Characterization of a Panel of Monoclonal Antibodies Targeting the F-Protein of the Respiratory Syncytial Virus (RSV) for the Typing of Contemporary Circulating Strains
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Interaction of the mAbs with Reference RSV Strains Using a Cell-ELISA
3.2. Relationship between Conformational Changes in the Structure of the F Protein and the Ability of mAbs to Interact with It
3.3. Determination of Epitope Targeting of mAbs
3.4. Analysis of Antigenic Variability of Currently Circulating RSV
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Staadegaard, L.; Caini, S.; Wangchuk, S.; Thapa, B.; de Almeida, W.A.F.; de Carvalho, F.C.; Njouom, R.; Fasce, R.A.; Bustos, P.; Kyncl, J.; et al. The Global Epidemiology of RSV in Community and Hospitalized Care: Findings From 15 Countries. Open Forum Infect. Dis. 2021, 8, ofab159. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, D.M.; Sominina, A.A.; Komissarov, A.B.; Pisareva, M.M.; Stolyarov, K.A.; Musaeva, T.D.; Eder, V.; Bakaev, M.I.; Komissarova, K.S.; Ivanova, A.A.; et al. Etiology of severe acute respiratory viral infection during the epidemic seasons 2015–2020. Therapy 2021, 4, 7–17. (In Russian) [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. RSV Global Epidemiology Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Tin Tin Htar, M.; Yerramalla, M.S.; Moïsi, J.C.; Swerdlow, D.L. The burden of respiratory syncytial virus in adults: A systematic review and meta-analysis. Epidemiol. Infect. 2020, 148, e48. [Google Scholar] [CrossRef] [PubMed]
- Bosco, E.; van Aalst, R.; McConeghy, K.W.; Silva, J.; Moyo, P.; Eliot, M.N.; Chit, A.; Gravenstein, S.; Zullo, A.R. Estimated Cardiorespiratory Hospitalizations Attributable to Influenza and Respiratory Syncytial Virus Among Long-term Care Facility Residents. JAMA Netw. Open 2021, 4, e2111806. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Gálvez, N.M.S.; Canedo-Marroquín, G.; Pizarro-Ortega, M.S.; Andrade-Parra, C.; Gómez-Santander, F.; Kalergis, A.M. Contribution of Cytokines to Tissue Damage During Human Respiratory Syncytial Virus Infection. Front. Immunol. 2019, 10, 452. [Google Scholar] [CrossRef]
- Zhou, Y.; Tong, L.; Li, M.; Wang, Y.; Li, L.; Yang, D.; Zhang, Y.; Chen, Z. Recurrent Wheezing and Asthma After Respiratory Syncytial Virus Bronchiolitis. Front. Pediatr. 2021, 9, 649003. [Google Scholar] [CrossRef]
- Bruning, A.H.L.; de Kruijf, W.B.; van Weert, H.C.P.M.; Willems, W.L.M.; de Jong, M.D.; Pajkrt, D.; Wolthers, K.C. Diagnostic performance and clinical feasibility of a point-of-care test for respiratory viral infections in primary health care. Fam. Pract. 2017, 34, 558–563. [Google Scholar] [CrossRef]
- Plotnikova, M.A.; Klotchenko, S.A.; Lebedev, K.I.; Lozhkov, A.A.; Taraskin, A.S.; Gyulikhandanova, N.E.; Ramsay, E.S.; Vasin, A.V. Antibody microarray immunoassay for screening and differential diagnosis of upper respiratory tract viral pathogens. J. Immunol. Methods 2020, 478, 112712. [Google Scholar] [CrossRef]
- Rogovik, A.L.; Carleton, B.; Solimano, A.; Goldman, R.D. Palivizumab for the prevention of respiratory syncytial virus infection. Can. Fam. Physician 2010, 56, 769–772. [Google Scholar] [PubMed]
- Liu, D.; Tang, Z.; Qiu, K.; Bajinka, O.; Wang, L.; Qin, L.; Tan, Y. RSV Promotes Epithelial Neuroendocrine Phenotype Differentiation through NODAL Signaling Pathway. BioMed Res. Int. 2021, 2021, 9956078. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Citron, M.; Bett, A.J.; Espeseth, A.S.; Vora, K.A.; Zhang, L.; DiStefano, D.J. Development and application of a higher throughput RSV plaque assay by immunofluorescent imaging. J. Virol. Methods 2019, 263, 88–95. [Google Scholar] [CrossRef]
