House Screening Reduces Exposure to Indoor Host-Seeking and Biting Malaria Vectors: Evidence from Rural South-East Zambia
Abstract
1. Introduction
2. Materials and Methods
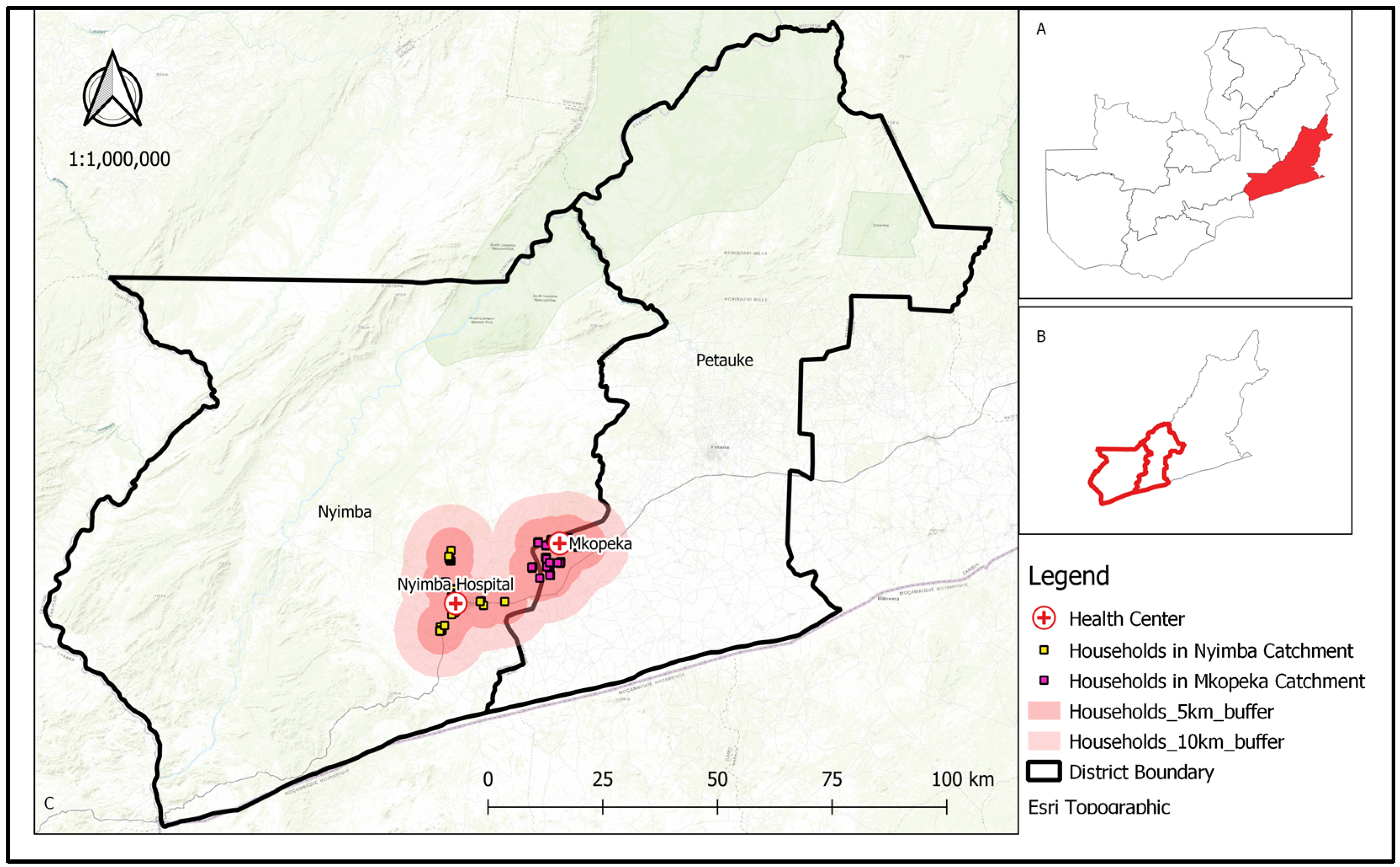
2.1. Study Area
2.2. Study Design
2.3. Community Sensitization and Consent to Participate
2.4. Study Households, Enumeration, and Participants
2.5. Installation of House Screens
2.6. Adult Mosquito Collections
2.6.1. Light Traps
2.6.2. Indoor Resting Collections
2.6.3. Human Landing Catches
2.7. Species Composition
2.8. Detection of Plasmodium falciparum Infection in Mosquitoes
2.9. Data Analysis
3. Results
3.1. Anopheles Species Composition
3.2. Impact of House Screening on Mosquito Densities
3.2.1. Indoor Host-Seeking
3.2.2. Indoor Resting Densities
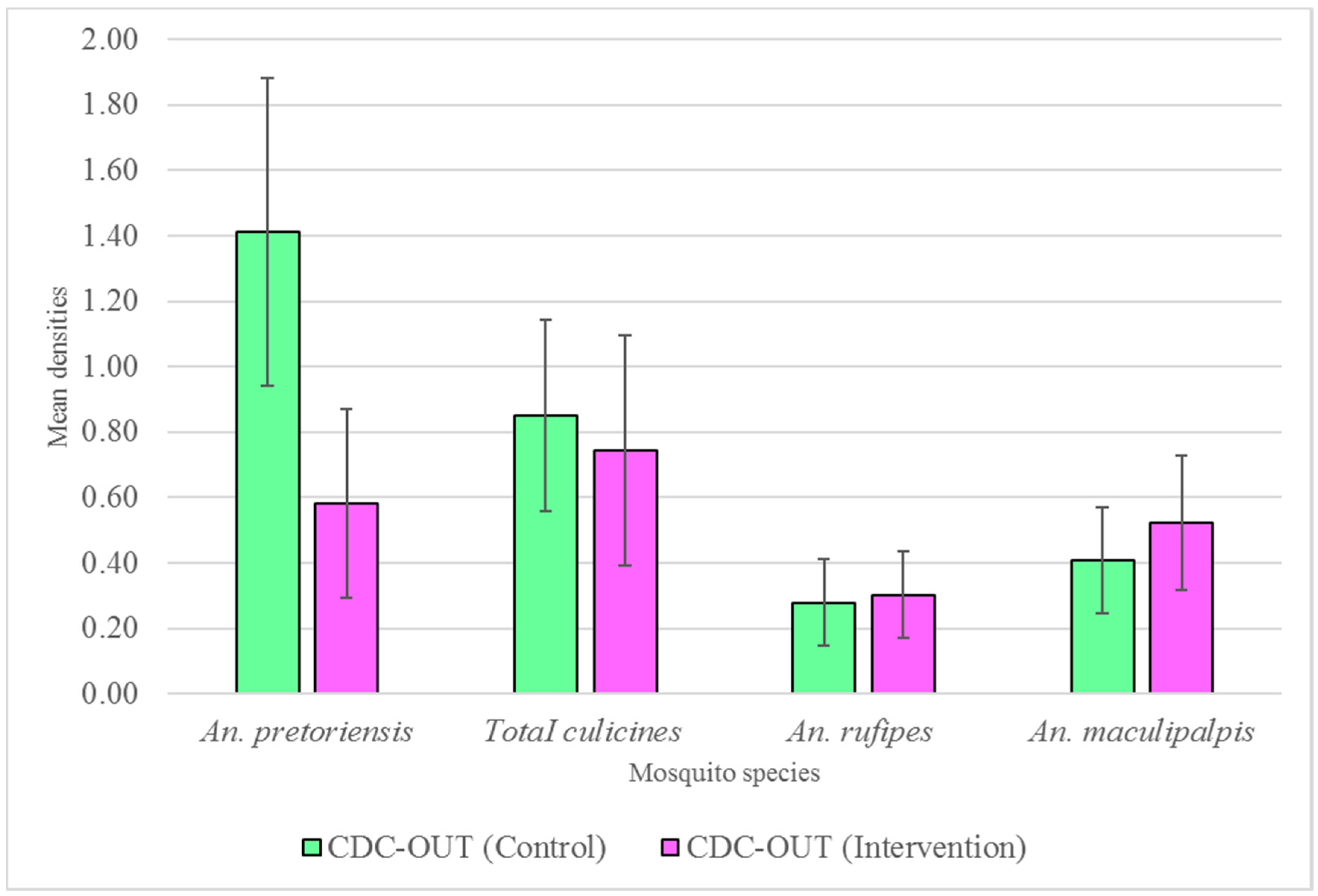
3.3. Outdoor Host-Seeking
3.4. Effect of House Screening on Vector Biting Behavior
3.4.1. Indoor Biting
3.4.2. Outdoor Biting
3.5. Sporozoite Infectivity Rates
3.6. Entomological Inoculation Rates
3.6.1. Indoors
3.6.2. Outdoors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Republic of Zambia, M.o.H. National Malaria Elimination Strategic Plan (2022–2026); Ministry of Health: Lusaka, Zambia, 2022. [Google Scholar]
- Republic of Zambia, M.o.H. Zambia National Malaria Indicator Survey 2018; Ministry of Health: Lusaka, Zambia, 2018. [Google Scholar]
- WHO. WHO Guidelines for Malaria, 3 June 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Okumu, F.; Gyapong, M.; Casamitjana, N.; Castro, M.C.; Itoe, M.A.; Okonofua, F.; Tanner, M. What Africa can do to accelerate and sustain progress against malaria. PLoS Glob. Public Health 2022, 2, e0000262. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasites Vectors 2010, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.W.; Davies, M.; Alabaster, G.; Altamirano, H.; Jatta, E.; Jawara, M.; Carrasco-Tenezaca, M.; von Seidlein, L.; Shenton, F.C.; Tusting, L.S. Recommendations for building out mosquito-transmitted diseases in sub-Saharan Africa: The DELIVER mnemonic. Philos. Trans. R. Soc. B 2021, 376, 20190814. [Google Scholar] [CrossRef]
