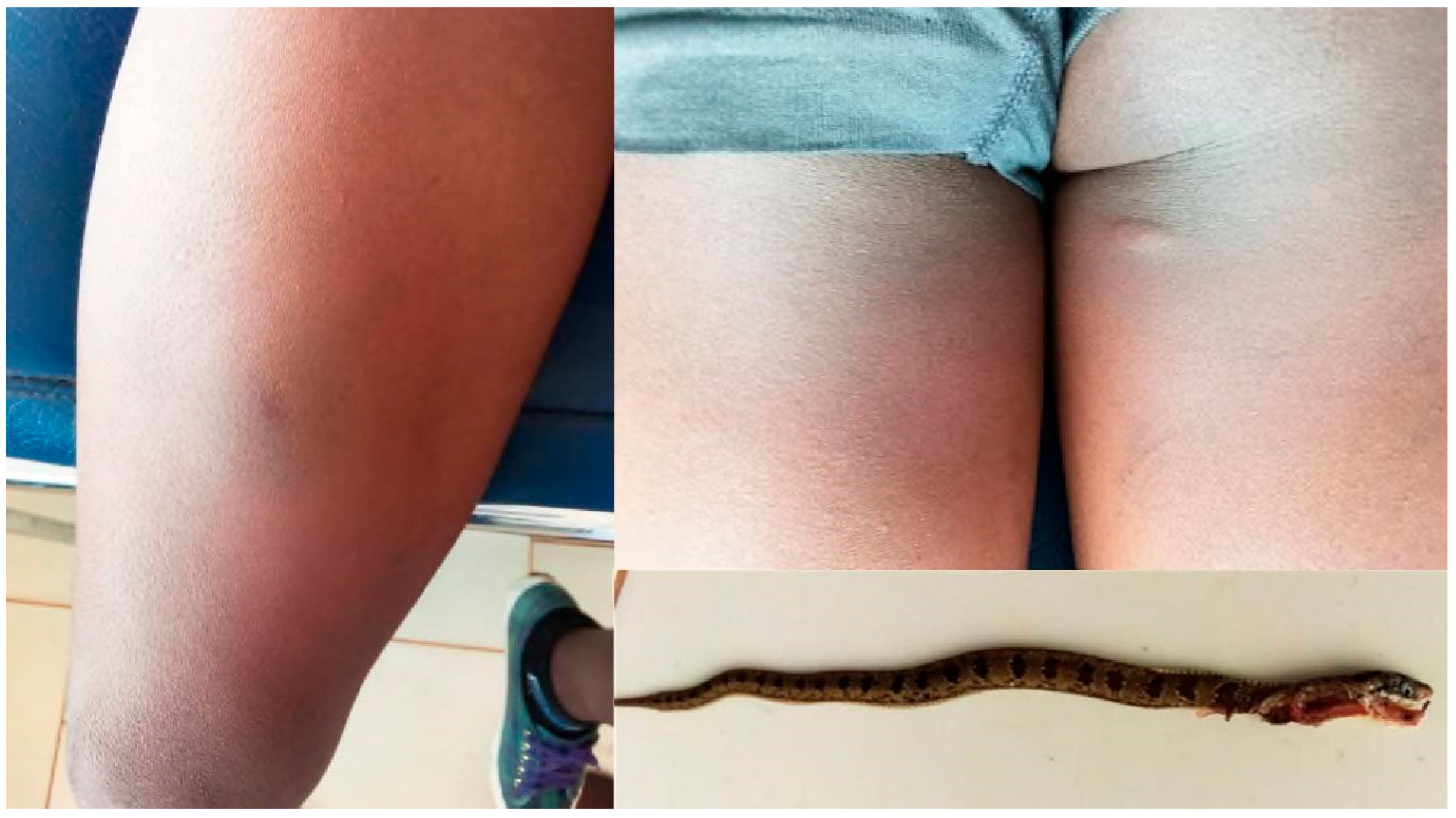
3.4. Causus maculatus (Table 5)
| # | Gender (Age) | Time to Hosp. (h) | Edema | WBCT | Bleeding | Neuro | Necrosis | # Vials AV | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| CM1 | F (8) | 16 | Grade 1 | Normal | No | No | No | 2 | Recovered |
| CM2 | M (63) | 7:30 | Grade 1 | Normal | No | No | No | 0 | Recovered |
| CM3 | F (10) | MD | No | Normal | No | No | No | 0 | Dry bite |
| CM4 | M (27) | 1:15 | Grade 2 | Normal | Grade 1 | No | No | 2 | Recovered |
| CM5 | M (10) | 2 | No | Normal | No | No | No | 0 | Dry bite |
| CM6 | F (80) | 0:20 | Grade 1 | Normal | No | No | No | 2 | Recovered |
| CM7 | M (6) | 3:30 | No | Normal | No | No | No | 0 | Dry bite |
| CM8 | F (27) | 1 | Grade 1 | Grade 1 | No | No | No | 2 | Recovered |
| CM9 | M (40) | 1:50 | Grade 2 | Normal | No | No | No | 2 | Recovered |
| CM10 | F (28) | 0:45 | Grade 1 | Normal | No | No | No | 2 | Recovered |
| CM11 | M (11) | 0:30 | No | Normal | No | No | No | 0 | Dry bite |
3.5. Dendroaspis jamesoni (Table 6)
| # | Gender (Age) | Time to Hosp. (h) | Edema | WBCT | Bleeding | Neuro | Necrosis | # Vials AV | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| DJ1 | M (14) | 1:40 | No | Normal | No | Grade 3 | No | 4 | Recovered |
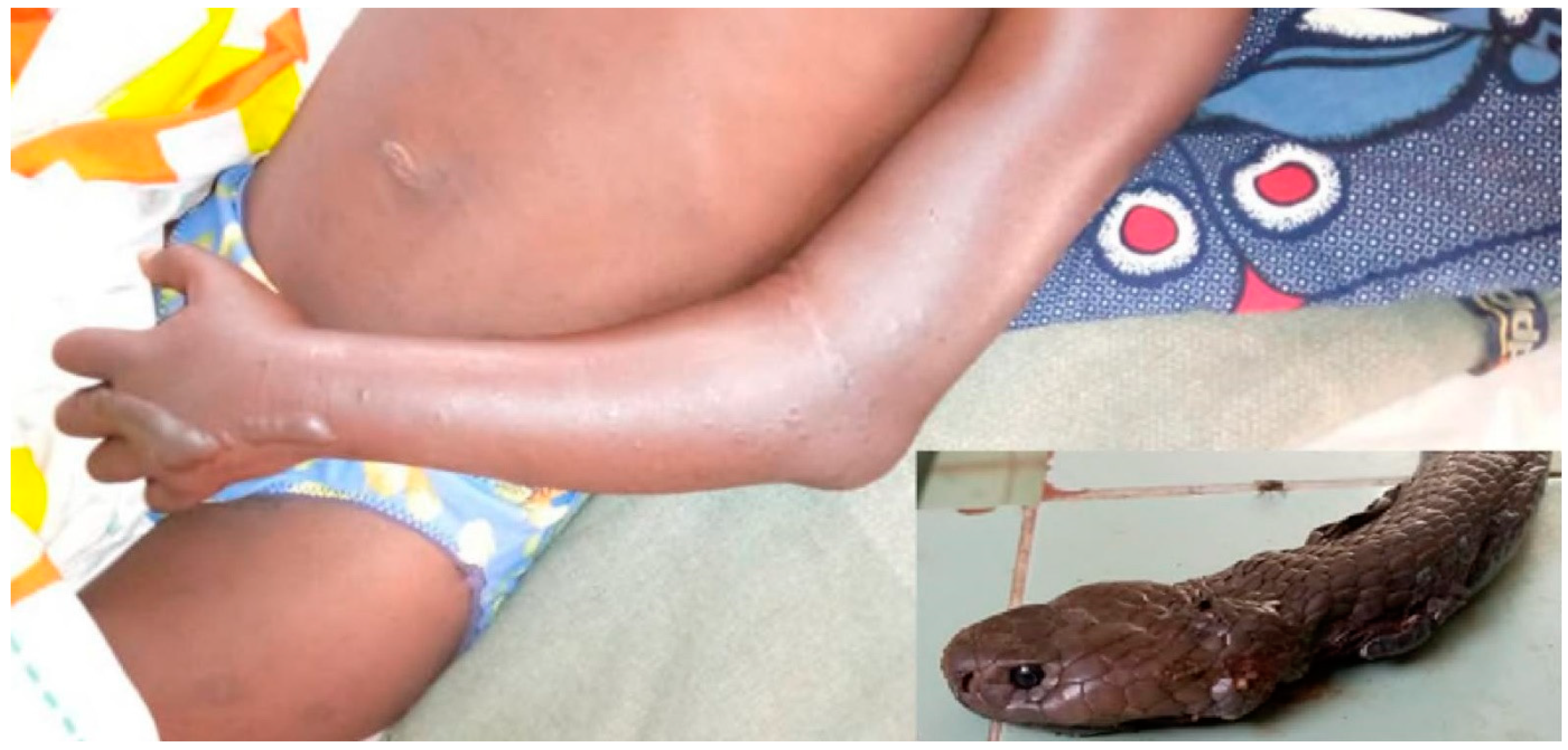