- Tabor, D.E.; Fernandes, F.; Langedijk, A.C.; Wilkins, D.; Lebbink, R.J.; Tovchigrechko, A.; Ruzin, A.; Kragten-Tabatabaie, L.; Jin, H.; Esser, M.T.; et al. Global Molecular Epidemiology of Respiratory Syncytial Virus from the 2017-2018 INFORM-RSV Study. J. Clin. Microbiol. 2020, 59, e01828-20. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; Drysdale, S.B.; Snape, M.D.; O’Connor, D.; Brown, A.; MacIntyre-Cockett, G.; Mellado-Gomez, E.; de Cesare, M.; Bonsall, D.; Ansari, M.A.; et al. Distinct patterns of within-host virus populations between two subgroups of human respiratory syncytial virus. Nat. Commun. 2021, 12, 5125. [Google Scholar] [CrossRef] [PubMed]
- Cantú-Flores, K.; Rivera-Alfaro, G.; Muñoz-Escalante, J.C.; Noyola, D.E. Global distribution of respiratory syncytial virus A and B infections: A systematic review. Pathog. Glob. Health 2022, 116, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Hierholzer, J.C.; Tsou, C.; Hendry, R.M.; Fernie, B.F.; Stone, Y.; McIntosh, K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 1985, 151, 626–633. [Google Scholar] [CrossRef]
- Pangesti, K.N.A.; Abd, E.l.; Ghany, M.; Walsh, M.G.; Kesson, A.M.; Hill-Cawthorne, G.A. Molecular epidemiology of respiratory syncytial virus. Rev. Med. Virol. 2018, 28, e1968. [Google Scholar] [CrossRef]
- Yu, J.M.; Fu, Y.H.; Peng, X.L.; Zheng, Y.P.; He, J.S. Genetic diversity and molecular evolution of human respiratory syncytial virus A and B. Sci. Rep. 2021, 11, 12941. [Google Scholar] [CrossRef]
- Lamb, R.A.; Parcs, G.D. Paramyxoviridae: The viruses and their replication. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer, Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 5.1, pp. 1449–1496. [Google Scholar]
- Okamoto, M.; Dapat, C.P.; Sandagon, A.M.D.; Batangan-Nacion, L.P.; Lirio, I.C.; Tamaki, R.; Saito, M.; Saito-Obata, M.; Lupisan, S.P.; Oshitani, H. Molecular Characterization of Respiratory Syncytial Virus in Children with Repeated Infections with Subgroup B in the Philippines. J. Infect Dis. 2018, 218, 1045–1053. [Google Scholar] [CrossRef]
- Vos, L.M.; Oosterheert, J.J.; Kuil, S.D.; Viveen, M.; Bont, L.J.; Hoepelman, A.I.M.; Coenjaerts, F.E.J. High epidemic burden of RSV disease coinciding with genetic alterations causing amino acid substitutions in the RSV G-protein during the 2016/2017 season in the Netherlands. J. Clin. Virol. 2019, 112, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Trento, A.; Ábrego, L.; Rodriguez-Fernandez, R.; González-Sánchez, M.I.; González-Martínez, F.; Delfraro, A.; Pascale, J.M.; Arbiza, J.; Melero, J.A. Conservation of G-Protein Epitopes in Respiratory Syncytial Virus (Group A) Despite Broad Genetic Diversity: Is Antibody Selection Involved in Virus Evolution? J. Virol. 2015, 89, 7776–7785. [Google Scholar] [CrossRef] [PubMed]
- Mousa, J.J.; Kose, N.; Matta, P.; Gilchuk, P.; Crowe, J.E., Jr. A novel pre-fusion conformation-specific neutralizing epitope on the respiratory syncytial virus fusion protein. Nat. Microbiol. 2017, 2, 16271. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, Z.; Ni, F.; Chen, X.; Ma, J.; Patel, N.; Lu, H.; Liu, Y.; Tian, J.H.; Flyer, D.; et al. Structure basis of neutralization by a novel site II/IV antibody against respiratory syncytial virus fusion protein. PLoS ONE 2019, 14, e0210749. [Google Scholar] [CrossRef]
- Djagbare, M.D.; Yu, L.; Parupudi, A.; Sun, J.; Coughlin, M.L.; Rush, B.S.; Sanyal, G. Monoclonal antibody based in vitro potency assay as a predictor of antigenic integrity and in vivo immunogenicity of a Respiratory Syncytial Virus post-fusion F-protein based vaccine. Vaccine 2018, 36, 1673–1680. [Google Scholar] [CrossRef]
- Patel, N.; Massare, M.J.; Tian, J.H.; Guebre-Xabier, M.; Lu, H.; Zhou, H.; Maynard, E.; Scott, D.; Ellingsworth, L.; Glenn, G.; et al. Respiratory syncytial virus prefusogenic fusion (F) protein nanoparticle vaccine: Structure, antigenic profile, immunogenicity, and protection. Vaccine 2019, 37, 6112–6124. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers (accessed on 28 September 2023).
