Meta-Analysis of Data from Four Clinical Trials in the Ivory Coast Assessing the Efficacy of Two Artemisinin-Based Combination Therapies (Artesunate-Amodiaquine and Artemether-Lumefantrine) between 2009 and 2016
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
- The first study was conducted from 2008 to 2009 in the health district of Dabakala, located in the central-eastern part of the Ivory Coast and Ayamé.
- The second study was conducted in 2012 in three sites: the health districts of Abengourou, located in the southeastern forest region, San Pedro in the southwest coastal and forest region, and Yamoussoukro in the country’s central Lake District.
- The third study occurred in the cities of Man, Korhogo, Abengourou, San-Pedro, Yamoussoukro, and Abidjan, in 2013.
- The last study was conducted in Abidjan, Man, Abengourou, San-Pedro, Yamoussoukro, and Korhogo from 2015 to 2016.
2.2. Study Design
2.3. Study Participants and Inclusion Criteria
- Inclusion criteria;
- Patients aged over six months;
- Without distinction by sex (male or female patient);
- Presenting fever with an axillary temperature of ≥37.5 °C or a history of fever within 24 h;
- With acute uncomplicated P. falciparum malaria (2000–200,000 parasites/μL), confirmed under the microscope (Giemsa-stained thick and thin films);
- Having the ability to ingest tablets orally;