- Massebo, F.; Lindtjørn, B. The effect of screening doors and windows on indoor density of Anopheles arabiensis in south-west Ethiopia: A randomized trial. Malar. J. 2013, 12, 319. [Google Scholar] [CrossRef]
- Scott, T.W.; Takken, W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012, 28, 114–121. [Google Scholar] [CrossRef]
- Huho, B.; Briet, O.; Seyoum, A.; Sikaala, C.; Bayoh, N.; Gimnig, J.; Okumu, F.; Diallo, D.; Abdulla, S.; Smith, T.; et al. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int. J. Epidemiol. 2013, 42, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Okumu, F.; Finda, M. Key characteristics of residual malaria transmission in two districts in south-eastern Tanzania—Implications for improved control. J. Infect. Dis. 2021, 223, S143–S154. [Google Scholar] [CrossRef]
- Seyoum, A.; Sikaala, C.H.; Chanda, J.; Chinula, D.; Ntamatungiro, A.J.; Hawela, M.; Miller, J.M.; Russell, T.L.; Briët, O.J.; Killeen, G.F. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, South-east Zambia. Parasites Vectors 2012, 5, 101. [Google Scholar] [CrossRef]
- Tusting, L.S.; Cairncross, S.; Ludolph, R.; Velayudhan, R.; Wilson, A.L.; Lindsay, S.W. Assessing the health benefits of development interventions. BMJ Glob. Health 2021, 6, e005169. [Google Scholar] [CrossRef]
- Abong’o, B.; Gimnig, J.E.; Omoke, D.; Ochomo, E.; Walker, E.D. Screening eaves of houses reduces indoor mosquito density in rural, western Kenya. Malar. J. 2022, 21, 377. [Google Scholar] [CrossRef]
- Getawen, S.K.; Ashine, T.; Massebo, F.; Woldeyes, D.; Lindtjørn, B. Exploring the impact of house screening intervention on entomological indices and incidence of malaria in Arba Minch town, southwest Ethiopia: A randomized control trial. Acta Trop. 2018, 181, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Killeen, G.F.; Govella, N.J.; Mlacha, Y.P.; Chaki, P.P. Suppression of malaria vector densities and human infection prevalence associated with scale-up of mosquito-proofed housing in Dar es Salaam, Tanzania: Re-analysis of an observational series of parasitological and entomological surveys. Lancet Planet. Health 2019, 3, e132–e143. [Google Scholar] [CrossRef]
