Hepatitis E Virus in the Wild Boar Population: What Is the Real Zoonotic Risk in Portugal?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Molecular Detection of HEV in Liver and Faeces
2.3. Serologic Detection of HEV
2.4. Hunters’ Survey
2.5. Statstistical Analysis
3. Results
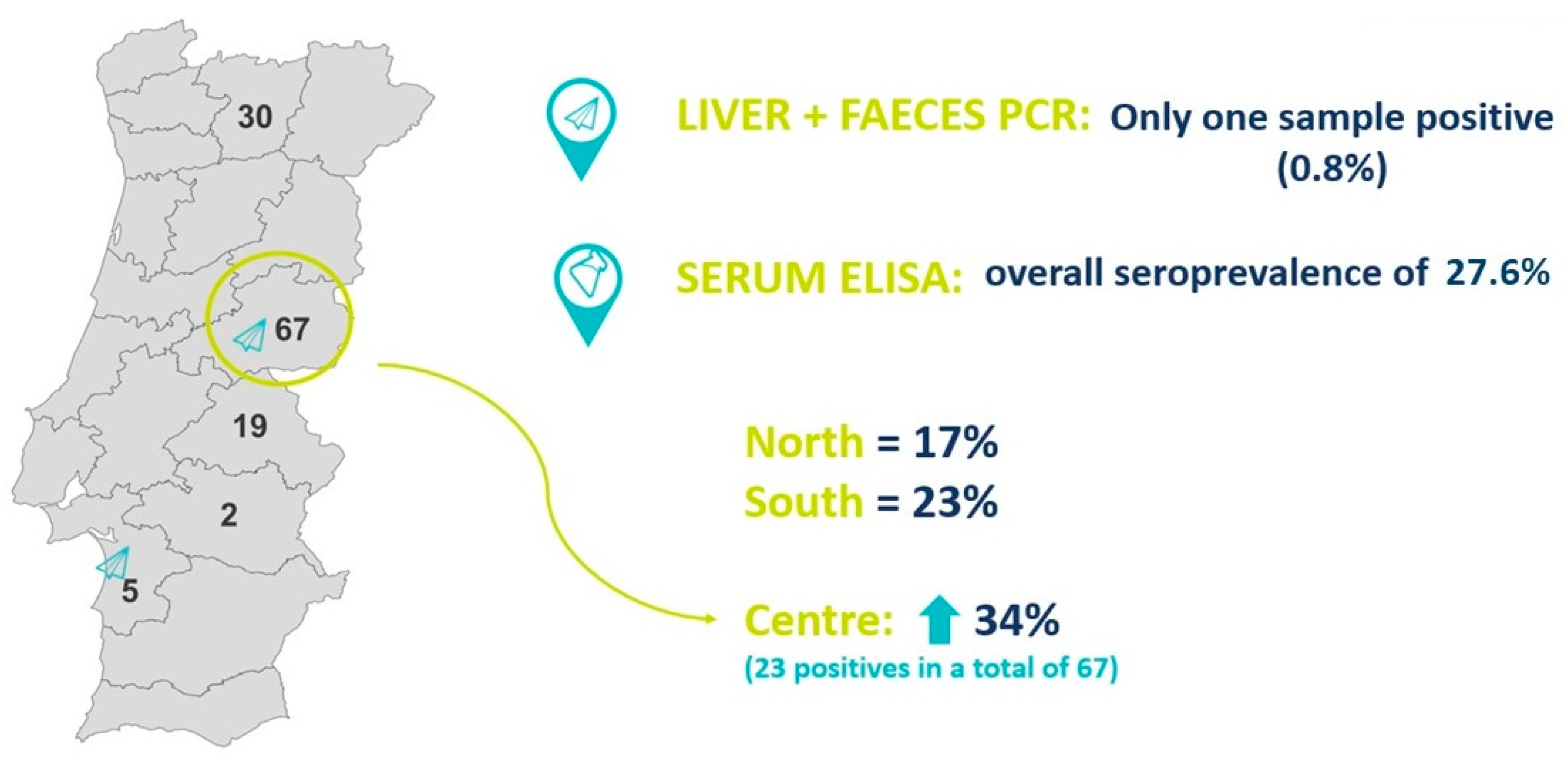
3.1. Molecular Detection of HEV in Liver and Faeces
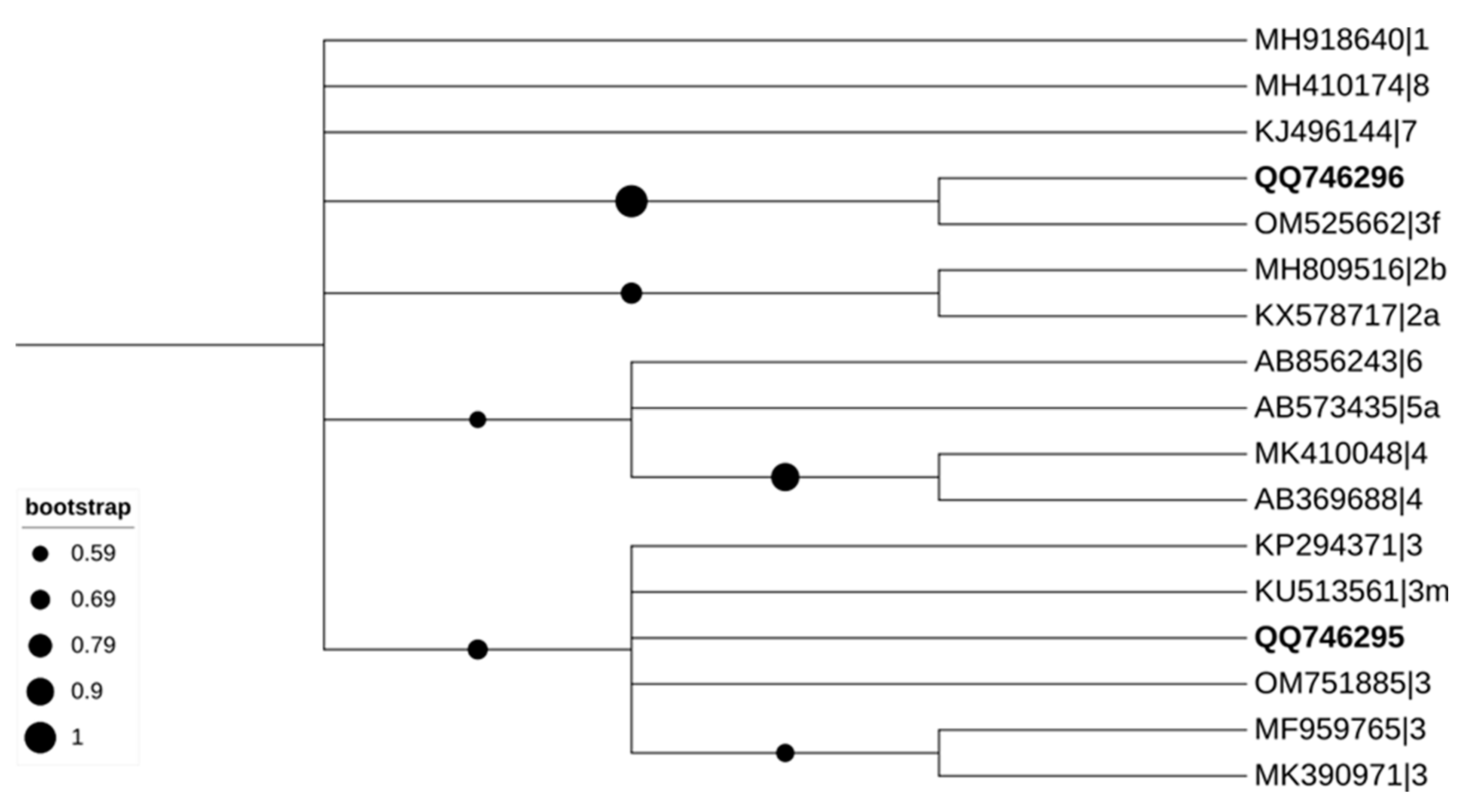
3.2. Serologic Detection of HEV
3.3. Results of the Hunters’ Survey
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz-Fons, F. A Review of the Current Status of Relevant Zoonotic Pathogens in Wild Swine (Sus scrofa) Populations: Changes Modulating the Risk of Transmission to Humans. Transbound. Emerg. Dis. 2017, 64, 68–88. [Google Scholar] [CrossRef]
- Abrantes, A.C.; Vieira-Pinto, M. 15 years overview of European zoonotic surveys in wild boar and red deer: A systematic review. One Health 2023, 16, 100519. [Google Scholar] [CrossRef]
- Aprea, G.; Amoroso, M.G.; Di Bartolo, I.; D’Alessio, N.; Di Sabatino, D.; Boni, A.; Cioffi, A.; D’Angelantonio, D.; Scattolini, S.; De Sabato, L.; et al. Molecular detection and phylogenetic analysis of hepatitis E virus strains circulating in wild boars in south-central Italy. Transbound. Emerg. Dis. 2018, 65, 25–31. [Google Scholar] [CrossRef]
- De Sabato, L.; Ostanello, F.; De Grossi, L.; Marcario, A.; Franzetti, B.; Monini, M.; Di Bartolo, I. Molecular survey of HEV infection in wild boar population in Italy. Transbound. Emerg. Dis. 2018, 65, 1749–1756. [Google Scholar] [CrossRef]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.J.; Norder, H.; Okamoto, H.; van der Poel, W.H.M.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef]
- Cunha, L.; Luchs, A.; Azevedo, L.S.; Silva, V.C.M.; Lemos, M.F.; Costa, A.C.; Compri, A.P.; França, Y.; Viana, E.; Malta, F.; et al. Detection of Hepatitis E Virus Genotype 3 in Feces of Capybaras (Hydrochoeris hydrochaeris) in Brazil. Viruses 2023, 15, 335. [Google Scholar] [CrossRef]
- Lo Presti, A.; Bruni, R.; Villano, U.; Marcantonio, C.; Equestre, M.; Ciuffetelli, M.; Grimaldi, A.; Suffredini, E.; Di Pasquale, S.; De Medici, D.; et al. Phylogenetic analysis and epidemiological history of Hepatitis E virus 3f and 3c in swine and wild boar, Italy. Heliyon 2020, 6, e05110. [Google Scholar] [CrossRef]