3.6. Naja haje (Table 7)
| # | Gender (Age) | Time to Hosp. (h) | Edema | WBCT | Bleeding | Neuro | Necrosis | # Vials AV | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| NH1 | F (8) | 16:45 | Grade 3 | Normal | No | No | No | 2 | Died H12 |
3.7. Naja katiensis (Table 8)
| # | Gender (Age) | Time to Hosp. (h) | Edema | WBCT | Bleeding | Neuro | Necrosis | # Vials AV | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| NK1 | F (60) | 5:30 | Grade 1 | Normal | No | Grade 1 | Yes | 4 | Local scar |
3.8. Naja melanoleuca (Table 9)
| # | Gender (Age) | Time to Hosp. (h) | Edema | WBCT | Bleeding | Neuro | Necrosis | # Vials AV | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| NM1 | F (20) | 2:20 | Grade 2 | Normal | No | No | No | 2 | Recovered |
| NM2 | M (36) | 0:10 | Grade 1 | Normal | No | No | No | 2 | Recovered |
| NM3 | F (11) | 8:15 | Grade 2 | Normal | No | Grade 3 | No | 4 | Recovered |
| NM4 | F (14) | 1:15 | Grade 1 | Normal | No | No | No | 2 | Recovered |
| NM5 | M (16) | 8:30 | No | Normal | No | Grade 3 | No | 4 | Recovered |
3.9. Naja nigricollis (Table 10)
| # | Gender (Age) | Time to Hosp. (h) | Edema | WBCT | Bleeding | Neuro | Necrosis | # Vials AV | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| NN1 | F (80) | 4:10 | Grade 2 | Normal | No | No | No | 2 | Died D20 |