- Kirby, M.J.; Ameh, D.; Bottomley, C.; Green, C.; Jawara, M.; Milligan, P.J.; Snell, P.C.; Conway, D.J.; Lindsay, S.W. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: A randomised controlled trial. Lancet 2009, 374, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Prokopec, G.M.; Lenhart, A.; Manrique-Saide, P. Housing improvement: A novel paradigm for urban vector-borne disease control? Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 567–569. [Google Scholar] [CrossRef][Green Version]
- Manrique-Saide, P.; Herrera-Bojórquez, J.; Medina-Barreiro, A.; Trujillo-Peña, E.; Villegas-Chim, J.; Valadez-González, N.; Ahmed, A.M.; Delfín-González, H.; Palacio-Vargas, J.; Che-Mendoza, A. Insecticide-treated house screening protects against Zika-infected Aedes aegypti in Merida, Mexico. PLoS Negl. Trop. Dis. 2021, 15, e0009005. [Google Scholar] [CrossRef] [PubMed]
- Ogoma, S.B.; Lweitoijera, D.W.; Ngonyani, H.; Furer, B.; Russell, T.L.; Mukabana, W.R.; Killeen, G.F.; Moore, S.J. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PLoS Negl. Trop. Dis. 2010, 4, e773. [Google Scholar] [CrossRef]
- Lupenza, E.; Gasarasi, D.B.; Minzi, O.M. Lymphatic filariasis, infection status in Culex quinquefasciatus and Anopheles species after six rounds of mass drug administration in Masasi District, Tanzania. Infect. Dis. Poverty 2021, 10, 20. [Google Scholar] [CrossRef]
- Saili, K.; de Jager, C.; Sangoro, O.P.; Nkya, T.E.; Masaninga, F.; Mwenya, M.; Sinyolo, A.; Hamainza, B.; Chanda, E.; Fillinger, U. Anopheles rufipes implicated in malaria transmission both indoors and outdoors alongside Anopheles funestus and Anopheles arabiensis in rural south-east Zambia. Malar. J. 2023, 22, 95. [Google Scholar] [CrossRef]