- Patients having provided their consent to participate in the study or parents or guardians consented to children participating in the study.
- Exclusion criteria;
- Severe malnutrition;
- Pregnant women and nursing mothers;
- Signs of altered general condition or signs of severe malaria;
- Fever due to a disease other than malaria;
- History of a hypersensitive reaction to the combination of artemether–lumefantrine and/or artesunate–amodiaquine or to one of the constituents;
- Taking an antimalarial drug (or a drug with known antiplasmodial activity) in the week preceding inclusion.
2.4. Randomization, Treatment, and Follow-Up
2.5. Data Collection
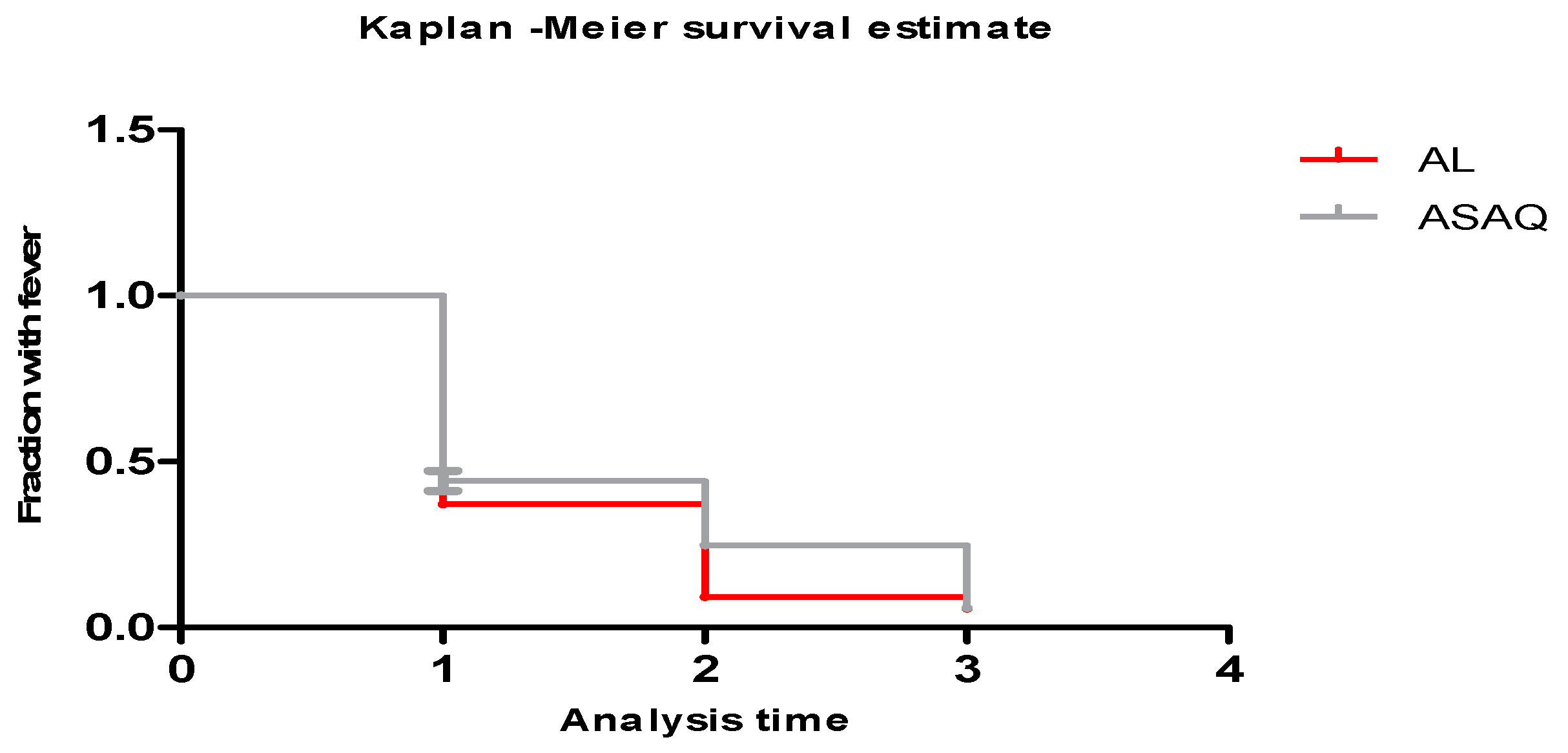
2.6. Outcome Assessment
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Period of 2009–2012
3.3. Period of 2013–2016
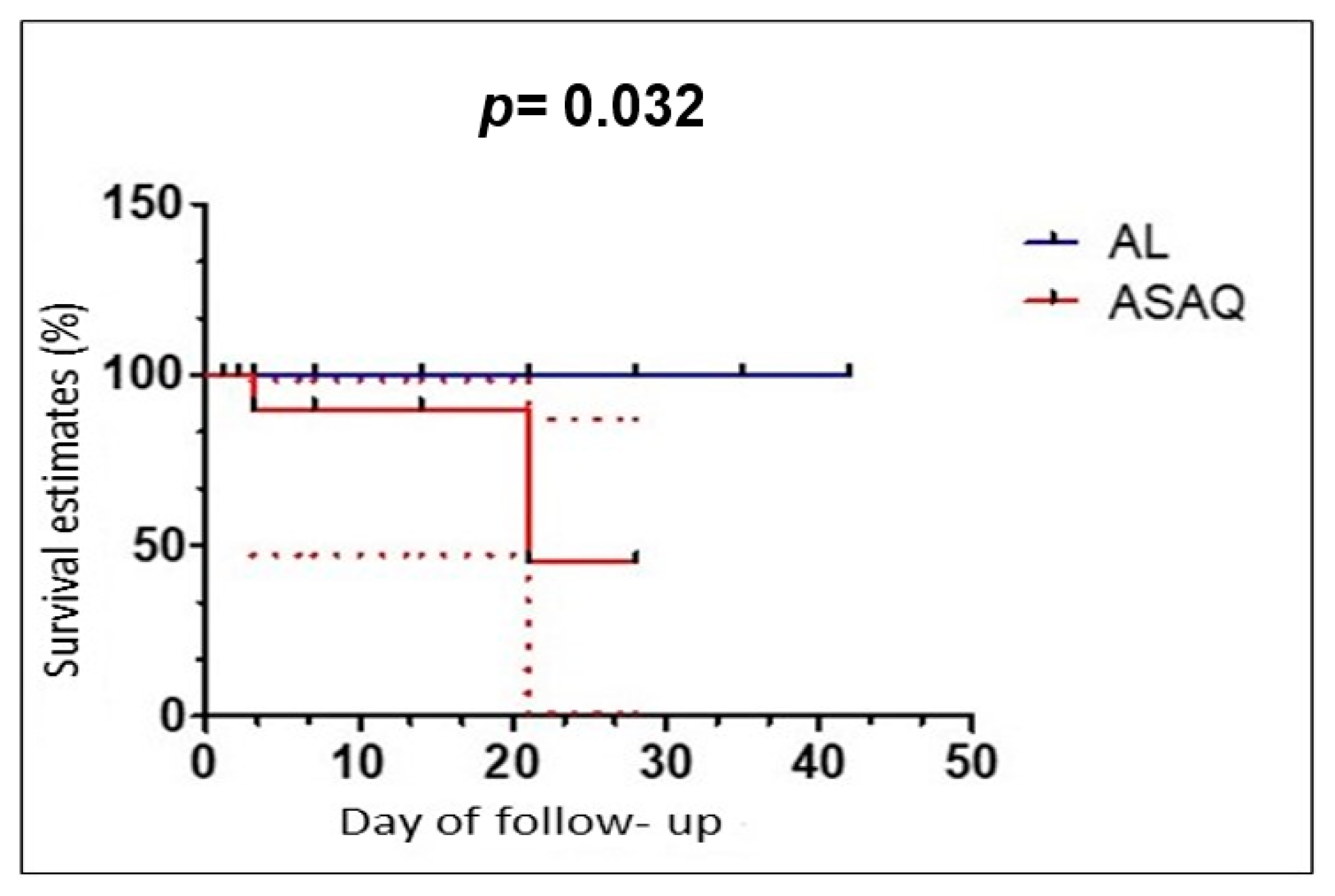
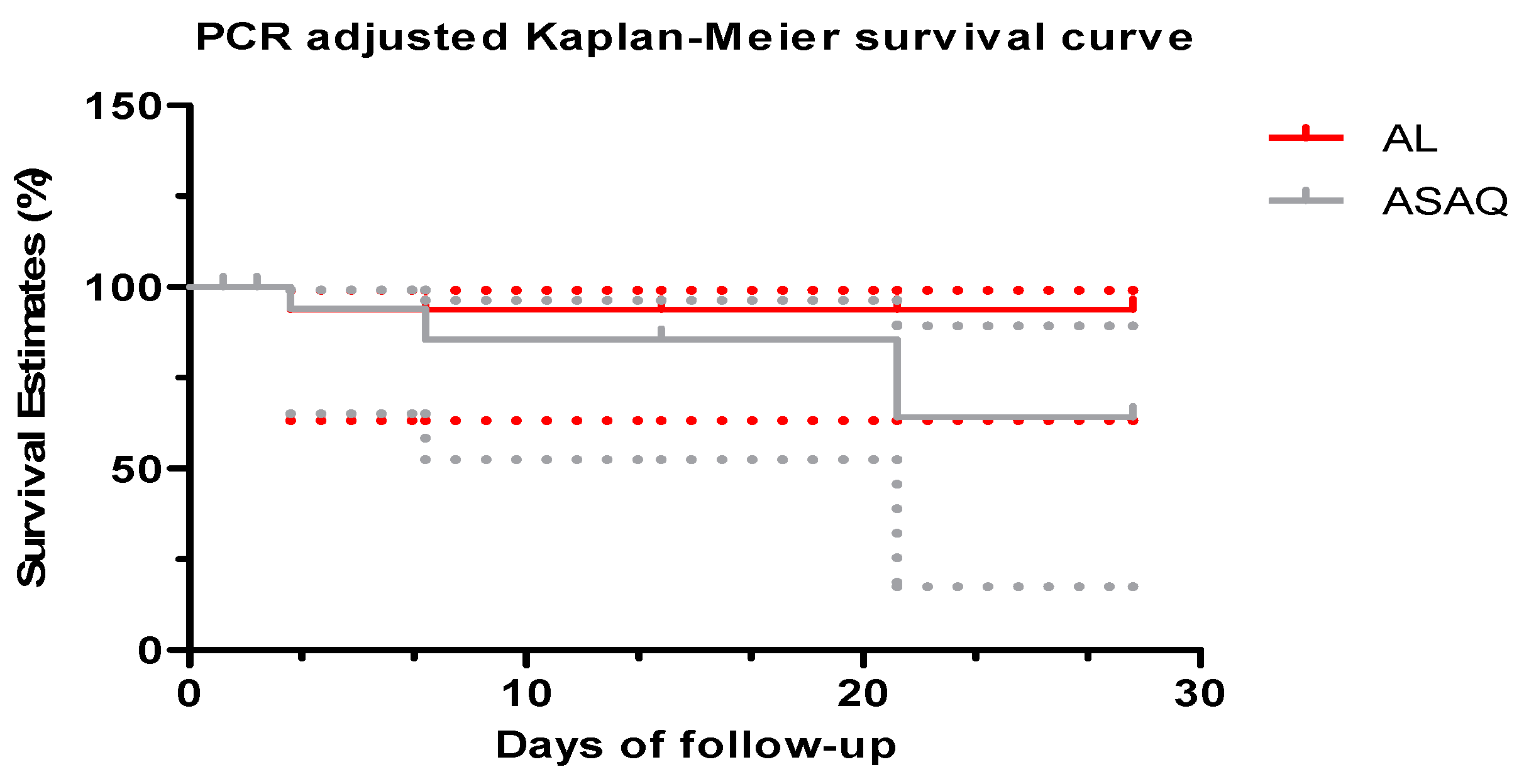
3.4. Period of 2009–2016
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO World Malaria Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. World Malaria Report 2021, Key Messages; WHO: Geneva, Switzerland, 2021; p. 24. [Google Scholar]
- Yavo, W.; Konaté, A.; Kassi, F.K.; Djohan, V.; Angora, E.K.; Kiki-Barro, P.C.; Vanga-Bosson, H.; Menan, E.I.H. Efficacy and Safety of Artesunate-Amodiaquine versus Artemether-Lumefantrine in the Treatment of Uncomplicated Plasmodium falciparum Malaria in Sentinel Sites across Ivory Coast. Malar. Res. Treat. 2015, 2015, 878132. [Google Scholar] [CrossRef] [PubMed]