| NN2 | M (37) | 2:30 | No | Normal | No | No | No | 0 | Dry bite |
4. Discussion
4.1. Atheris squamigera
4.2. Atractaspis sp.
4.3. Bitis arietans
4.4. Causus maculatus
4.5. Dendroaspis jamesoni
4.6. Naja haje
4.7. Naja katiensis
4.8. Naja melanoleuca
4.9. Naja nigricollis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
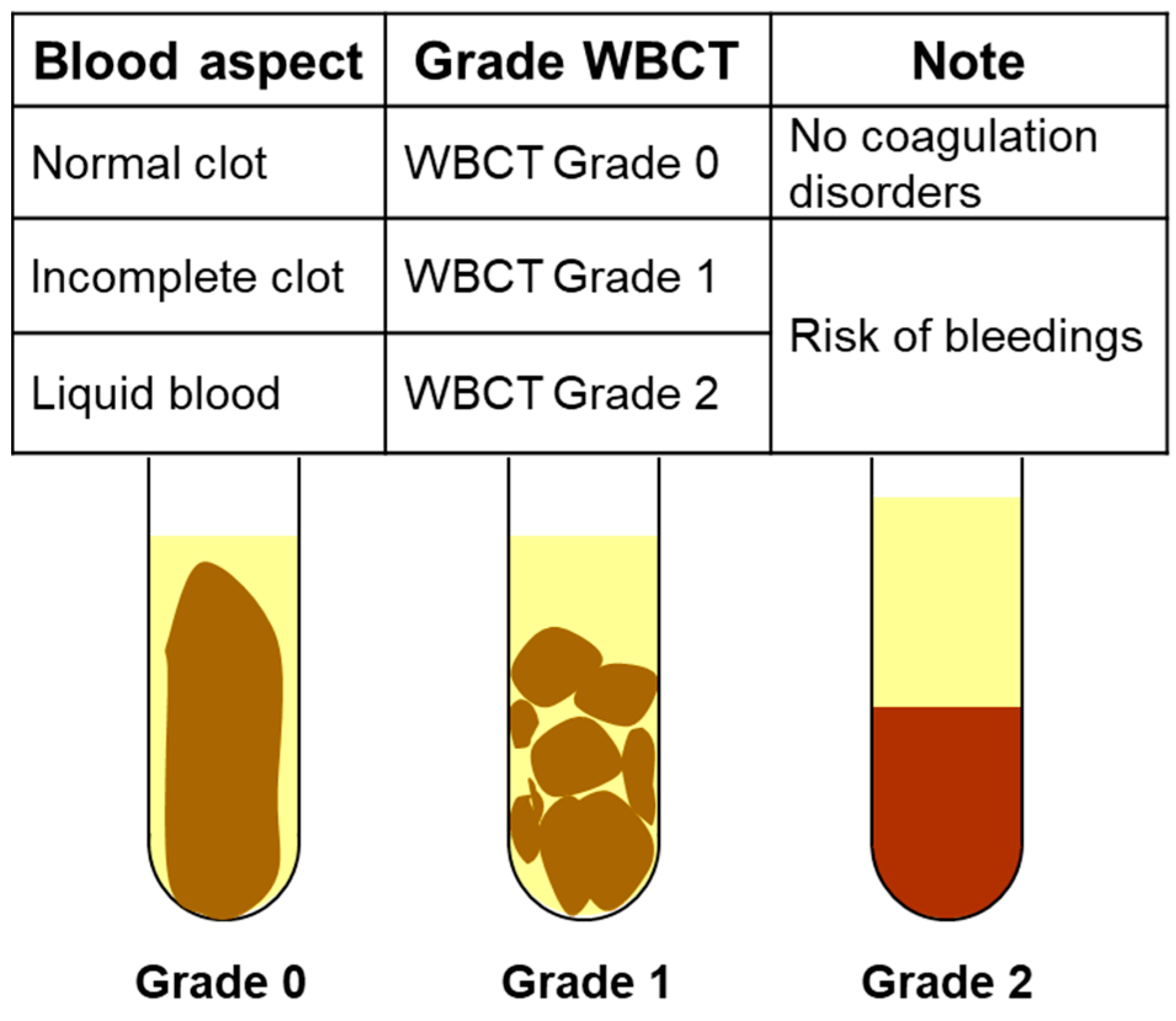
Appendix A. Management Algorithm Recommended by Cameroonian Ministry of Envenomation Patients (From 12, 13)
- Sample 2 mL blood in a clean dry glass tube.
- Let the tube stand without shaking it.
- Read the result after 20 and 60 min of waiting.
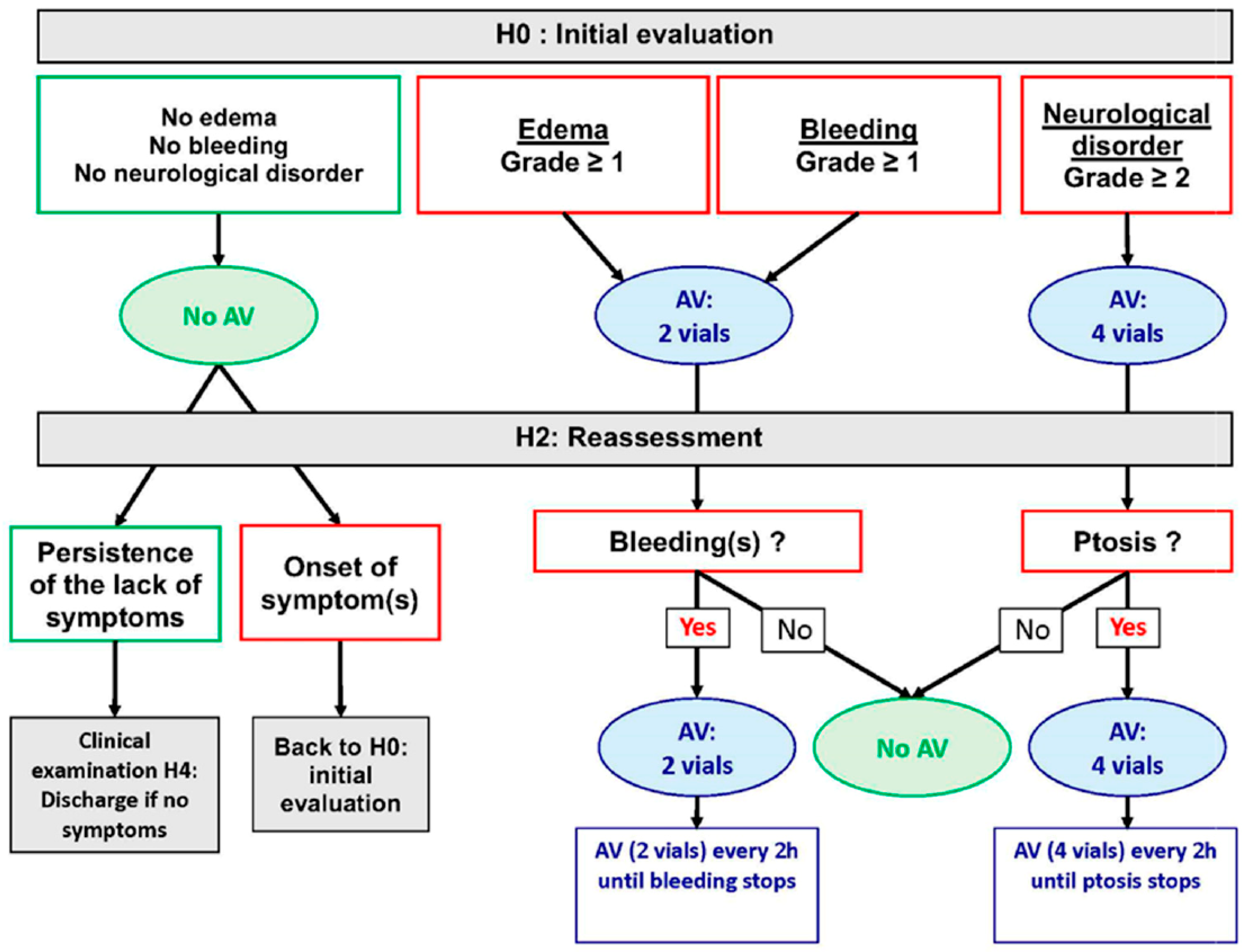
Appendix B. Management Algorithm Recommended by Cameroonian Ministry of Envenomation Patients (From 12, 13)
References
- Chippaux, J.P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Faiz, M.A.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Fan, H.W.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, e0007059. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P. Estimate of the burden of snakebites in sub-Saharan Africa: A meta-analytic approach. Toxicon 2011, 57, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Tchoffo, D.; Kamgno, J.; Kekeunou, S.; Yadufashije, C.; Nana Djeunga, H.C.; Nkwescheu, A.S. High snakebite underreporting rate in the Centre Region of Cameroon: An observational study. BMC Public Health 2019, 19, 1040. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef]
- Halilu, S.; Iliyasu, G.; Hamza, M.; Chippaux, J.P.; Kuznik, A.; Habib, A.G. Snakebite burden in Sub-Saharan Africa: Estimates from 41 countries. Toxicon 2019, 159, 1–4. [Google Scholar] [CrossRef]
- Chippaux, J.P. Les Serpents d’Afrique Occidentale et Centrale; IRD: Paris, France, 2006; 311p. [Google Scholar]
- Chippaux, J.P.; Jackson, K. Snakes from Central & Western Africa; John Hopkins University Press: Baltimore, MD, USA, 2019; 419p. [Google Scholar]
- Trape, J.F. Guide des Serpents d’Afrique Occidentale, Centrale et d’Afrique du Nord; IRD: Marseille, France, 2023; 896p. [Google Scholar]
- Stark, M.A. 1986. Geographical distribution. Reptilia. Serpentes. Elapidae. Dendroaspis polylepis Günther 1864. J. Herpetol. Assoc. Afr. 1986, 32, 31. [Google Scholar]
- LeBreton, M.; Chirio, L. Dendroaspis polylepis (black mamba) Cameroon. Herpetol. Rev. 2004, 35, 191. [Google Scholar]
- Chippaux, J.P.; Ntone, R.; Benhammou, D.; Madec, Y.; Noël, G.; Perilhou, A.; Karl, F.; Amta, P.; Sanchez, M.; Matchim, L.; et al. Real-life condition evaluation of Inoserp PAN-AFRICA antivenom effectiveness in Cameroon. PLoS Negl. Trop. Dis. 2023, 17, e0011707. [Google Scholar] [CrossRef]
- Benhammou, D.; Chippaux, J.P.; Ntone, R.; Madec, Y.; Amta, P.; Noel, G.; Karl, F.N.; Perilhou, A.; Matchim, L.; Sanchez, M.; et al. Snakebites in Cameroon: Tolerance of a Snake Antivenom (Inoserp™ PAN-AFRICA) in Africa in Real-Life Conditions. Toxins 2024, 16, 165. [Google Scholar] [CrossRef]
- Mathé, H. Clinical review of a polyvalent F(ab’)2 antivenom (InoserpTM PAN-AFRICA) in the management of snakebite envenomation in sub-Saharan Africa: Clinical studies and actual use since its introduction in 2012. Biol. Life Sci. Forum 2023, 24, 13. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D.; Holada, K.; Kornalík, F.; Simák, J.; Vanková, H.; Müller, D.; Schoenemann, H.; Lange, H.; Herrmann, H.W. Severe coagulopathy after a bite of a green bush viper (Atheris squamiger): Case report and biochemical analysis of the venom. Toxicon 1998, 36, 1333–1340. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; König, E.; Zhou, M.; Wang, L.; Chen, T.; Shaw, C. Comparative Profiling of Three Atheris Snake Venoms: A. squamigera, A. nitschei and A. chlorechis. Protein J. 2018, 37, 353–360. [Google Scholar] [CrossRef]