- Chisanga, B.; Bulte, E.; Kassie, M.; Mutero, C.; Masaninga, F.; Sangoro, O.P. The economic impacts of house screening against malaria transmission: Experimental evidence from eastern Zambia. Soc. Sci. Med. 2023, 321, 115778. [Google Scholar] [CrossRef]
- Sangoro, O.P.; Fillinger, U.; Saili, K.; Nkya, T.E.; Marubu, R.; Masaninga, F.; Trigo, S.C.; Tarumbwa, C.; Hamainza, B.; Baltazar, C. Evaluating the efficacy, impact, and feasibility of community-based house screening as a complementary malaria control intervention in southern Africa: A study protocol for a household randomized trial. Trials 2021, 22, 883. [Google Scholar] [CrossRef]
- World Health Organization. Malaria Entomology and Vector Control; 924150580X; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Coetzee, M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar. J. 2020, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Brogdon, W.G.; Collins, F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Koekemoer, L.L.; Lochouarn, L.; Hunt, R.H.; Coetzee, M. Single-strand conformation polymorphism analysis for identification of four members of the Anopheles funestus (Diptera: Culicidae) group. J. Med. Entomol. 1999, 36, 125–130. [Google Scholar] [CrossRef]
- Wirtz, R.; Zavala, F.; Charoenvit, Y.; Campbell, G.; Burkot, T.; Schneider, I.; Esser, K.; Beaudoin, R.L.; Andre, R. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull. World Health Organ. 1987, 65, 39. [Google Scholar]
- Benedict, M. Methods in Anopheles Research; Malaria Research and Reference Reagent Resource Center (MR4): 2007. Available online: https://www.beiresources.org/Publications/MethodsinAnophelesResearch.aspx (accessed on 8 January 2024).
- Durnez, L.; Van Bortel, W.; Denis, L.; Roelants, P.; Veracx, A.; Trung, H.D.; Sochantha, T.; Coosemans, M. False positive circumsporozoite protein ELISA: A challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar. J. 2011, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2013. Available online: https://www.R-project.org/ (accessed on 8 January 2024).