- Konaté, A.; Barro-Kiki, P.C.M.; Angora, K.E.; Bédia-Tanoh, A.V.; Djohan, V.; Kassi, K.F.; Vanga-Bosson, H.; Miézan, A.J.S.; Assi, S.B.; Menan, E.I.H.; et al. Efficacy and Tolerability of Artesunate-Amodiaquine versus Artemether-Lumefantrine in the Treatment of Uncomplicated Plasmodium falciparum Malaria at Two Sentinel Sites across Côte d’Ivore. Ann. Parasitol. 2018, 64, 49–57. [Google Scholar]
- NMCP. National Malaria Control Program: National Strategic Plan for Malaria Control 2016–2021; NMCP: Abidjan, Ivory Coast, 2016; p. 88. [Google Scholar]
- Ali, I.M.; Netongo, P.; Atogho-Tiedeu, B.; Ngongang, E.-O.; Ajua, A.; Achidi, E.A.; Mbacham, W.F. Amodiaquine-Artesunate versus Artemether-Lumefantrine against Uncomplicated Malaria in Children Less Than 14 Years in Ngaoundere, North Cameroon. Efficacy, Safety, and Baseline Drug Resistant Mutations in Pfcrt, Pfmdr1, and Pfdhfr Genes. Malar. Res. Treat. 2013, 2013, 234683. [Google Scholar] [CrossRef] [PubMed]
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M. Evidence of Artemisinin-Resistant Malaria in Western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Methods for Surveillance of Antimalarial Drug Efficacity; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Leang, R.; Taylor, W.R.J.; Bouth, D.M.; Song, L.; Tarning, J.; Char, M.C.; Kim, S.; Witkowski, B.; Duru, V.; Domergue, A.; et