Endogenous Bacteremia Caused by Intestinal Colonization of Carbapenem-Resistant Enterobacteriaceae (CRE) in Immunocompromised Children
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients and Sampling
2.2. Bacterial Culture and Characterization
2.3. Antimicrobial Susceptibility Testing
2.4. Detection of Carbapenemase Genes in the CRE Isolates
2.5. Molecular Typing by ERIC-PCR
2.6. Data Analysis
3. Results
3.1. Patients
3.2. Bacterial Isolates
3.3. Antimicrobial Resistance and Frequency of CRE among the Blood and Stool Samples of Febrile Children
3.4. Diversity of Carbapenem Resistance Genes among CRE
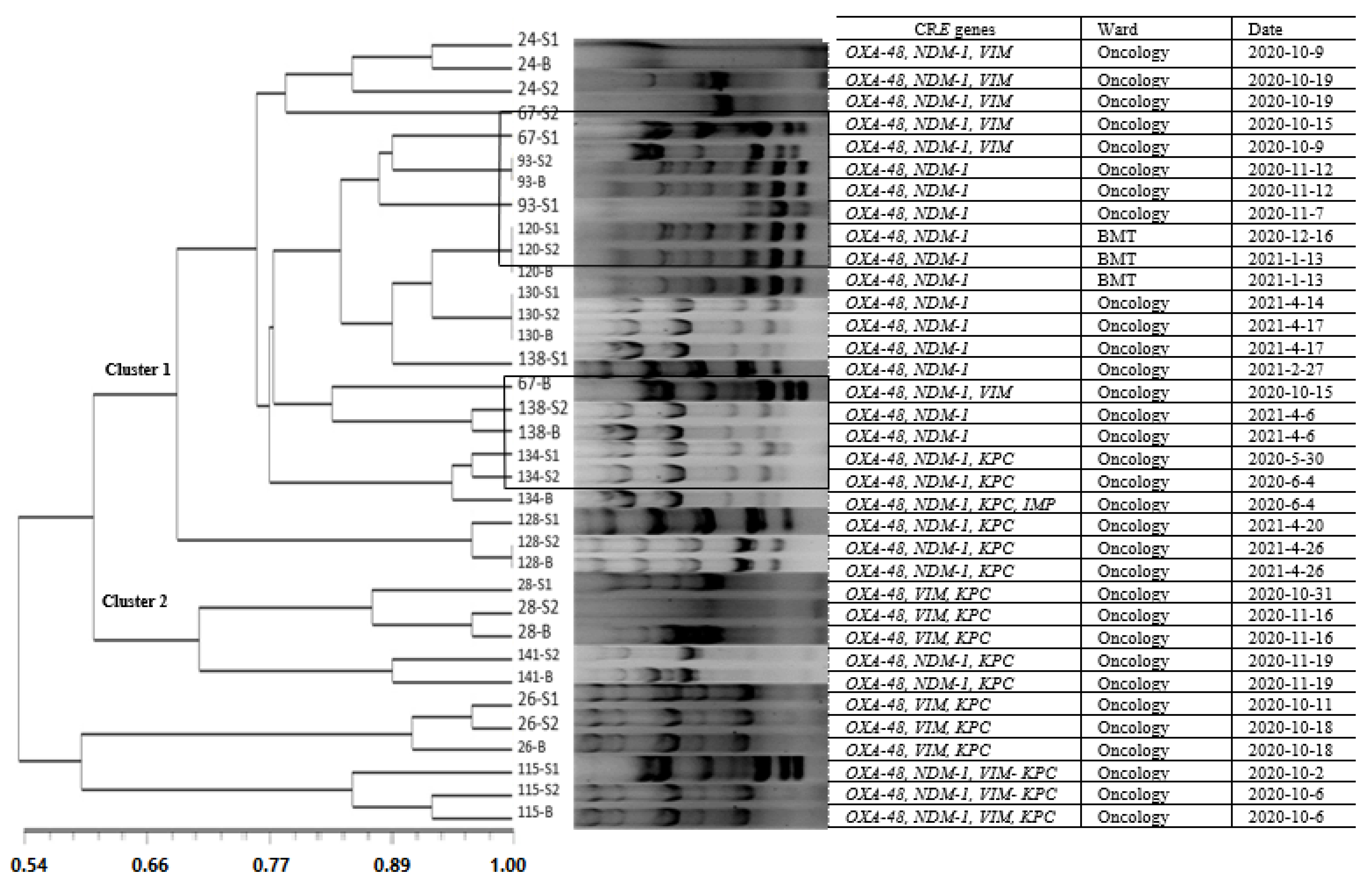
3.5. Molecular Typing of CRE in the Blood Samples and Their Similarity with the Stool Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, S.; Das, D.; Niranjan, D.K.; Singh, N.P.; Kaur, I.R. Carriage prevalence of carbapenem-resistant Enterobacteriaceae in stool samples: A surveillance study. Australas. Med. J. 2014, 7, 64. [Google Scholar] [CrossRef]
- Nagarjuna, D.; Mittal, G.; Dhanda, R.; Verma, P.; Gaind, R.; Yadav, M. Faecal Escherichia coli isolates show potential to cause endogenous infection in patients admitted to the ICU in a tertiary care hospital. New Microbes New Infect. 2015, 7, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Habib, I.; Al-Rifai, R.H.; Mohamed, M.-Y.I.; Ghazawi, A.; Abdalla, A.; Lakshmi, G.; Agamy, N.; Khan, M. Contamination Levels and Phenotypic and Genomic Characterization of Antimicrobial Resistance in Escherichia coli Isolated from Fresh Salad Vegetables in the United Arab Emirates. Trop. Med. Infect. Dis. 2023, 8, 294. [Google Scholar] [CrossRef]
- Donskey, C.J. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 2004, 39, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokur, M.E.; Korkmaz, P.; Alkan, S.; Yıldız, E.; Arık, Ö.; Renders, D.P.; Balcı, C. Mortality predictors on the day of healthcare-associated Acinetobacter baumanni bacteremia in intensive care unit. J. Infect. Dev. Ctries. 2022, 16, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Tabone, M.; Moissenet, D.; Leverger, G. Nosocomial infections in immunocompromised children. Pathol.-Biol. 2000, 48, 893–900. [Google Scholar] [PubMed]
- Mittal, G.; Gaind, R.; Kumar, D.; Kaushik, G.; Gupta, K.B.; Verma, P.; Deb, M. Risk factors for fecal carriage of carbapenemase producing Enterobacteriaceae among intensive care unit patients from a tertiary care center in India. BMC Microbiol. 2016, 16, 138. [Google Scholar] [CrossRef] [Green Version]
- Agyeman, A.A.; Bergen, P.J.; Rao, G.G.; Nation, R.L.; Landersdorfer, C.B. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int. J. Antimicrob. Agents 2020, 55, 105833. [Google Scholar] [CrossRef] [PubMed]