- Di Pasquale, S.; De Santis, P.; La Rosa, G.; Di Domenico, K.; Iaconelli, M.; Micarelli, G.; Martini, E.; Bilei, S.; De Medici, M.; Suffredini, E. Quantification and genetic diversity of Hepatitis E virus in wild boar (Sus scrofa) hunted for domestic consumption in Central Italy. Food Microbiol. 2019, 82, 194–201. [Google Scholar] [CrossRef]
- De Sabato, L.; Di Bartolo, I.; Lapa, D.; Capobianchi, M.R.; Garbuglia, A.R. Molecular characterization of HEV genotype 3 in Italy at human/animal interface. Front. Microbiol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Hrazdilová, K.; Lesiczka, P.M.; Bardoň, J.; Vyroubalová, Š.; Šimek, B.; Zurek, L.; Modrý, D. Wild boar as a potential reservoir of zoonotic tick-borne pathogens. Ticks Tick-Borne Dis. 2021, 12, 101558. [Google Scholar] [CrossRef]
- Gomes-Neves, E.; Abrantes, A.C.; Vieira-Pinto, M.; Muller, A. Wild Game Meat—A Microbiological Safety and Hygiene Challenge? Curr. Clin. Microbiol. Rep. 2021, 8, 31–39. [Google Scholar] [CrossRef]
- Niewiadomska, K.; Kosicka-Gębska, M.; Gębski, J.; Gutkowska, K.; Jeżewska-Zychowicz, M.; Sułek, M. Game meat consumption-conscious choice or just a game? Foods 2020, 9, 1357. [Google Scholar] [CrossRef]
- Porea, D.; Anita, A.; Demange, A.; Raileanu, C.; Oslobanu, L.; Anita, D.; Savuta, G.; Pavio, N. Molecular detection of hepatitis E virus in wild boar population in eastern Romania. Transbound. Emerg. Dis. 2017, 65, 527–533. [Google Scholar] [CrossRef]
- Risalde, M.A.; Rivero-Juarez, A.; Romero-Palomo, F.; Frıas, M.; Lopez-Lopez, P.; Cano-Terriza, D.; Garcıa-Bocanegra, I.; Jimenez-Ruiz, S.; Camacho, A.; Machuca, I.; et al. Persistence of hepatitis E virus in the liver of non-viremic naturally infected wild boar. PLoS ONE 2017, 12, e0186858. [Google Scholar] [CrossRef]
- Choi, C.; Chae, C. Localization of swine hepatitis E virus in liver and extrahepatic tissues from naturally infected pigs by in situ hybridization. J. Hepatol. 2003, 38, 827–832. [Google Scholar] [CrossRef]
- Ferri, G.; Lauteri, C.; Festino, A.R.; Piccinini, A.; Olivastri, A.; Vergara, A. Hepatitis E Virus Detection in Hunted Wild Boar Liver and Muscle Tissues in Central Italy. Microorganisms 2022, 10, 1628. [Google Scholar] [CrossRef]
- Schlosser, J.; Rodríguez, A.V.; Fast, C.; Groschup, M.; Eiden, M. Chronically infected wild boar can transmit genotype 3 hepatitis E virus to domestic pigs. Vet. Microbiol. 2015, 180, 15–21. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Martinez-Peinado, A.; Risalde, M.A.; Rodriguez-Cano, D.; Camacho, A.; García-Bocanegra, I.; Cuenca-Lopez, F.; Gomez-Villamandos, J.C.; Rivero, A. Familial Hepatitis E Outbreak Linked to Wild Boar Meat Consumption. Zoonoses Public Health 2017, 64, 561–565. [Google Scholar] [CrossRef]