al. Evidence of Plasmodium falciparum Malaria Multidrug Resistance to Artemisinin and Piperaquine in Western Cambodia: Dihydroartemisinin-Piperaquine Open-Label Multicenter Clinical Assessment. Antimicrob. Agents Chemother. 2015, 59, 471926. [Google Scholar] [CrossRef] [PubMed]
- Djaman, A.J. Evaluation of Plasmodium falciparum Chemoresistance to Different Antimalarial Drugs (Chloroquine, Sulfadoxine-Pyrimethamine, Quinine) and Genetic Profile of Corresponding Isolates. These Doctorate. Paris 12. 2003. Available online: https://www.theses.fr/2003PA120021 (accessed on 29 September 2023).
- World Health Organization. World Malaria Report 2001; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Toure, O.A.; Landry, T.N.; Assi, S.B.; Kone, A.A.; Gbessi, E.A.; Ako, B.A.; Coulibaly, B.; Kone, B.; Ouattara, O.; Beourou, S.; et al. Malaria Parasite Clearance from Patients Following Artemisinin-Based Combination Therapy in Côte d’Ivoire. Infect. Drug Resist. 2018, 11, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- MSHPCMU. Coast Ministry of Health and the Control of HIV/AIDS Order N°00111/CAB/MSHPCMU of 16 June 2022. In A Redefining the Therapeutic and Prevention Regimen for Malaria in Ivory Coast; NMCP: Abidjan, Ivory Coast, 2023; p. 4. Available online: https://www.pnlpcotedivoire.org (accessed on 23 March 2023).