- Njie, M.; Dilger, E.; Lindsay, S.W.; Kirby, M.J. Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J. Med. Entomol. 2009, 46, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Takken, W. The mosquito and malaria: Would mosquito control alone eliminate the disease? Mosquitopia 2021, 109–122. [Google Scholar]
- Shaukat, A.M.; Breman, J.G.; McKenzie, F.E. Using the entomological inoculation rate to assess the impact of vector control on malaria parasite transmission and elimination. Malar. J. 2010, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Boelaert, M.; Kleinschmidt, I.; Pinder, M.; Scott, T.W.; Tusting, L.S.; Lindsay, S.W. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 2015, 31, 380–390. [Google Scholar] [PubMed]
- Pinder, M.; Bradley, J.; Jawara, M.; Affara, M.; Conteh, L.; Correa, S.; Jeffries, D.; Jones, C.; Kandeh, B.; Knudsen, J. Improved housing versus usual practice for additional protection against clinical malaria in The Gambia (RooPfs): A household-randomised controlled trial. Lancet Planet. Health 2021, 5, e220–e229. [Google Scholar] [CrossRef] [PubMed]
- Ndebele, P.; Musesengwa, R. Ethical dilemmas in malaria vector research in Africa: Making the difficult choice between mosquito, science and humans. Malawi Med. J. 2012, 24, 65–68. [Google Scholar]
- Mukabana, W.R.; Takken, W.; Coe, R.; Knols, B.G. Host-specific cues cause differential attractiveness of Kenyan men to the African malaria vector Anopheles gambiae. Malar. J. 2002, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, M.E.; Searle, K.M.; Kobayashi, T.; Shields, T.M.; Hamapumbu, H.; Simubali, L.; Mudenda, T.; Thuma, P.E.; Stevenson, J.C.; Moss, W.J. Understudied Anophelines Contribute to Malaria Transmission in a Low-Transmission Setting in the Choma District, Southern Province, Zambia. Am. J. Trop. Med. Hyg. 2022, 106, 1406. [Google Scholar] [CrossRef]
- Lobo, N.F.; St Laurent, B.; Sikaala, C.H.; Hamainza, B.; Chanda, J.; Chinula, D.; Krishnankutty, S.M.; Mueller, J.D.; Deason, N.A.; Hoang, Q.T.; et al. Unexpected diversity of Anopheles species in Eastern Zambia: Implications for evaluating vector behavior and interventions using molecular tools. Sci. Rep. 2015, 5, 17952. [Google Scholar] [CrossRef]
- Ranson, H.; N’Guessan, R.; Lines, J.; Moiroux, N.; Nkuni, Z.; Corbel, V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol 2011, 27, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sougoufara, S.; Doucouré, S.; Sembéne, P.M.B.; Harry, M.; Sokhna, C. Challenges for malaria vector control in sub-Saharan Africa: Resistance and behavioral adaptations in Anopheles populations. J. Vector Borne Dis. 2017, 54, 4. [Google Scholar]





| Species | Unscreened Houses (Control) | Screened Houses (Intervention) | ||
|---|---|---|---|---|
| N | Mean (95% CI) | N | Mean (95% CI) | |
| An. funestus | 121 | 0.65 (0.42–0.89) | 19 | 0.11 (0.05–0.16) |
| An. arabiensis | 76 | 0.41 (0.26–0.56) | 15 | 0.08 (0.01–0.16) |
| An. pretoriensis | 171 | 0.92 (0.62–1.23) | 46 | 0.26 (0.10–0.42) |
| An. rufipes | 63 | 0.34 (0.16–0.52) | 23 | 0.13 (0.05–0.21) |
| An. maculipalapis | 43 | 0.23 (0.12–0.34) | 43 | 0.24 (0.14–0.35) |
| An. coustani | 27 | 0.15 0.03–0.26) | 5 | 0.03 (0–0.05) |
| An. gibbinsi | 13 | 0.07 (0.02–0.12) | 8 | 0.05 (0–0.01) |
| An. squamosus | 8 | 0.04 (0–0.09) | 2 | 0.01 (0–0.03) |
| Total Anopheles | 522 | 2.82 | 161 | 0.91 |
| Total Culicines | 111 | 0.6 | 48 | 0.27 |
| Species | Unscreened Houses (Control) | Screened (Intervention) | ||