- Chowdhury, A.; Lewin, M.R.; Carter, R.; Soria, R.; Aldridge, M.; Fry, B.G. Extreme Procoagulant Potency in Human Plasma of Venoms from the African Viperid Genera Atheris, Cerastes, and Proatheris and the Relative Efficacy of Antivenoms and Synthetic Enzyme-Inhibitors. Toxins 2022, 14, 836. [Google Scholar] [CrossRef]
- Knoepffler, L.P. Autoobservation d’envenimation par morsure d’Atheris sp. Toxicon 1965, 2, 275–276. [Google Scholar] [CrossRef]
- Imperato, N.S.; Amaducci, A.M.; Abo, B.N.; Koons, A.L.; Fikse, D.J.; Katz, K.D. African Bush Viper Envenomation: A Case Report. Cureus 2022, 14, e28040. [Google Scholar] [CrossRef]
- Ontiveros, S.T.; Srihari, P.; Winkler, G.A.; Del Rosso, J.; Sobel, J.; Clark, R.F.; Minns, A.B. Envenomation by the Green Bush Viper Atheris squamigera. Toxicol. Rep. 2022, 9, 2018–2019. [Google Scholar] [CrossRef]
- Robinson, R.F.; Baker, R.S.; Martin, S.; Casavant, M.C. Use of “Near Middle East Antivenom” to treat African bush viper envenomation. Vet. Hum. Toxicol. 2004, 46, 264–265. [Google Scholar]
- Branch, W.R.; Haagner, G.V.; Morgan, D.R.; Lanoie, L.O. Venoms and snakebite. J. Herpetol. Assoc. Afr. 1991, 39, 8–29. [Google Scholar] [CrossRef]
- Weinstein, S.A.; Warrell, D.A. The African and Middle Eastern Burrowing asps (Atractaspis spp.) and Their Allies. Biology, Venom and Envenoming; Chimaira: Frankfurt am Main, Germany, 2019; 391p. [Google Scholar]
- Corkill, N.L.; Kirk, R. Poisoning by the Sudan mole viper Atractaspis microlepidota Günther. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Gunders, A.E.; Walter, H.J.; Etzel, E. Case of snakebite by Atractaspis corpulenta. Trans. R. Soc. Trop. Med. Hyg. 1960, 54, 279–280. [Google Scholar] [CrossRef]
- Tilbury, C.R.; Verster, J. A fatal bite from the burrowing asp Atractaspis corpulenta (Hallowell 1854). Toxicon 2016, 118, 21–26. [Google Scholar] [CrossRef]
- Quinton, L.; Le Caer, J.P.; Phan, G.; Ligny-Lemaire, C.; Bourdais-Jomaron, J.; Ducancel, F.; Chamot-Rooke, J. Characterization of toxins within crude venoms by combined use of Fourier transform mass spectrometry and cloning. Anal. Chem. 2005, 77, 6630–6639. [Google Scholar] [CrossRef]
- Fry, B.G.; Scheib, H.; de LM Junqueira de Azevedo, I.; Silva, D.A.; Casewell, N.R. Novel transcripts in the maxillary venom glands of advanced snakes. Toxicon 2012, 59, 696–708. [Google Scholar] [CrossRef]
- Terrat, Y.; Sunagar, K.; Fry, B.G.; Jackson, T.N.; Scheib, H.; Fourmy, R.; Verdenaud, M.; Blanchet, G.; Antunes, A.; Ducancel, F. Atractaspis aterrima toxins: The first insight into the molecular evolution of venom in side-stabbers. Toxins 2013, 5, 1948–1964. [Google Scholar] [CrossRef]
- Oulion, B.; Dobson, J.S.; Zdenek, C.N.; Arbuckle, K.; Lister, C.; Coimbra, F.C.P.; Op den Brouw, B.; Debono, J.; Rogalski, A.; Violette, A.; et al. Factor X activating Atractaspis snake venoms and the relative coagulotoxicity neutralising efficacy of African antivenoms. Toxicol. Lett. 2018, 288, 119–128. [Google Scholar] [CrossRef]