- Sylla, K.; Abiola, A.; Tine, R.C.K.; Faye, B.; Sow, D.; Ndiaye, J.L.; Ndiaye, M.; Lo, A.C.; Folly, K.; Ndiaye, L.A.; et al. Monitoring the Efficacy and Safety of Three Artemisinin Based-Combinations Therapies in Senegal: Results from Two Years Surveillance. BMC Infect. Dis. 2013, 13, 598. [Google Scholar] [CrossRef]
- The Four Artemisinin-Based Combinations (4ABC) Study Group. A Head-to-Head Comparison of Four Artemisinin-Based Combinations for Treating Uncomplicated Malaria in African Children: A Randomized Trial. PLoS Med. 2011, 8, e1001119. [Google Scholar] [CrossRef]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef]
- Cooper Cooper, R.A.; Conrad, M.D.; Watson, Q.D.; Huezo, S.J.; Ninsiima, H.; Tumwebaze, P.; Nsobya, S.L.; Rosenthal, P.J. Lack of Artemisinin Resistance in Plasmodium falciparum in Uganda Based on Parasitological and Molecular Assays. Antimicrob. Agents Chemother. 2015, 59, 5061–5064. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2016; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Toure, O.A.; Assi, S.B.; N’Guessan, T.L.; Adji, G.E.; Ako, A.B.; Brou, M.J.; Ehouman, M.F.; Gnamien, L.A.; Coulibaly, M.A.; Coulibaly, B.; et al. Open-Label, Randomized, Non-Inferiority Clinical Trial of Artesunate-Amodiaquine versus Artemether-Lumefantrine Fixed-Dose Combinations in Children and Adults with Uncomplicated falciparum Malaria in Côte d’Ivoire. Malar. J. 2014, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Menan, H.; Faye, O.; Same-Ekobo, A.; Oga, A.S.S.; Faye, B.; Kiki Barro, C.P.; Kuete, T.; N’diaye, J.-L.; Vicky, A.-M.; Tine, R.; et al. Comparative Study of the Efficacy and Tolerability of Dihydroartemisinin-Piperaquine-Trimethoprim versus Artemether-Lumefantrine in the Treatment of Uncomplicated Plasmodium falciparum Malaria in Cameroon, Ivory Coast and Senegal. Malar. J. 2011, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Faye, B.; Kuete, T.; Kiki Barro, C.P.; Tine, R.C.; Nkoa, T.; NDiaye, J.L.; Kakpo, C.; Sylla; Menan, E.I.H.; Gaye, O.; et al. Multicentre Study Evaluating the Non-Inferiority of the New Paediatric Formulation of Artesunate/Amodiaquine versus Artemether/Lumefantrine for the Management of Uncomplicated Plasmodium falciparum Malaria in Children in Cameroon, Ivory Coast and Senegal. Malar. J. 2012, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Siribié, M.; Diarra, A.; Tiono, A.B.; Soulama, I.; Sirima, S.B. Efficacy of artemether-lumefantrine in the treatment of uncomplicated malaria in children living in a rural area of Burkina Faso in 2009. Bull. Soc. Pathol. Exot. 2012, 105, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Yeka, A.; Kigozi, R.; Conrad, M.D.; Lugemwa, M.; Okui, P.; Katureebe, C.; Belay, K.; Kapella, B.K.; Chang, M.A.; Kamya, M.R.; et al. Artesunate/Amodiaquine Versus Artemether/Lumefantrine for the Treatment of Uncomplicated Malaria in Uganda: A Randomized Trial. J. Infect. Dis. 2016, 213, 1134–1142. [Google Scholar] [CrossRef]
- Adjei, G.O.; Goka, B.Q.; Enweronu-Laryea, C.C.; Rodrigues, O.P.; Renner, L.; Sulley, A.M.; Alifrangis, M.; Khalil, I.; Kurtzhals, J.A. A Randomized Trial of Artesunate-Amodiaquine versus Artemether-Lumefantrine in Ghanaian Paediatric Sickle Cell and Non-Sickle Cell Disease Patients with Acute Uncomplicated Malaria. Malar. J. 2014, 13, 369. [Google Scholar] [CrossRef][Green Version]