- PATH. RSV Vaccine and mAb Snapshot. Available online: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ (accessed on 28 September 2023).
- Sominina, A.A.; Sukhubaevskaya, L.P.; Samoilovich, M.P.; Klimovich, V.B.; Monaenkov, A.O.; Amosova, I.V.; Markina, I.A.; Bubnova, S.D.; Zibina, E.A.; Ostrova, I.V. Characteristics of monoclonal antibodies to the RS virus in immunoenzyme and immunofluorescent reactions. Bull. Russ. Acad. Med. Sci. 1995, 9, 49–54. (In Russian) [Google Scholar]
- Krivitskaya, V.; Petrova, E.; Sorokin, E.; Tsareva, T.; Sverlova, M.; Fadeev, A.; Komissarov, A.; Sominina, A. Design and Characteristics of Monoclonal Antibodies Specific to Respiratory Syncytial Virus. Biotechnology 2016, 32, 65–75. (In Russian) [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 53, 33–79. [CrossRef]
- Laemmli, U.R. Cleveage of structural proteins during the assembly of the head of bacteriophage T 4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Nakane, P.K.; Kawaoi, A. Peroxidase-labeled antibody. A new method of conjugation. J. Histochem. Cytochem. 1974, 22, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Mottet, G. Post-translational processing and oligomerization of the fusion glycoprotein of human respiratory syncytial virus. J. Gen. Virol. 1991, 72, 3095–3101. [Google Scholar] [CrossRef] [PubMed]
- Rixon, H.W.; Brown, C.; Brown, G.; Sugrue, R.J. Multiple glycosylated forms of the respiratory syncytial virus fusion protein are expressed in virus-infected cells. J. Gen. Virol. 2002, 83, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Brandriss, M.W.; Schlesinger, J.J. Purification and characterization of the respiratory syncytial virus fusion protein. J. Gen. Virol. 1985, 66, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nagasawa, K.; Tsukagoshi, H.; Matsushima, Y.; Fujita, K.; Yoshida, L.M.; Tanaka, R.; Ishii, H.; Shimojo, N.; Kuroda, M.; et al. Molecular evolution of the fusion protein gene in human respiratory syncytial virus subgroup A. Infect. Genet. Evol. 2016, 43, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nagasawa, K.; Kimura, R.; Tsukagoshi, H.; Matsushima, Y.; Fujita, K.; Hirano, E.; Ishiwada, N.; Misaki, T.; Oishi, K.; et al. Molecular evolution of the fusion protein (F) gene in human respiratory syncytial virus subgroup B. Infect. Genet. Evol. 2017, 52, 1–9. [Google Scholar] [CrossRef]
- Palomo, C.; Mas, V.; Thom, M.; Vázquez, M.; Cano, O.; Terrón, M.; Luque, D.; Taylor, G.; Melero, J.A. Influence of respiratory syncytial virus F glycoprotein conformation on induction of protective immune responses. J. Virol. 2016, 90, 5485–5498. [Google Scholar] [CrossRef]
- Adams, O.; Bonzel, L.; Kovacevic, A.; Mayatepek, E.; Hoehn, T.; Vogel, M. Palivizumab-resistant human respiratory syncytial virus infection in infancy. Clin. Infect. Dis. 2010, 51, 185–188. [Google Scholar] [CrossRef]
- Zhu, Q.; McAuliffe, J.M.; Patel, N.K.; Palmer-Hill, F.J.; Yang, C.F.; Liang, B.; Su, L.; Zhu, W.; Wachter, L.; Wilson, S.; et al. Analysis of respiratory syncytial virus preclinical and clinical variants resistant to neutralization by monoclonal antibodies palivizumab and/or motavizumab. J. Infect. Dis. 2011, 203, 674–682. [Google Scholar] [CrossRef]
- McLellan, J.S. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr. Opin. Virol. 2015, 11, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Adams, O.; Werzmirzowsky, J.; Hengel, H. Genetic analysis and antigenic characterization of human respiratory syncytial virus group A viruses isolated in Germany 1996-2008. Virus Genes 2013, 47, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Tapia, L.I.; Shaw, C.A.; Aideyan, L.O.; Jewell, A.M.; Dawson, B.C.; Haq, T.R.; Piedra, P.A. Gene sequence variability of the three surface proteins of human respiratory syncytial virus (HRSV) in Texas. PLoS ONE 2014, 9, e90786. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.J.; Cameron, R.; Lawrence, L.J.; Lin, B.; Lowe, M.; Luttick, A.; Mason, A.; McKimm-Breschkin, J.; Parker, M.W.; Ryan, J.; et al. Structural characterization of respiratory syncytial virus fusion inhibitor escape mutants: Homology model of the F protein and a syncytium formation assay. Virology 2003, 311, 275–288. [Google Scholar] [CrossRef]