- Corkill, N.L.; Ionides, C.J.; Pitman, C.R. Biting and poisoning by the mole vipers of the genus Atractaspis. Trans. R. Soc. Trop. Med. Hyg. 1959, 53, 95–101. [Google Scholar] [CrossRef]
- Pasqual, J.R.H. The biting mechanism of Atractaspis. Niger. Field 1962, 27, 137–142. [Google Scholar]
- Loveridge, A. On a collection of reptiles and amphibians from Liberia. Proc. N. Engl. Zool. Club 1938, 42, 49–74. [Google Scholar]
- Chippaux, J.P. Symptomatologie clinique des envenimations. Etudes Médicales 1981, 1981, 143–167. [Google Scholar]
- Kochva, E. Atractaspis (Serpentes, Atractaspididae) the burrowing asp; a multidisciplinary minireview. Bull. Nat. Hist. Museum. Zool. Ser. 2002, 68, 91–99. [Google Scholar] [CrossRef]
- Weiser, E.; Wollberg, Z.; Kochva, E.; Lee, S.Y. Cardiotoxic effects of the venom of the burrowing asp, Atractaspis engaddensis (Atractaspididae, Ophidia). Toxicon 1984, 22, 767–774. [Google Scholar] [CrossRef]
- Wollberg, Z.; Shabo-Shina, R.; Intrator, N.; Bdolah, A.; Kochva, E.; Shavit, G.; Oron, Y.; Vidne, B.A.; Gitter, S. A novel cardiotoxic polypeptide from the venom of Atractaspis engaddensis (burrowing asp): Cardiac effects in mice and isolated rat and human heart preparations. Toxicon 1988, 26, 525–534. [Google Scholar] [CrossRef]
- Abd-Elsalam, M.A. Bosentan, a selective and more potent antagonist for Atractaspis envenomation than the specific antivenom. Toxicon 2011, 57, 861–870. [Google Scholar] [CrossRef]
- Boulenger, E.G. Reptiles and Batrachians; JM Dent & Sons: London, UK, 1913; p. 180. [Google Scholar]
- Lepesme, P.; Doucet, J. Sur un cas d’envenimation par Atractaspis, vipéridé Ouest-Africain. Bull. Soc. Path. Exot. 1953, 46, 207–211. [Google Scholar]
- Warrell, D.A.; Ormerod, L.D.; Davidson, N.M. Bites by the night adder (Causus maculatus) and burrowing vipers (genus Atractaspis) in Nigeria. Am. J. Trop. Med. Hyg. 1976, 25, 517–524. [Google Scholar] [CrossRef]
- Britt, D.P. Death following the bite of a burrowing viper. Niger. Field 1978, 43, 41–42. [Google Scholar]
- Spawls, S. Notes on a bite by a West African Atractaspis (Colubridae: Aparallactini). J. Herpetol. Assoc. Afr. 1980, 28, 21–22. [Google Scholar]
- Wallach, V. Report on a bite by a side-stabbing snake Atractaspis irregularis, with notes on Elapid bites. J. Herpetol. Assoc. Afr. 1980, 24, 15–18. [Google Scholar]
- Barrıère, P.; Ineich, I.; Fretey, T. Un cas de morsure par Atractaspis irregularis (Serpentes: Atractaspididae) en République centrafricaine. Bull. Soc. Herpétol. Fr. 2005, 116, 51–56. [Google Scholar]
- Pauwels, O.; Mbini, J.C.; Péaud, P.; Tobi, E. Human envenoming by Atractaspis corpulenta corpulenta (Reptilia: Atractaspididae) in Gabon, Western Central Africa: A first case report. Hamadryad 2008, 32, 112–115. [Google Scholar]
- Burmeister, B.R.; Schabold, J.A.; Zosel, A.E. Envenomation by Atractaspis irregularis (variable burrowing asp), a case report. Clin. Toxicol. 2018, 56, 1169–1170. [Google Scholar] [CrossRef]
- Wagner, P.; Townsend, E.; Barej, M.; Rödder, D.; Spawls, S. First record of human envenomation by Atractaspis congica Peters, 1877 (Squamata: Atractaspididae). Toxicon 2009, 54, 368–372. [Google Scholar] [CrossRef]