- Al-Tonbary, Y.A.; Soliman, O.E.; Sarhan, M.M.; Hegazi, M.A.; El-Ashry, R.A.; El-Sharkawy, A.A.; Salama, O.S.; Yahya, R. Nosocomial infections and fever of unknown origin in pediatric hematology/oncology unit: A retrospective annual study. World J. Pediatr. 2011, 7, 60–64. [Google Scholar] [CrossRef]
- Grabowski, M.E.; Kang, H.; Wells, K.M.; Sifri, C.D.; Mathers, A.J.; Lobo, J.M. Provider role in transmission of carbapenem-resistant Enterobacteriaceae. Infect. Control. Hosp. Epidemiol. 2017, 38, 1329–1334. [Google Scholar] [CrossRef]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetiere, M.; Batard, E. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavaro, D.F.; Pizzutilo, P.; Catino, A.; Signorile, F.; Pesola, F.; Di Gennaro, F.; Cassiano, S.; Marech, I.; Lamorgese, V.; Angarano, G.; et al. Incidence of infections and predictors of mortality during checkpoint inhibitor immunotherapy in patients with advanced lung cancer: A retrospective cohort study. Open. Forum. Infect. Dis. 2021, 8, ofab187. [Google Scholar] [CrossRef] [PubMed]
- Balkan, I.I.; Aygün, G.; Aydın, S.; Mutcalı, S.I.; Kara, Z.; Kuşkucu, M.; Midilli, K.; Şemen, V.; Aras, Ş.; Yemişen, M. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: Treatment and survival. Int. J. Infect. Dis. 2014, 26, 51–56. [Google Scholar] [CrossRef]
- Bower, C.; Adelman, M.; Howard-Anderson, J.; Ansari, U.; Lutgring, J.; Tiga, G.; Jacob, J. Carbapenem-Resistant Enterobacteriaceae Resistant Only to Ertapenem: An Epidemiologically Distinct Cohort, Atlanta, 2016–2018. Infect. Control Hosp. Epidemiol. 2020, 41, s463–s464. [Google Scholar] [CrossRef]
- Thomas, S.; Espeisse, O. Antimicrobial resistance. N. Engl. J. Med. 2020, 17, 330–333. [Google Scholar]
- Li, Z.; Lin, X.X.; Liu, C.X.; Ye, W.J.; Liu, P.N.; Li, H.Y.; Dong, L. Epidemiological characteristics and risk factors of nosocomial carbapenem-resistant Enterobacteriaceae infections in children. Chin. Med. J. 2020, 133, 2756–2758. [Google Scholar] [CrossRef]
- Cherak, Z.; Loucif, L.; Moussi, A.; Bendjama, E.; Benbouza, A.; Rolain, J.-M. Emergence of Metallo-β-Lactamases and OXA-48 Carbapenemase Producing Gram-Negative Bacteria in Hospital Wastewater in Algeria: A Potential Dissemination Pathway Into the Environment. Microb. Drug Resist. 2021, 28, 23–30. [Google Scholar] [CrossRef]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Nabarro, L.E.; Shankar, C.; Pragasam, A.K.; Mathew, G.; Jeyaseelan, V.; Veeraraghavan, B.; Verghese, V.P. Clinical and bacterial risk factors for mortality in children with carbapenem-resistant Enterobacteriaceae bloodstream infections in India. Pediatr. Infect. Dis. J. 2017, 36, e161–e166. [Google Scholar] [CrossRef]