- Widén, F.; Sundqvist, L.; Matyi-Toth, A.; Metreveli, G.; Belák, S.; Hallgren, G.; Norder, H. Molecular epidemiology of hepatitis E virus in humans, pigs and wild boars in Sweden. Epidemiol. Infect. 2011, 139, 361–371. [Google Scholar] [CrossRef]
- Abrantes, A.C.; Ferreira, M.P.; Ruano, Z.; Vinhas, B.; Vaz, Y.; Vieira-Pinto, M. Hygiene and biosecurity conditions of initial examination on-spot in Portugal: One step toward game meat safety. Vet. World 2023, 16, 882–887. [Google Scholar] [CrossRef]
- Vieira-Pinto, M.; Fernandes, A.R.G.; Santos, M.H.; Marucci, G. Trichinella britovi infection in wild boar in Portugal. Zoonoses Public Health 2021, 68, 103–109. [Google Scholar] [CrossRef]
- Nascimento, M.S.J.; Pereira, S.S.; Teixeira, J.; Abreu-Silva, J.; Oliveira, R.M.S.; Myrmel, M.; Stene-Johansen, K.; Øverbø, J.; Gonçalves, G.; Mesquita, J.R. A nationwide serosurvey of hepatitis e virus antibodies in the general population of Portugal. Eur. J. Public Health. 2018, 28, 720–724. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Li, D. Optimization and implementation of the virus extraction method for hepatitis E virus detection from raw pork liver. Food Environ. Virol. 2021, 13, 74–83. [Google Scholar] [CrossRef]
- Johne, R.; Plenge-Bönig, A.; Hess, M.; Ulrich, R.G.; Reetz, J.; Schielke, A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J. Gen. Virol. 2010, 91, 750–758. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computingplatforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, 256–259. [Google Scholar]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Caruso, C.; Modesto, P.; Bertolini, S.; Peletto, S.; Acutis, P.L.; Dondo, A.; Robetto, S.; Mignone, W.; Orusa, R.; Ru, G.; et al. Serological and virological survey of Hepatitis E virus in wild boar populations in northwestern Italy: Detection of HEV subtype 3e and 3f. Arch. Virol. 2015, 160, 153–160. [Google Scholar] [CrossRef]
- Serracca, l.; Battistini, R.; Rossini, I.; Mignone, W.; Peletto, S.; Boin, C.; Pistone, G.; Ercolini, R.; Ercolini, C. Molecular Investigation on the Presence of Hepatitis E Virus (HEV) in Wild Game in North-Western Italy. Food Environ. Virol. 2015, 7, 206–212. [Google Scholar] [CrossRef]
- Mesquita, J.R.; Oliveira, R.M.S.; Coelho, C.; Vieira-Pinto, M.; Nascimento, M.S.J. Hepatitis E virus in sylvatic and captive wild boar from Portugal. Transbound. Emerg. Dis. 2016, 63, 574–578. [Google Scholar] [CrossRef]