- Mutabingwa, T.K.; Anthony, D.; Heller, A.; Hallett, R.; Ahmed, J.; Drakeley, C.; Greenwood, B.M.; Whitty, C.J.M. Amodiaquine Alone, Amodiaquine+sulfadoxine-Pyrimethamine, Amodiaquine+artesunate, and Artemether-Lumefantrine for Outpatient Treatment of Malaria in Tanzanian Children: A Four-Arm Randomised Effectiveness Trial. Lancet 2005, 365, 1474–1480. [Google Scholar] [CrossRef]
- Kabanywanyi, A.M.; Mwita, A.; Sumari, D.; Mandike, R.; Mugittu, K.; Abdulla, S. Efficacy and Safety of Artemisinin-Based Antimalarial in the Treatment of Uncomplicated Malaria in Children in Southern Tanzania. Malar. J. 2007, 6, 146. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.-C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A Molecular Marker of Artemisinin-Resistant Plasmodium falciparum Malaria. Nature 2013, 505, 50–55. [Google Scholar] [CrossRef]
- Tun, K.M.; Imwong, M.; Lwin, K.M.; Win, A.A.; Hlaing, T.M.; Hlaing, T.; Lin, K.; Kyaw, M.P.; Plewes, K.; Faiz, M.A.; et al. Spread of Artemisinin-Resistant Plasmodium falciparum in Myanmar: A Cross-Sectional Survey of the K13 Molecular Marker. Lancet Infect. Dis. 2015, 15, 415–421. [Google Scholar] [CrossRef]
- Sow, D.; Ndiaye, J.-L.; Sylla, K.; Ba, M.S.; Tine, R.C.K.; Faye, B.; Pene, M.; Ndiaye, M.; Seck, A.; Lo, A.C.; et al. Evaluation of the Efficacy and Safety of Three 2-Drug Combinations for the Treatment of Uncomplicated Plasmodium falciparum Malaria in Senegal: Artesunate-Amodiaquine, Dihydroartemisinin-Piperaquine, and Artemether-Lumefantrine. Med. Sante Trop. 2016, 26, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Sondo, P.; Derra, K.; Diallo-Nakanabo, S.; Tarnagda, Z.; Zampa, O.; Kazienga, A.; Valea, I.; Sorgho, H.; Owusu-Dabo, E.; Ouedraogo, J.-B.; et al. Effectiveness and Safety of Artemether-Lumefantrine versus Artesunate-Amodiaquine for Unsupervised Treatment of Uncomplicated falciparum Malaria in Patients of All Age Groups in Nanoro, Burkina Faso: A Randomized Open Label Trial. Malar. J. 2015, 14, 325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laminou, M.L.; Sadou, F.; Daou, M. Comparison of the efficacy and safety of artemether-lumefantrine with artesunate-amodiaquine in Niger. Mali Med. 2016, 31, 7. [Google Scholar]
- Adjei, G.O.; Kurtzhals, J.A.; Rodrigues, O.P.; Alifrangis, M.; Hoegberg, L.C.; Kitcher, E.D.; Badoe, E.V.; Lamptey, R.; Goka, B.Q. Amodiaquine-Artesunate vs. Artemether-Lumefantrine for Uncomplicated Malaria in Ghanaian Children: A Randomized Efficacy and Safety Trial with One Year Follow-Up. Malar. J. 2008, 7, 127. [Google Scholar] [CrossRef]
- Falade, C.O.; Ogundele, A.O.; Yusuf, B.O.; Ademowo, O.G.; Ladipo, S.M. High Efficacy of Two Artemisinin-Based Combinations (Artemether-Lumefantrine and Artesunate plus Amodiaquine) for Acute Uncomplicated Malaria in Ibadan, Nigeria. Trop. Med. Int. Health 2008, 13, 635–643. [Google Scholar] [CrossRef]