- Rashidi, A.; Kaiser, T.; Graiziger, C.; Holtan, S.G.; Rehman, T.U.; Weisdorf, D.J.; Khoruts, A.; Staley, C. Specific gut microbiota changes heralding bloodstream infection and neutropenic fever during intensive chemotherapy. Leukemia 2020, 34, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wang, H.; Min, C.; Yu, T.; Luo, Y.; Li, J.; Hu, Y.; Yan, Q.; Liu, W.E.; Zou, M. A retrospective study on Escherichia coli bacteremia in immunocompromised patients: Microbiological features, clinical characteristics, and risk factors for shock and death. J. Clin. Lab. Anal. 2020, 34, e23319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Medical Microbiology E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2020. [Google Scholar]
- Wolter, D.J.; Khalaf, N.; Robledo, I.E.; Vázquez, G.J.; Santé, M.I.; Aquino, E.E.; Goering, R.V.; Hanson, N.D. Surveillance of carbapenem-resistant Pseudomonas aeruginosa isolates from Puerto Rican Medical Center Hospitals: Dissemination of KPC and IMP-18 β-lactamases. Antimicrob. Agents Chemother. 2009, 53, 1660–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Wang, Y.; Xu, J.; Li, Y.; Guo, J.; Ke, Y.; Yuan, X.; Wang, L.; Du, X.; Wang, Z. Genome sequence of an OXA23-producing, carbapenem-resistant Acinetobacter baumannii strain of sequence type ST75. Am. Soc. Microbiol. 2012, 194, 6000–6001. [Google Scholar] [CrossRef] [Green Version]
- Szejbach, A.; Mikucka, A.; Bogiel, T.; Gospodarek, E. Usefulness of phenotypic and genotypic methods for metallo-beta-lactamases detection in carbapenem-resistant Acinetobacter baumannii strains. Med. Sci. Monit. Basic Res. 2013, 19, 32. [Google Scholar] [CrossRef] [Green Version]
- Lowings, M.; Ehlers, M.M.; Dreyer, A.W.; Kock, M.M. High prevalence of oxacillinases in clinical multidrug-resistant Acinetobacter baumannii isolates from the Tshwane region, South Africa—An update. BMC Infect. Dis. 2015, 15, 521. [Google Scholar] [CrossRef] [Green Version]
- Azimi, L.; Rastegar-Lari, A.; Talebi, M.; Ebrahimzadeh-Namvar, A.; Soleymanzadeh-Moghadam, S. Evaluation of phenotypic methods for detection of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in Tehran. J. Med. Bacteriol. 2013, 2, 26–31. [Google Scholar]
- Brkić, S.; Božić, D.; Stojanović, N.; Vitorović, T.; Topalov, D.; Jovanović, M.; Stepanović, M.; Ćirković, I. Antimicrobial Susceptibility and Molecular Characterization of Carbapenemase-Producing Enterobacter spp. Community Isolates in Belgrade, Serbia. Microb. Drug Resist. 2020, 26, 378–384. [Google Scholar] [CrossRef]