- Dingwoke, E.J.; Adamude, F.A.; Mohamed, G.; Klein, A.; Salihu, A.; Abubakar, M.S.; Sallau, A.B. Venom proteomic analysis of medically important Nigerian viper Echis ocellatus and Bitis arietans snake species. Biochem. Biophys. Rep. 2021, 28, 101164. [Google Scholar] [CrossRef]
- Visser, J.; Chapman, D.S. Snakes and Snakebite; Centaur Publishers: Johannesburg, South Africa, 1978; 152p. [Google Scholar]
- Warrell, D.A.; Ormerod, L.D.; Davidson, N.M. Bites by puff-adder (Bitis arietans) in Nigeria, and value of antivenom. Br. Med. J. 1975, 4, 697–700. [Google Scholar] [CrossRef]
- Pugh, R.N.; Theakston, R.D. A clinical study of viper bite poisoning. Ann. Trop. Med. Parasitol. 1987, 81, 135–149. [Google Scholar] [CrossRef]
- Lavonas, E.J.; Tomaszewski, C.A.; Ford, M.D.; Rouse, A.M.; Kerns, W.P., 2nd. Severe puff adder (Bitis arietans) envenomation with coagulopathy. J. Toxicol. Clin. Toxicol. 2002, 40, 911–918. [Google Scholar] [CrossRef]
- Le Dantec, P.; Hervé, Y.; Niang, B.; Chippaux, J.P.; Bellefleur, J.P.; Boulesteix, G.; Diatta, B. Morsure par vipère Bitis arietans au Sénégal, intérêt de la mesure de pression intracompartimentale. Med. Trop. 2004, 64, 187–191. [Google Scholar]
- Husain, Z.; Wicaksono, A.C.; Renault, A.; Md Zhahir, S.S.; Ismail, A.K. A case of fatal envenomation by a captive puff adder (Bitis arietans) in Malaysia. Toxicon 2023, 224, 107023. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P.; Bressy, C. L’endémie ophidienne des plantations de Côte-d’Ivoire. Bull. Soc. Pathol. Exot. Filiales 1981, 74, 458–467. [Google Scholar] [PubMed]
- Gray, H.H. Green mamba envenomation: Case report. Trans. R. Soc. Trop. Med. Hyg. 1962, 56, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Stahel, E. Epidemiological aspects of snake bites on a Liberian rubber plantation. Acta Trop. 1980, 37, 367–374. [Google Scholar]
- Ainsworth, S.; Petras, D.; Engmark, M.; Süssmuth, R.D.; Whiteley, G.; Albulescu, L.O.; Kazandjian, T.D.; Wagstaff, S.C.; Rowley, P.; Wüster, W.; et al. The medical threat of mamba envenoming in sub-Saharan Africa revealed by genus-wide analysis of venom composition, toxicity and antivenomics profiling of available antivenoms. J. Proteom. 2018, 172, 173–189. [Google Scholar] [CrossRef]
- Malih, I.; Ahmad Rusmili, M.R.; Tee, T.Y.; Saile, R.; Ghalim, N.; Othman, I. Proteomic analysis of Moroccan cobra Naja haje legionis venom using tandem mass spectrometry. J. Proteom. 2014, 96, 240–252. [Google Scholar] [CrossRef]
- Adamude, F.A.; Dingwoke, E.J.; Abubakar, M.S.; Ibrahim, S.; Mohamed, G.; Klein, A.; Sallau, A.B. Proteomic analysis of three medically important Nigerian Naja (Naja haje, Naja katiensis and Naja nigricollis) snake venoms. Toxicon 2021, 197, 24–32. [Google Scholar] [CrossRef]
- Warrell, D.A.; Barnes, H.J.; Piburn, M.F. Neurotoxic effects of bites by the Egyptian cobra (Naja haje) in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1976, 70, 78–79. [Google Scholar] [CrossRef]
- Zouari, N.; Choyakh, F. Les effets neurotoxiques du venin de cobra (Naja haje haje) sur la jonction neuromusculaire. Etude électroclinique de deux cas en Tunisie. Neurophysiol. Clin. 1995, 25, 59–65. [Google Scholar] [CrossRef]
- Petras, D.; Sanz, L.; Segura, A.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; León, G.; Warrell, D.A.; Theakston, R.D.; et al. Snake venomics of African spitting cobras: Toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef]