- Faye, B.; Offianan, A.T.; Ndiaye, J.L.; Tine, R.C.; Touré, W.; Djoman, K.; Sylla, K.; Ndiaye, P.S.; Penali, L.; Gaye, O. Efficacy and Tolerability of Artesunate-Amodiaquine (Camoquin plus) versus Artemether-Lumefantrine (Coartem) against Uncomplicated Plasmodium falciparum Malaria: Multisite Trial in Senegal and Ivory Coast. Trop. Med. Int. Health 2010, 15, 608–613. [Google Scholar] [CrossRef]


| Characteristics | AL (n = 791) | ASAQ (n = 784) | p-Value |
|---|---|---|---|
| Sex (Sex-ratio) | (0.9) | (1) | 1 |
| M | 374 | 392 | |
| F | 417 | 392 | |
| Age, year mean (±SD) | 9 (9.9) | 9.2 (11.4) | 1 |
| [0–5] | 427 | 401 | |
| [6–15] | 232 | 270 | |
| >15 | 122 | 113 | |
| Temperature °C mean (±SD) | 38.4 (1) | 38.5 (1) | 0.9 |
| Parasite density, parasites/μL, mean (±SD) | 40,354.3 (52,446.9) | 41,562.5 (58,417) | 0.5 |
| Treatment Outcome | AL (n1 = 270) | ASAQ (n2 = 272) | Odd Ratio (CI 95%) | p-Value |
|---|---|---|---|---|
| Lost to follow up | 5 (2.57%) | 7 (1.85%) | 0.72 (0.20 2.52) | 0.7897 |
| Intention To Treat (ITT) analysis | ||||
| PCR unadjusted | ||||
| ETF | 4 (1.50%) | 2 (0.73%) | 0.5 (0.06–3.17) | 0.6857 |
| LTF | 6 (2.22%) | 3 (1.10%) | 0.5 (0.10–2.25) | 0.5042 |
| LPF | 1 (0.37%) | 0 (0%) | 0 (0–17.29) | 0.4990 |
| ACPR | 254 (94.07%) | 260 (95.59%) | 1.02 (0.79–1.30) | 0.8457 |
| PCR adjusted | ||||
| ETF | 0 (0%) | 1 (0.37%) | - | 1 |
| LTF | 0 (0%) | 0 (0%) | - | - |
| LPF | 1 (0.37%) | 0 (0%) | 0 (0–17.29) | 0.4990 |
| ACPR | 264 (97.78%) | 264 (97.06%) | 0.99 (0.78–1.27) | 0.9993 |
| Per-Protocol (PP) analysis | ||||
| PCR unadjusted | ||||
| ETF | 4(1.51%) | 1(0.38%) | 0.25 (0.01–2.38) | 0.3726 |
| LTF | 6 (2.26%) | 3 (1.13%) | 0.5 (0.10–2.27) | 0.5041 |
| LPF | 1 (0.38%) | 0 (0%) | 0 (0–17.42) | 1 |
| ACPR | 254 (95.85%) | 261 (98.49%) | 1.03 (0.80–1.32) | 0.8746 |
| PCR adjusted | ||||
| ETF | 0 (0%) | 0 (0%) | - | - |
| LTF | 0 (0%) | 0 (0%) | - | - |
| LPF | 0 (0%) | 1 (0.38%) | 0 (0–17.42) | 1 |
| ACPR | 265 (100%) | 264 (99.62%) | 1 (0.78–1.29) | 0.9755 |
| Treatment Outcome | AL (n1 = 521) | ASAQ (n2 = 512) | Odd Ratio (CI 95%) | p-Value |
|---|---|---|---|---|
| Lost to follow up | 21 (4.03%) | 15 (2.92%) | 1.38 (0.67–2.84) | 0.4459 |
| Intention to Treat (ITT) analysis | ||||
| PCR unadjusted | ||||
| ETF | 20 (3.84%) | 13 (2.54%) | 0.66 (0.31–1.41) | 0.3302 |
| LTF | 7 (1.34%) | 6 (1.17%) | 0.87 (0.26–2.90) | 0.9723 |
| LPF | 0 (0%) | 1 (0.20%) | 0.4961 | |
| ACPR | 473 (90.79%) | 477 (93.16%) | 1.03 (0.86–1.23) | 0.8083 |
| PCR adjusted | ||||
| ETF | 0 (0%) | 0 (0%) | ||
| LTF | 0 (0%) | 1 (0.20%) | 0.4961 | |
| LPF | 0 (0%) | 1 (0.20%) | 0.4961 | |
| ACPR | 500 (95.97%) | 495 (96.68%) | 1.01 (0.84–1.20) | 0.9692 |
| Per-Protocol (PP) analysis | ||||
| PCR unadjusted | ||||
| ETF | 20 (4%) | 13 (2.61%) | 0.64 (0.30–1.39) | 0.3149 |
| LTF | 7 (1.40%) | 6 (1.21%) | 0.93 (0.29–2.87) | 0.9885 |
| LPF | 0 (0%) | 1 (0.20%) | 0.4989 | |
| ACPR | 473 (94.6%) | 477 (95.98%) | 1.01 (0.85–1.22) | 0.9093 |
| PCR unadjusted | ||||
| ETF | 0 (0%) | 1 (0.20%) | 0.4989 | |
| LTF | 0 (0%) | 1 (0.20%) | 0.4989 | |
| LPF | 0 (0%) | 0 (0%) | ||
| ACPR | 500 (100%) | 495 (99.60%) | 1 (0.83–1.19) | 1 |
| Treatment Outcome | AL (n1 = 791) | ASAQ (n2 = 784) | Odd Ratio (CI 95%) | p-Value |
|---|---|---|---|---|
| Lost to follow up | 23 (2.91%) | 22 (2.80%) | 1.04 (0.55–1.95) | 0.9731 |