- Tümmler, B. Molecular epidemiology in current times. Environ. Microbiol. 2020, 22, 4909–4918. [Google Scholar] [CrossRef]
- Van Belkum, A.; Tassios, P.; Dijkshoorn, L.; Haeggman, S.; Cookson, B.; Fry, N.; Fussing, V.; Green, J.; Feil, E.; Gerner-Smidt, P. Guidelines for the validation and application of typing methods for use in bacterial epidemiology. Clin. Microbiol. Infect. 2007, 13, 1–46. [Google Scholar] [CrossRef]
- Ghalavand, Z.; Alebouyeh, M.; Ghanati, K.; Azimi, L.; Rashidan, M. Genetic relatedness of the Enterococcus faecalis isolates in stool and urine samples of patients with community-acquired urinary tract infection. Gut Pathog. 2020, 12, 42. [Google Scholar] [CrossRef]
- Moore, D.L. Essentials of paediatric infection control. Paediatr. Child Health 2001, 6, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Ara-Montojo, M.F.; Escosa-García, L.; Alguacil-Guillén, M.; Seara, N.; Zozaya, C.; Plaza, D.; Schuffelmann-Gutierrez, C.; De la Vega, A.; Fernandez-Camblor, C.; Ramos-Boluda, E. Predictors of mortality and clinical characteristics among carbapenem-resistant or carbapenemase-producing Enterobacteriaceae bloodstream infections in Spanish children. J. Antimicrob. Chemother. 2021, 76, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, C.; Prato, M.; Scolfaro, C.; Colombo, S.; Esposito, S.; Tagliabue, C.; Lo Vecchio, A.; Bruzzese, E.; Loy, A.; Cursi, L. Carbapenem-resistant Enterobacteriaceae infections in children. Pediatr. Infect. Dis. J. 2016, 35, 862–868. [Google Scholar] [CrossRef]
- Pan, F.; Tian, D.; Wang, B.; Zhao, W.; Qin, H.; Zhang, T.; Zhang, H. Fecal carriage and molecular epidemiology of carbapenem-resistant Enterobacteriaceae from outpatient children in Shanghai. BMC Infect. Dis. 2019, 19, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazeli, H.; Moghim, S.; Zare, D. Antimicrobial resistance pattern and spectrum of multiple-drug-resistant enterobacteriaceae in Iranian hospitalized patients with cancer. Adv. Biomed. Res. 2018, 7, 69. [Google Scholar]
- McConville, T.H.; Sullivan, S.B.; Gomez-Simmonds, A.; Whittier, S.; Uhlemann, A.-C. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS ONE 2017, 12, e0186195. [Google Scholar] [CrossRef] [Green Version]
- Azimi, L.; Nordmann, P.; Lari, A.R.; Bonnin, R.A. First report of OXA-48-producing Klebsiella pneumoniae strains in Iran. GMS Hyg. Infect. Control 2014, 9, Doc07. [Google Scholar]
- Kamel, N.A.; El-Tayeb, W.N.; El-Ansary, M.R.; Mansour, M.T.; Aboshanab, K.M. Phenotypic screening and molecular characterization of carbapenemase-producing Gram-negative bacilli recovered from febrile neutropenic pediatric cancer patients in Egypt. PLoS ONE 2018, 13, e0202119. [Google Scholar] [CrossRef]