- Spancerniene, U.; Grigas, J.; Buitkuviene, J.; Zymantiene, J.; Juozaitiene, V.; Stankeviciute, M.; Razukevicius, D.; Zienius, D.; Stankevicius, A. Prevalence and phylogenetic analysis of hepatitis E virus in pigs, wild boars, roe deer, red deer and moose in Lithuania. Acta Vet. Scand. 2018, 60, 13. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Caprioli, A.; Zengarini, M.; Marata, A.; Fiegna, C.; Di Bartolo, I.; Ruggeri, F.M.; Delogu, M.; Ostanello, F. Detection of Hepatitis E virus (HEV) in a demographic managed wild boar (Sus scrofa scrofa) population in Italy. Vet. Microbiol. 2008, 126, 74–81. [Google Scholar] [CrossRef]
- Pierini, I.; Di Bartolo, I.; Manuali, E.; Pirani, S.; Bazzucchi, M.; Moscati, L.; De Mia, G.M.; Giammarioli, M. Hepatitis E virus (HEV) genotype 3 diversity: Identification of a novel HEV subtype in wild boar in Central Italy. Transbound. Emerg. Dis. 2021, 68, 2121–2129. [Google Scholar] [CrossRef]
- Lhomme, S.; Top, S.; Bertagnoli, S.; Dubois, M.; Guerin, J.L.; Izopet, J. Wildlife Reservoir for Hepatitis E Virus, Southwestern France. Emerg. Infect. Dis. 2015, 21, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Mauroy, A.; Saegerman, C.; Licoppe, A.; Fett, T.; Thomas, I.; Brochier, B.; Thiry, E.; Linden, A. Belgian Wildlife as Potential Zoonotic Reservoir of Hepatitis E Virus. Transbound. Emerg. Dis. 2017, 64, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, S.; Moraes, D.F.d.S.D.; López-López, P.; Palmeira, J.D.; Torres, R.T.; São José Nascimento, M.; Dashti, A.; Carmena, D.; Rivero-Juarez, A.; Mesquita, J.R. Survey of Zoonotic Diarrheagenic Protist and Hepatitis E Virus in Wild Boar (Sus scrofa) of Portugal. Animals 2023, 13, 256. [Google Scholar] [CrossRef]
- Mazzei, M.; Nardini, R.; Verin, R.; Forzan, M.; Poli, A.; Tolari, F. Serologic and molecular survey for hepatitis E virus in wild boar (Sus scrofa) in Central Italy. New Microbes New Infect. 2015, 7, 41–47. [Google Scholar] [CrossRef]
- Wang, H.; Castillo-Contreras, R.; Saguti, F.; López-Olvera, J.; Karlsson, M.; Mentaberre, G.; Lindh, M.; Serra-Cobo, J.; Norder, H. Genetically similar hepatitis E virus strains infect both humans and wild boars in the Barcelona area, Spain, and Sweden. Transbound. Emerg. Dis. 2019, 66, 978–985. [Google Scholar] [CrossRef]