- Fuentes, S.; Coyle, E.M.; Beeler, J.; Golding, H.; Khurana, S. Antigenic Fingerprinting following Primary RSV Infection in Young Children Identifies Novel Antigenic Sites and Reveals Unlinked Evolution of Human Antibody Repertoires to Fusion and Attachment Glycoproteins. PLoS Pathog. 2016, 12, e1005554. [Google Scholar] [CrossRef]
- Karpova, L.S.; Smorodintseva, E.A.; Sysoeva, T.P.; Stolyarova, T.P.; Popovtseva, N.M.; Stolyarov, K.A.; Danilenko, D.M.; Tsybalova, L.M. The Spread of RS-virus Infection and other ARVI not Influenza Etiology in Children and Adults in the Regions of Russia from 2014 to 2016. Epidemiol. Vaccinal Prev. 2018, 17, 16–26. (In Russian) [Google Scholar] [CrossRef]
- Esposito, S.; Piralla, A.; Zampiero, A.; Bianchini, S.; Di Pietro, G.; Scala, A.; Pinzani, R.; Fossali, E.; Baldanti, F.; Principi, N. Characteristics and Their Clinical Relevance of Respiratory Syncytial Virus Types and Genotypes Circulating in Northern Italy in Five Consecutive Winter Seasons. PLoS ONE 2015, 10, e0129369. [Google Scholar] [CrossRef]

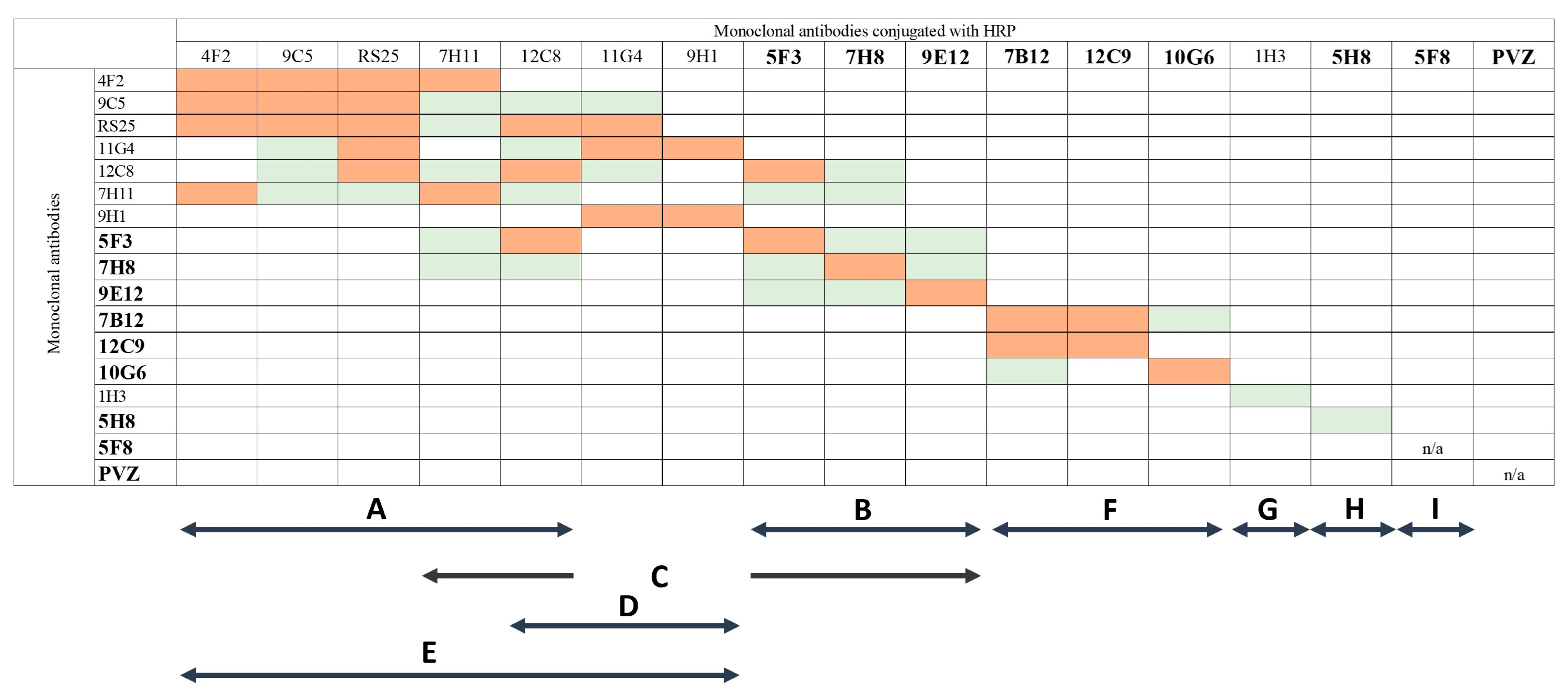
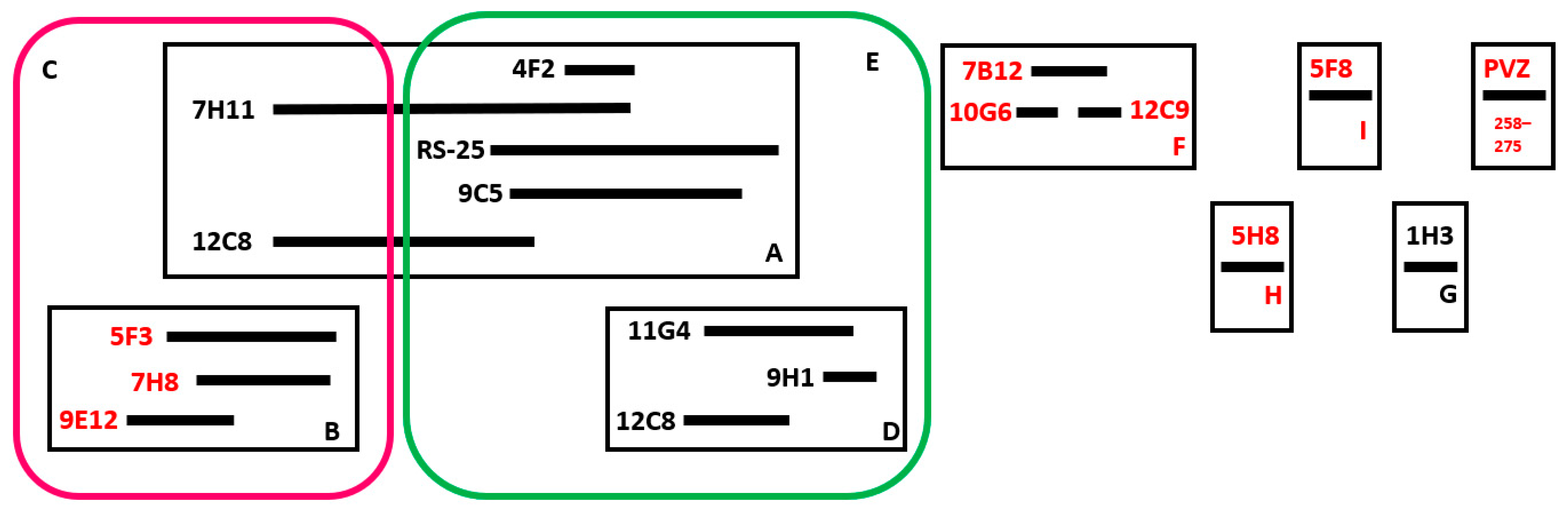
| RSV Strain | Subtype | OD450 for mAbs: | ||||||||||||||||
| 5F3 | 7H8 | 9E12 | 12C8 | 4F2 | 7H11 | RS25 | 9C5 | 11G4 | 9H1 | 10G6 | 7B12 | 12C9 | 5F8 | 5H8 | 1H3 | PVZ | ||
| Long | RSV-A | 0.89 ± 0.08 | 0.91 ± 0.08 | 1.10 ± 0.09 | 0.87 ± 0.09 | 0.90 ± 0.02 | 0.98 ± 0.10 | 0.98 ± 0.07 | 0.95 ± 0.09 | 0.84 ± 0.07 | 0.98 ± 0.05 | 0.90 ± 0.09 | 0.73 ± 0.03 | 0.95 ± 0.10 | 0.58 ± 0.02 | 0.59 ± 0.02 | 0.95 ± 0.02 | 1.11 ± 0.11 |
| A2 | RSV-A | 0.80 ± 0.06 | 0.72 ± 0.07 | 0.75 ± 0.03 | 0.77 ± 0.05 | 0.87 ± 0.03 | 0.70 ± 0.07 | 0.92 ± 0.05 | 0.84 ± 0.07 | 0.73 ± 0.04 | 0.79 ± 0.06 | 0.71 ± 0.03 | 0.75 ± 0.05 | 0.71 ± 0.03 | 0.53 ± 0.06 | 0.56 ± 0.04 | 0.75 ± 0.02 | 1.25 ± 0.09 |