- Bocanegra-Ibarias, P.; Garza-González, E.; Morfín-Otero, R.; Barrios, H.; Villarreal-Treviño, L.; Rodríguez-Noriega, E.; Garza-Ramos, U.; Petersen-Morfin, S.; Silva-Sanchez, J. Molecular and microbiological report of a hospital outbreak of NDM-1-carrying Enterobacteriaceae in Mexico. PLoS ONE 2017, 12, e0179651. [Google Scholar] [CrossRef]
- Hudson, C.M.; Bent, Z.W.; Meagher, R.J.; Williams, K.P. Resistance determinants and mobile genetic elements of an NDM-1-encoding Klebsiella pneumoniae strain. PLoS ONE 2014, 9, e99209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Bush, K. Success and Challenges Associated with Large Scale Collaborative Surveillance for Carbapenemase Genes in Gram Negative Bacteria. Antimicrob. Agents Chemother. 2021, 66, e02299-21. [Google Scholar] [CrossRef] [PubMed]
| Carbapenemase Genes | PCR Product (bp) | Sequence (5′–3′) | PCR Conditions | Reference |
|---|---|---|---|---|
| OXA-48 | 392 | F: CCAAGCATTTTTACCCGCATCKACC R: GYTTGACCATACGCTGRCTGCG | Initial denaturation at 95 °C for 1 min; 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 75 °C for 1 min; and final extension at 72 °C for 7 min | [24] |
| NDM-1 | 129 | F: CCCCGCCACACCAGTGACANCTC R: GTAGTGCTCAGTGTGGGCAT | Initial denaturation at 95 °C for 1 min; 32 cycles of denaturation at 95 °C for 30 s, annealing at 61 °C for 30 s, extension at 7 °C for 1 min; and final extension at 72 °C for 5 min | [25] |
| VIM | 390 | F: GATGGTGTTTGGTCGCATA R: CGAATGCGCAGCACCAG | Initial denaturation at 94 °C for 10 min; 35 cycles of denaturation at 94 °C for 30 s, annealing at 61 °C for 40 s, extension at 72 °C for 1 min; and final extension at 72 °C for 7 min | [26] |
| IMP | 139 | F: TTGACACTCCATTTACDG R: GATYGAGAATTAAGCCACYCT | Initial denaturation at 94 °C for 10 min; 33 cycles of denaturation at 94 °C for 30 s, annealing at 61 °C for 40 s, axtension at 72 °C for 40 s; and final extension at 72 °C for 7 min | [27] |
| KPC | 636 | F: CTGTCTTGTCTCTCATGGCC R: CCTCGCTGTGCTTGTCATCC | Initial denaturation at 94 °C for 5 min; 32 cycles of denaturation at 94 °C for 35 s, annealing at 62 °C for 35 s, extension at 72 °C for 32 s; and final extension at 72 °C for 5 min | [28] |
| ERIC-PCR | F: ATGTAAGCTCCTGGGGATTCAC R: AAGTAAGTGACTGGGGTGAGCG | Initial denaturation at 94 °C for 3 min; 35 cycles of denaturation at 94 °C for 1 min, annealing at 48 °C for 1 min, extension at 72 °C for 2 min; and final extension at 72 °C for 5 min | [29] |
| Bacterial Species | Primary Stool a N = 44 (n, %) | Secondary Stool b N = 45 (n, %) | Blood c N = 45 (n, %) |
|---|---|---|---|
| Escherichia coli | 28, (63.63) | 28, (62.22) | 3, (6.66) |