- Kozyra, I.; Jabłoński, A.; Bigoraj, E.; Rzeżutka, A. Wild boar as a sylvatic reservoir of hepatitis E virus in Poland: A cross-sectional population study. Viruses 2020, 12, 1113. [Google Scholar] [CrossRef]
- Weigand, K.; Weigand, K.; Schemmerer, M.; Müller, M.; Wenzel, J.J. Hepatitis E seroprevalence and genotyping in a cohort of wild boars in Southern Germany and Eastern Alsace. Food Environ. Virol. 2018, 10, 167–175. [Google Scholar]
- Kureljušić, B.; Savić, B.; Jezdimirović, N.; Kureljušić, J.; Milićević, V.; Karabasil, N.; Vesković Moračanin, S.; Žutić, J. Seroprevalence of hepatitis E in pigs and wild boars in the region of the city Belgrade. J. Infect. Dev. Ctries. 2020, 14, 669–673. [Google Scholar] [CrossRef]
- Caballero-Gómez, J.; Jiménez-Ruiz, S.; Lopez-Lopez, P.; Vicente, J.; Risalde, M.A.; Cano-Terriza, D.; Frias, M.; Barasona, J.A.; Rivero, A.; García-Bocanegra, I.; et al. Emergent subtype of hepatitis E virus genotype 3 in wild boar in Spain. Transbound. Emerg. Dis. 2019, 66, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.; Pereira-Vaz, J.; Duque, V.; Bandeira, V.; Fonseca, C.; Donato, A.; Luxo, C.; Matos, A.M. First serological evidence on endemicity of HEV infection in wild boar (Sus scrofa) populations from Portugal. Virol. Sin. 2018, 33, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Anheyer-Behmenburg, H.E.; Szabo, K.; Schotte, U.; Binder, A.; Klein, G.; Johne, R. Hepatitis E Virus in Wild Boars and Spillover Infection in Red and Roe Deer, Germany, 2013–2015. Emerg. Infect. Dis. 2017, 23, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Schotte, U.; Martin, A.; Brogden, S.; Schilling-Loeffler, K.; Schemmerer, M.; Anheyer-Behmenburg, H.E.; Szabo, K.; Müller-Graf, C.; Wenzel, J.J.; Kehrenberg, C.; et al. Phylogeny and spatiotemporal dynamics of hepatitis E virus infections in wild boar and deer from six areas of Germany during 2013–2017. Transbound. Emerg. Dis. 2022, 69, e1992–e2005. [Google Scholar] [CrossRef]
- Aranha, J.; Abrantes, A.C.; Gonçalves, R.; Miranda, R.; Serejo, J.; Vieira-Pinto, M. GIS as na Epidemiological Tool to Monitor the Spatial–Temporal Distribution of Tuberculosis in Large Game in a High-Risk Area in Portugal. Animals 2021, 11, 2374. [Google Scholar] [CrossRef]
- Cunha, M.V.; Matos, F.; Canto, A.; Albuquerque, T.; Alberto, J.; Aranha, J.; Vieira-Pinto, M.; Botelho, A. Implications and Challenges of Tuberculosis in Wildlife Ungulates: A Molecular Epidemiology Perspective. Res. Vet. Sci. 2012, 92, 225–235. [Google Scholar] [CrossRef]