| 9320 | RSV-B | 0.49 ± 0.02 | 0.48 ± 0.04 | 0.72 ± 0.04 | 0.74 ± 0.04 | 0.89 ± 0.07 | 0.74 ± 0.04 | 0.90 ± 0.08 | 0.76 ± 0.06 | 0.86 ± 0.06 | 0.81 ± 0.07 | 0.76 ± 0.05 | 0.78 ± 0.03 | 0.80 ± 0.03 | 0.51 ± 0.02 | 0.43 ± 0.03 | 0.51 ± 0.03 | 1.14 ± 0.08 |
| mAb | mAb Isotype | Neutralizing mAb Activity (μg/mL) upon Interaction with RSV in Cell Culture (Cell-ELISA Data): | Color Intensity of F Protein Interaction with mAbs on Nitrocellulose Membranes after the Following Treatment: | |||||
|---|---|---|---|---|---|---|---|---|
| Non-Reducing Treatment | Reducing Treatment | |||||||
| RSV-A Long | RSV-A A2 | RSV-B 9320 | Heat for 20 min, 37 °C w/o β-ME 1 | Heat for 90 min, 37 °C w/o β-ME 2 | Boiling for 2 min, 100 °C, w/o β-ME 3 | Boiling for 2 min, 100 °C, with β-ME 4 | ||
| 9C5 | IgG2a | ˃800 | ˃800 | ˃800 | ++++ | ++ | + | − |
| RS25 | IgG2b | ˃650 | ˃650 | ˃650 | ++++ | +++ | +++ | − |
| 4F2 | IgG1 | ˃700 | ˃700 | ˃700 | ++++ | + | − | − |
| 5F3 | IgG1 | 16 | 26 | 74 | ++++ | + | − | − |
| 5H8 | IgG1 | 135 | 135 | 270 | ++++ | +++ | − | − |
| 1H3 | IgG1 | ˃900 | ˃900 | ˃900 | ++++ | + | − | − |
| 5F8 | IgG2a | 9 | 13 | 54 | ++++ | ++ | − | − |
| 7B12 | IgG2a | 63 | 63 | 126 | ++++ | ++ | − | − |
| 11G4 | IgG1 | ˃650 | ˃650 | ˃650 | ++++ | ++ | ++ | − |
| 12C8 | IgG2b | ˃650 | ˃650 | ˃650 | ++++ | + | − | − |
| 12C9 | IgG1 | 56 | 56 | 56 | ++++ | + | − | − |
| 9E12 | IgG1 | 63 | 63 | 252 | ++++ | + | − | − |
| 10G6 | IgG1 | 125 | 125 | 250 | ++++ | ++ | ++ | − |
| 7H8 | IgG1 | 75 | 75 | 300 | ++++ | +++ | − | − |
| 7H11 | IgG2a | ˃800 | ˃800 | ˃800 | ++++ | + | − | − |
| 9H1 | IgG1 | ˃550 | ˃550 | ˃550 | ++++ | +++ | ++ | − |
| PVZ | IgG1 | 0.1 | 0.1 | 0.2 | ++++ | ++ | + | − |
| Antigenic Group | Number of Strains | Proportion of Strains with Reduced Interaction with F-Specific mAbs (%) *: | ||||||||||||||||
| 4F2 | 9C5 | RS25 | 7H11 | 12C8 | 11G4 | 9H1 | 5F3 | 7H8 | 9E12 | 7B12 | 12C9 | 10G6 | 5H8 | 5F8 | 1H3 | PVZ | ||
| RSV-A | 73 | 0 | 32.8 | 0 | 0 | 1.4 | 1.4 | 0 | 42.5 | 52.1 | 24.7 | 79.5 | 80.8 | 72.6 | 58.9 | 75.3 | 41.1 | 0 |