- Warrell, D.A.; Ormerod, L.D. Snake venom ophthalmia and blindness caused by the spitting cobra (Naja nigricollis) in Nigeria. Am. J. Trop. Med. Hyg. 1976, 25, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A.; Greenwood, B.M.; Davidson, N.M.; Ormerod, L.D.; Prentice, C.R. Necrosis, haemorrhage and complement depletion following bites by the spitting cobra (Naja nigricollis). Q. J. Med. 1976, 45, 1–22. [Google Scholar] [PubMed]
- Tilbury, C.R. Observations on the bite of the Mozambique spitting cobra (Naja mossambica mossambica). S. Afr. Med. J. 1982, 61, 308–313. [Google Scholar] [PubMed]
- Wüster, W.; Chirio, L.; Trape, J.F.; Ineich, I.; Jackson, K.; Greenbaum, E.; Barron, C.; Kusamba, C.; Nagy, Z.T.; Storey, R.; et al. Integration of nuclear and mitochondrial gene sequences and morphology reveals unexpected diversity in the forest cobra (Naja melanoleuca) species complex in Central and West Africa (Serpentes: Elapidae). Zootaxa 2018, 4455, 68–98. [Google Scholar] [CrossRef]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutiérrez, J.M. Exploring the venom of the forest cobra snake: Toxicovenomics and antivenom profiling of Naja melanoleuca. J. Proteomics 2017, 150, 98–108. [Google Scholar] [CrossRef]
- Wang, C.R.; Harlington, A.C.; Snel, M.F.; Pukala, T.L. Characterisation of the forest cobra (Naja melanoleuca) venom using a multifaceted mass spectrometric-based approach. Biochim. Biophys. Acta Proteins Proteom. 2024, 1872, 140992. [Google Scholar] [CrossRef]
- Casasola, A.; Ramos-Cerrillo, B.; de Roodt, A.R.; Carbajal Saucedo, A.; Chippaux, J.P.; Alagón, A.; Stock, R.P. Paraspecific neutralization of the venom of African species of cobra by an equine antiserum against Naja melanoleuca: A comparative study. Toxicon 2009, 53, 602–608. [Google Scholar] [CrossRef]
- Trape, J.F.; Peelman, P.; Carme, B. La gravité d’une morsure de serpent. A propos de trois observations au Congo. Ann. Soc. Belg. Med. Trop. 1992, 72, 155–157. [Google Scholar]
- Nkwescheu, A.; Donfack, L.C.; Ba, F.B.; Dzudie, A.; Billong, S.C.; Ngouakam, H.; Le Doux, J.; Eyongorock, H.; Mbacham, W. Snakebite in bedroom kills a physician in Cameroon: A case report. Pan Afr. Med. J. 2016, 24, 231. [Google Scholar] [CrossRef]
- Chippaux, J.P.; N’Guessan, G.; Parie, F.X.; Roland, G.; Keba, M. Spitting cobra (Naja nigricollis) bite. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 106. [Google Scholar] [CrossRef]
- Pugh, R.N.; Theakston, R.D.; Reid, H.A.; Bhar, I.S. Malumfashi Endemic Diseases Research Project, XIII. Epidemiology of human encounters with the spitting cobra, Naja nigricollis, in the Malumfashi area of northern Nigeria. Ann. Trop. Med. Parasitol. 1980, 74, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Sennwald, G. Nécrose toxique sur morsure de Naja nigricollis, un défi thérapeutique. Schweiz. Med. Wochenschr. 1992, 122, 696–701. [Google Scholar] [PubMed]
- Kuzon, W.M.; Marcus, J.R.; Kerluke, L.D.; Phillips, J.H. African Spitting Cobra (Naja nigricollis) Bite of the Hand. Can. J. Plast. Surg. 1994, 2, 90–92. [Google Scholar] [CrossRef]
- Soro, L.; Traoré, A.; Ayé, Y.D.; Sissoko, J.; Guiégui, C.P.; Ncho-Mottoh, M.P.; Monemo, P.; Babo, J.C.; Yéo, T.L.P.; Amonkou, A. Envenimation atypique par Naja nigricollis en Côte d’Ivoire. Reanoxyo 2010, 26, 14–15. Available online: https://sofia.medicalistes.fr/spip/IMG/pdf/Reanoxyo_26-volume_1_septembre_2010.pdf (accessed on 1 April 2013).