- Mrzljak, A.; Balen, I.; Barbic, L.; Ilic, M.; Vilibic-Cavlek, T. Hepatitis E virus in professionally exposed: A reason for concern? World J. Hepatol. 2021, 13, 723. [Google Scholar] [CrossRef]
- Montagnaro, S.; De Martinis, C.; Sasso, S.; Ciarcia, R.; Damiano, S.; Auletta, L.; Iovane, V.; Zottola, T.; Pagnini, U. Viral and Antibody Prevalence of Hepatitis E in European Wild Boars (Sus scrofa) and Hunters at Zoonotic Risk in the Latium Region. J. Comp. Pathol. 2015, 153, 1–8. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Segalés, J.; Gortázar, C. A review of viral diseases of the European wild boar: Effects of population dynamics and reservoir role. Vet. J. 2008, 176, 158–169. [Google Scholar] [CrossRef]

| Question | Alternative Responses | Number (Total = 106) | Percentage (%) |
|---|---|---|---|
| “Do you usually consume the liver of the carcasses that you eviscerate and prepare?” | Never | 81 | 76.40% |
| Always | 4 | 3.80% | |
| Sometimes | 21 | 19.80% | |
| “If so, is there any possibility of consuming the liver undercooked?” (Only for hunters that respond always or sometimes in the previous question (n = 25)) | Never Always | 22 0 | 88% 0% |
| Sometimes Not applicable | 3 81 | 2.8% 76.4% | |
| “Have you ever seen a hunter’s baptism, the liver being rubbed on the hunter’s face?” | Never | 52 | 49% |
| Always | 4 | 3.80% | |
| Sometimes | 50 | 47.20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrantes, A.C.; Santos-Silva, S.; Mesquita, J.; Vieira-Pinto, M. Hepatitis E Virus in the Wild Boar Population: What Is the Real Zoonotic Risk in Portugal? Trop. Med. Infect. Dis. 2023, 8, 433. https://doi.org/10.3390/tropicalmed8090433
Abrantes AC, Santos-Silva S, Mesquita J, Vieira-Pinto M. Hepatitis E Virus in the Wild Boar Population: What Is the Real Zoonotic Risk in Portugal? Tropical Medicine and Infectious Disease. 2023; 8(9):433. https://doi.org/10.3390/tropicalmed8090433
Chicago/Turabian StyleAbrantes, Ana Carolina, Sérgio Santos-Silva, João Mesquita, and Madalena Vieira-Pinto. 2023. "Hepatitis E Virus in the Wild Boar Population: What Is the Real Zoonotic Risk in Portugal?" Tropical Medicine and Infectious Disease 8, no. 9: 433. https://doi.org/10.3390/tropicalmed8090433
APA StyleAbrantes, A. C., Santos-Silva, S., Mesquita, J., & Vieira-Pinto, M. (2023). Hepatitis E Virus in the Wild Boar Population: What Is the Real Zoonotic Risk in Portugal? Tropical Medicine and Infectious Disease, 8(9), 433. https://doi.org/10.3390/tropicalmed8090433