|---|---|---|---|---|
| N | Mean (95% CI) | N | Mean (95% CI) | |
| An. arabiensis | 19 | 0.09 (0.04–0.13) | 14 | 0.06 (0.02–0.09) |
| An. funestus | 25 | 0.11 (0.06–0.17) | 13 | 0.05 (0.02–0.08) |
| An. gibbinsi | 10 | 0.05 (0.03–0.07) | 7 | 0.03 (0–0.05) |
| An. rufipes | 46 | 0.21 (0.12–0.30) | 34 | 0.13 (0.07–0.20) |
| An. coustani | 7 | 0.03 (0.01–0.06) | 5 | 0.02 (0–0.04) |
| An. maculipalapis | 56 | 0.26 (0.15–0.36) | 49 | 0.19 (0.11–0.28) |
| An. pretoriensis | 45 | 0.21 (0.12–0.30) | 66 | 0.26 (0.15–0.37) |
| Total Anopheles | 208 | 0.95 | 188 | 0.75 |
| Total Culicines | 135 | 0.62 | 48 | 0.19 |
| Species | Unscreened (Control) | Screened (Intervention) | ||
|---|---|---|---|---|
| N | Mean (95% CI) | N | Mean (95% CI) | |
| An. pretoriensis | 219 | 1.41 (0.94–1.88) | 77 | 0.55 (0.30–0.87) |
| An. funestus | 141 | 0.91 (0.60–1.22) | 54 | 0.41 (0.20–0.62) |
| An. coustani | 15 | 0.1 (0.04–0.15) | 7 | 0.05 (0.01–0.10) |
| An. arabiensis | 59 | 0.38 (0.22–0.54) | 39 | 0.30 (0.15–0.45) |
| An. gibbinsi | 11 | 0.07 (0.01–0.13) | 9 | 0.07 (0.02–0.12) |
| An. rufipes | 43 | 0.28 (0.15–0.41) | 40 | 0.30 (0.17–0.44) |
| An. maculipalapis | 63 | 0.41 (0.25–0.57) | 69 | 0.52 (0.32–0.73) |
| Total Anopheles | 551 | 3.56 | 296 | 2.24 |
| Total Culicines | 132 | 0.85 | 48 | 0.36 |
| Trap Location | Treatment | Species | # Assayed | # CSP Positive | Sporozoite Rate | Human Biting Rates 1 | EIR (ib/p/y) |
|---|---|---|---|---|---|---|---|
| Indoors | Unscreened | An. funestus | 81 | 2 | 0.02 | 0.65 | 2.91 |
| An. arabiensis | 66 | 0 | 0.00 | 0.25 | 0.00 | ||
| Screened | An. funestus | 21 | 2 | 0.10 | 0.11 | 1.88 | |
| An arabiensis | 19 | 0 | 0.00 | 0.06 | 0.00 | ||
| Outdoors | Unscreened | An. funestus | 40 | 1 | 0.03 | 0.91 | 4.09 |
| An. arabiensis | 22 | 0 | 0.00 | 0.25 | 0.00 | ||
| Screened | An. funestus | 20 | 0 | 0.00 | 0.42 | 0.00 | |
| An arabiensis | 16 | 0 | 0.00 | 0.30 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saili, K.; de Jager, C.; Masaninga, F.; Sangoro, O.P.; Nkya, T.E.; Likulunga, L.E.; Chirwa, J.; Hamainza, B.; Chanda, E.; Fillinger, U.; et al. House Screening Reduces Exposure to Indoor Host-Seeking and Biting Malaria Vectors: Evidence from Rural South-East Zambia. Trop. Med. Infect. Dis. 2024, 9, 20. https://doi.org/10.3390/tropicalmed9010020
Saili K, de Jager C, Masaninga F, Sangoro OP, Nkya TE, Likulunga LE, Chirwa J, Hamainza B, Chanda E, Fillinger U, et al. House Screening Reduces Exposure to Indoor Host-Seeking and Biting Malaria Vectors: Evidence from Rural South-East Zambia. Tropical Medicine and Infectious Disease. 2024; 9(1):20. https://doi.org/10.3390/tropicalmed9010020
Chicago/Turabian StyleSaili, Kochelani, Christiaan de Jager, Freddie Masaninga, Onyango P. Sangoro, Theresia E. Nkya, Likulunga Emmanuel Likulunga, Jacob Chirwa, Busiku Hamainza, Emmanuel Chanda, Ulrike Fillinger, and et al. 2024. "House Screening Reduces Exposure to Indoor Host-Seeking and Biting Malaria Vectors: Evidence from Rural South-East Zambia" Tropical Medicine and Infectious Disease 9, no. 1: 20. https://doi.org/10.3390/tropicalmed9010020
APA StyleSaili, K., de Jager, C., Masaninga, F., Sangoro, O. P., Nkya, T. E., Likulunga, L. E., Chirwa, J., Hamainza, B., Chanda, E., Fillinger, U., & Mutero, C. M. (2024). House Screening Reduces Exposure to Indoor Host-Seeking and Biting Malaria Vectors: Evidence from Rural South-East Zambia. Tropical Medicine and Infectious Disease, 9(1), 20. https://doi.org/10.3390/tropicalmed9010020