| Intention To Treat (ITT) analysis | ||||
| PCR unadjusted | ||||
| ETF | 17 (2.15%) | 9 (1.15%) | 0.53 (0.22–1.27) | 0.1815 |
| LTF | 12 (1.51%) | 7 (0.89%) | 0.59 (0.21–1.61) | 0.3733 |
| LPF | 1 (0.13%) | 1 (0.13%) | 1.01 (0–36.88) | 1 |
| ACPR | 738 (93.30%) | 745 (95.03%) | 1.02 (0.88–1.18) | 0.8281 |
| PCR adjusted | ||||
| Lost to follow up | 26 (3.28%) | 22 (2.80%) | 1.17 (0.64–2.16) | 0.6951 |
| ETF | 0 (0%) | 1 (0.13%) | 0.4980 | |
| LTF | 0 (0%) | 1 (0.13%) | 0.4980 | |
| LPF | 1 (0.13%) | 1 (0.13%) | 1.01 (0–36.88) | 1 |
| ACPR | 764 (96.59%) | 759 (96.81%) | 1 (0.87–1.16) | 0.9971 |
| Per-Protocol (PP) analysis | ||||
| PCR unadjusted | ||||
| ETF | 17 (2.21%) | 9 (1.18%) | 0.53 (0.22–1.27) | 0.808 |
| LTF | 12 (1.56%) | 7 (0.92%) | 0.59 (0.21–1.61) | 0.3722 |
| LPF | 1 (0.13%) | 1 (0.13%) | 1.01 (0–36.85) | 1 |
| ACPR | 738 (96.1%) | 745 (97.77%) | 1.02 (0.88–1.18) | 0.8409 |
| PCR adjusted | ||||
| ETF | 0 (0%) | 1 (0.13%) | 0.4983 | |
| LTF | 0 (0%) | 1 (0.13%) | 0.4983 | |
| LPF | 1 (0.13%) | 1 (0.13%) | 1.01 (0–36.85) | 1 |
| ACPR | 764 (99.48%) | 759 (99.61%) | 0.98 (0.76–1.25) | 0.8985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bédia-Tanoh, A.V.; Kassi, K.F.; Touré, O.A.; Assi, S.B.; Gnagne, A.P.; Adoubryn, K.D.; Bissagnene, E.; Konaté, A.; Miezan, J.S.; Angora, K.E.; et al. Meta-Analysis of Data from Four Clinical Trials in the Ivory Coast Assessing the Efficacy of Two Artemisinin-Based Combination Therapies (Artesunate-Amodiaquine and Artemether-Lumefantrine) between 2009 and 2016. Trop. Med. Infect. Dis. 2024, 9, 10. https://doi.org/10.3390/tropicalmed9010010
Bédia-Tanoh AV, Kassi KF, Touré OA, Assi SB, Gnagne AP, Adoubryn KD, Bissagnene E, Konaté A, Miezan JS, Angora KE, et al. Meta-Analysis of Data from Four Clinical Trials in the Ivory Coast Assessing the Efficacy of Two Artemisinin-Based Combination Therapies (Artesunate-Amodiaquine and Artemether-Lumefantrine) between 2009 and 2016. Tropical Medicine and Infectious Disease. 2024; 9(1):10. https://doi.org/10.3390/tropicalmed9010010
Chicago/Turabian StyleBédia-Tanoh, Akoua Valérie, Kondo Fulgence Kassi, Offianan André Touré, Serge Brice Assi, Akpa Paterne Gnagne, Koffi Daho Adoubryn, Emmanuel Bissagnene, Abibatou Konaté, Jean Sebastien Miezan, Kpongbo Etienne Angora, and et al. 2024. "Meta-Analysis of Data from Four Clinical Trials in the Ivory Coast Assessing the Efficacy of Two Artemisinin-Based Combination Therapies (Artesunate-Amodiaquine and Artemether-Lumefantrine) between 2009 and 2016" Tropical Medicine and Infectious Disease 9, no. 1: 10. https://doi.org/10.3390/tropicalmed9010010
APA StyleBédia-Tanoh, A. V., Kassi, K. F., Touré, O. A., Assi, S. B., Gnagne, A. P., Adoubryn, K. D., Bissagnene, E., Konaté, A., Miezan, J. S., Angora, K. E., Vanga-Bosson, H., Kiki-Barro, P. C., Djohan, V., Yavo, W., & Hervé Menan, E. I. (2024). Meta-Analysis of Data from Four Clinical Trials in the Ivory Coast Assessing the Efficacy of Two Artemisinin-Based Combination Therapies (Artesunate-Amodiaquine and Artemether-Lumefantrine) between 2009 and 2016. Tropical Medicine and Infectious Disease, 9(1), 10. https://doi.org/10.3390/tropicalmed9010010