| RSV-B | 32 | 0 | 18.8 | 0 | 0 | 3.1 | 0 | 0 | 18.8 | 18.8 | 34.4 | 3.1 | 18.8 | 12.5 | 68.8 | 25.0 | 15.6 | 0 |
| Level of significance of differences (p) between the RSV-A and RSV-B groups | p ˃ 0.05 | p ˃ 0.05 | p ˃ 0.05 | p ˃ 0.05 | p ˃ 0.05 | p ˃ 0.05 | p ˃ 0.05 | p < 0.001 | p < 0.001 | p ˃ 0.05 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p ˃ 0.05 | p < 0.0001 | p < 0.01 | p ˃ 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivitskaya, V.; Petrova, E.; Sorokin, E.; Tsareva, T.; Sverlova, M.; Komissarova, K.; Sominina, A.; Danilenko, D. Characterization of a Panel of Monoclonal Antibodies Targeting the F-Protein of the Respiratory Syncytial Virus (RSV) for the Typing of Contemporary Circulating Strains. Trop. Med. Infect. Dis. 2024, 9, 1. https://doi.org/10.3390/tropicalmed9010001
Krivitskaya V, Petrova E, Sorokin E, Tsareva T, Sverlova M, Komissarova K, Sominina A, Danilenko D. Characterization of a Panel of Monoclonal Antibodies Targeting the F-Protein of the Respiratory Syncytial Virus (RSV) for the Typing of Contemporary Circulating Strains. Tropical Medicine and Infectious Disease. 2024; 9(1):1. https://doi.org/10.3390/tropicalmed9010001
Chicago/Turabian StyleKrivitskaya, Vera, Ekaterina Petrova, Evgeniy Sorokin, Tatyana Tsareva, Maria Sverlova, Kseniia Komissarova, Anna Sominina, and Daria Danilenko. 2024. "Characterization of a Panel of Monoclonal Antibodies Targeting the F-Protein of the Respiratory Syncytial Virus (RSV) for the Typing of Contemporary Circulating Strains" Tropical Medicine and Infectious Disease 9, no. 1: 1. https://doi.org/10.3390/tropicalmed9010001
APA StyleKrivitskaya, V., Petrova, E., Sorokin, E., Tsareva, T., Sverlova, M., Komissarova, K., Sominina, A., & Danilenko, D. (2024). Characterization of a Panel of Monoclonal Antibodies Targeting the F-Protein of the Respiratory Syncytial Virus (RSV) for the Typing of Contemporary Circulating Strains. Tropical Medicine and Infectious Disease, 9(1), 1. https://doi.org/10.3390/tropicalmed9010001