- Rivel, M.; Solano, D.; Herrera, M.; Vargas, M.; Villalta, M.; Segura, Á.; Arias, A.S.; León, G.; Gutiérrez, J.M. Pathogenesis of dermonecrosis induced by venom of the spitting cobra, Naja nigricollis: An experimental study in mice. Toxicon 2016, 119, 171–179. [Google Scholar] [CrossRef]
- Tan, C.H.; Wong, K.Y.; Tan, N.H.; Ng, T.S.; Tan, K.Y. Distinctive Distribution of Secretory Phospholipases A₂ in the Venoms of Afro-Asian Cobras (Subgenus: Naja, Afronaja, Boulengerina and Uraeus). Toxins 2019, 11, 116. [Google Scholar] [CrossRef]

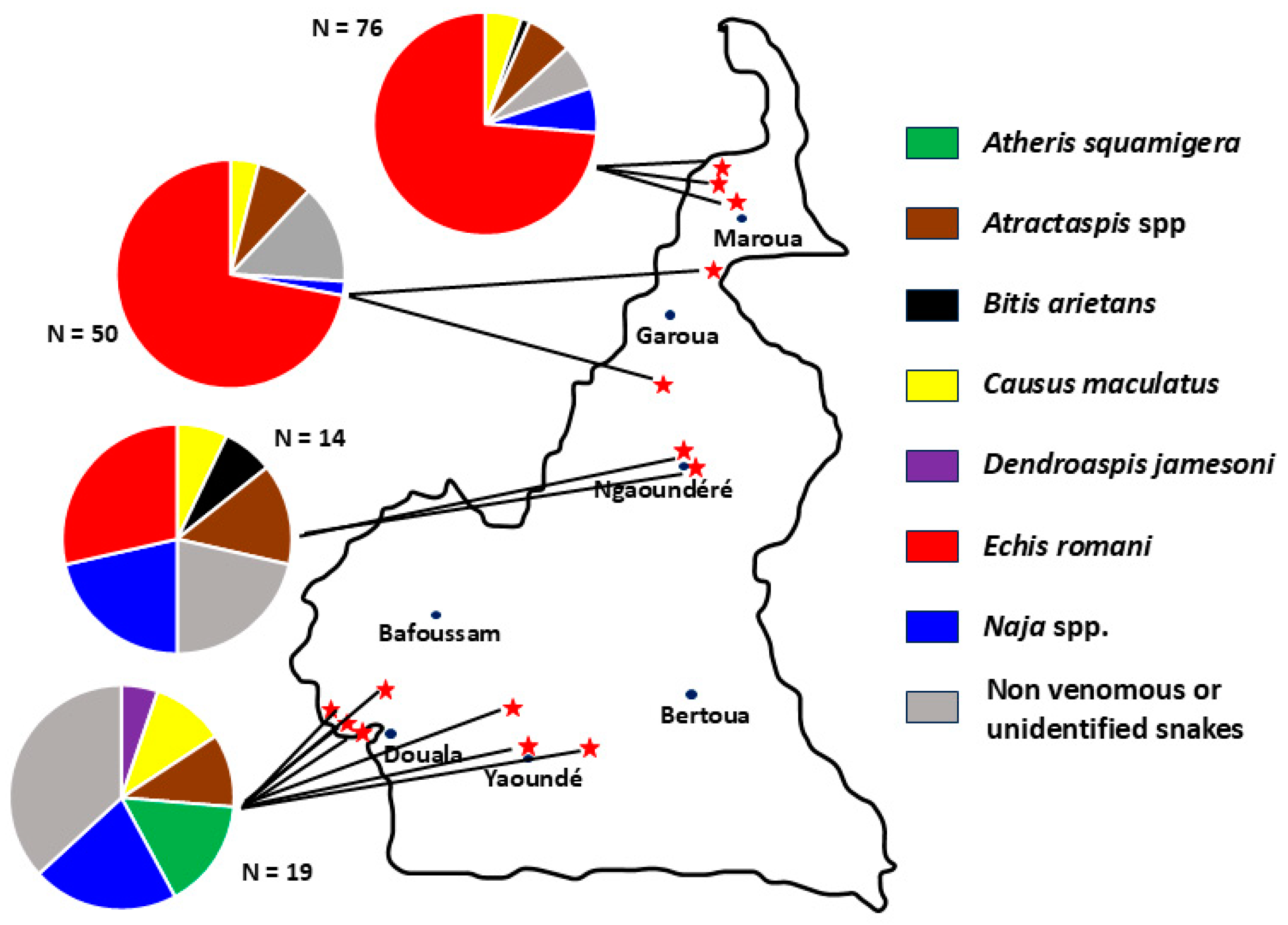

| Snake Species | Number, N (%) |
|---|---|
| Boidae | |
| Eryx colubrinus | 2 (1.3) |
| Colubridae | |
| Crotaphopeltis hotamboeia | 4 (2.5) |
| Telescopus variegatus | 1 (0.6) |
| Elapidae | |
| Dendroaspis jamesoni * | 1 (0.6) |
| Naja haje * | 1 (0.6) |
| Naja katiensis * | 1 (0.6) |
| Naja melanoleuca species complex * | 5 (3.1) |
| Naja nigricollis * | 2 (1.3) |
| Lamprophiidae | |
| Atractaspis spp. * | 12 (7.5) |
| Boaedon spp. | 5 (3.1) |
| Psammophis spp. | 6 (3.8) |
| Pythonidae | |
| Python sebae | 1 (0.6) |
| Viperidae | |
| Atheris squamigera * | 2 (1.3) |
| Bitis arietans * | 2 (1.3) |
| Causus maculatus * | 11 (6.9) |
| Echis romani * | 95 (59.7) |
| Unidentified species | 8 (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chippaux, J.-P.; Madec, Y.; Amta, P.; Ntone, R.; Noël, G.; Clauteaux, P.; Boum, Y., II; Nkwescheu, A.S.; Taieb, F. Snakebites in Cameroon by Species Whose Effects Are Poorly Described. Trop. Med. Infect. Dis. 2024, 9, 300. https://doi.org/10.3390/tropicalmed9120300
Chippaux J-P, Madec Y, Amta P, Ntone R, Noël G, Clauteaux P, Boum Y II, Nkwescheu AS, Taieb F. Snakebites in Cameroon by Species Whose Effects Are Poorly Described. Tropical Medicine and Infectious Disease. 2024; 9(12):300. https://doi.org/10.3390/tropicalmed9120300
Chicago/Turabian StyleChippaux, Jean-Philippe, Yoann Madec, Pierre Amta, Rodrigue Ntone, Gaëlle Noël, Pedro Clauteaux, Yap Boum, II, Armand S. Nkwescheu, and Fabien Taieb. 2024. "Snakebites in Cameroon by Species Whose Effects Are Poorly Described" Tropical Medicine and Infectious Disease 9, no. 12: 300. https://doi.org/10.3390/tropicalmed9120300
APA StyleChippaux, J.-P., Madec, Y., Amta, P., Ntone, R., Noël, G., Clauteaux, P., Boum, Y., II, Nkwescheu, A. S., & Taieb, F. (2024). Snakebites in Cameroon by Species Whose Effects Are Poorly Described. Tropical Medicine and Infectious Disease, 9(12), 300. https://doi.org/10.3390/tropicalmed9120300