| Klebsiella pneumoniae | 9, (20.45) | 12, (26.66) | 7, (15.55) |
| Klebsiella ozaenae | 1, (2.27) | 0, (0) | - |
| Enterobacter. agglomerans | 1, (2.27) | 0, (0) | - |
| Escherichia herminie | 1, (2.27) | 1, 2.22) | - |
| Klebsiella oxytoca | 1, (2.27) | 1, (2.22) | 2, (4.44) |
| Enterobacter cloacae | 1, (2.27) | 1, (2.22) | 1, (2.22) |
| Serratia rubidaea | 1, (2.27) | 1, (2.22) | 1, (2.22) |
| Antibiotics a | Primary Stool Sample b N = 44 (n, %) | Secondary Stool Samples N = 45 (n, %) | Blood Samples N = 45 (n, %) |
|---|---|---|---|
| IMP (10 µg) | 12, (27.27) | 19, (42.2) | 13, (28.88) |
| MEM (10 µg) | 14, (31.83) | 18, (40) | 13, (28.88) |
| AN (30 µg) | 13, (29.95) | 15, (33) | 8, (17.77) |
| GM (10 µg) | 22, (50) | 24, (53.3) | 11, (24.44) |
| CAZ (30 µg) | 29, (65.9) | 39, (86.6) | 12, (26.66) |
| LVX (5 µg) | 9, (20.4) | 15, (33) | 4, (8.88) |
| CTX (30 µg) | 33, (75) | 37, (82.2) | 13, (28.88) |
| CEF (30 µg) | 23, (52.27) | 26, (57.5) | 12, (26.66) |
| AZT (30 µg) | 29, (65.9) | 32, (71.1) | 11, (24.44) |
| TGC (15 µg) | 3, (6.8) | 7, (15.5) | 3, (6.66) |
| Variables | Frequency (n = 45) | CRE (n = 14) | p-Value |
|---|---|---|---|
| Gender | 0.99 | ||
| Female | 20 (20/45, 44.4%) | 6 (6/20, 30%) | |
| Male | 25 (25/45, 55.5%) | 8 (8/25, 32%) | |
| Age | 0.99 | ||
| 1–5 years old | 22 (22/45, 48%) | 7 (7/22, 31.81%) | |
| 6–10 years old | 11 (11/45, 24%) | 3 (3/11, 0.27%) | |
| 11–15 years old | 12 (12/45, 26%) | 4 (4/12, 0.33%) | |
| Fever day | 0.022 b | ||
| 7 days (first week) | 36 (36/45, 80%) | 8 (8/36, 0.22%) | |
| 14 days (second week) | 5 (5/45, 11%) | 3 (3/5, 0.6%) | |
| 21 days (third week) | 4 (4/45, 8%) | 3 (3/4, 0.75%) | |
| LOS | 0.018 b | ||
| 7 days (first week) | 13 (13/45, 28.8%) | 2 (2/13, 15.38%) | |
| 14 days (second week) | 17 (17/45, 37.7%) | 3 (3/17, 17.64%) | |
| Over 14 days (third week) | 15 (15/45, 33.3%) | 9 (9/15, 0.6%) | |
| Underlying diseases | 0.83 | ||
| AML | 12 (12/45, 26%) | 0 | |
| ALL | 6 (4/45, 13%) | 1 (1/6, 16.66%) | |
| Burkitt’s lymphoma | 3 (3/45, 6.6%) | 3 (3/3, 100%) | |
| Neuroblastoma | 5 (5/45, 11.1%) | 2 (2/5, 0.4%) | |
| CGD | 2 (2/45, 4.4%) | 0 | |
| Histiocytic disorders | 1 (1/45, 2.2%) | 0 | |
| Lymphoma | 7 (7/45, 15.5%) | 3 (3/7, 42.85%) | |
| PNH | 1 (1/45, 2.2%) | 0 | |
| RMS | 1 (1/45, 2.2%) | 1 (1/1, 100%) | |
| Wilms tumor | 3 (3/45, 6.6%) | 1 (1/3, 33.33%) | |
| HLH | 1 (1/45, 2.2%) | 0 | |
| YST | 1 (1/45, 2.2%) | 1 (1/1, 100%) | |
| RES | 1 (1/45, 2.2%) | 1 (1/1, 100%) | |
| Aplastic anemia | 1 (1/45, 2.2%) | 1 (1/1, 100%) | |
| Ward | 0.46 | ||
| BMT | 11 (11/45, 24.44%) | 2 (2/11, 18.18%) | |
| Oncology | 34 (34/45, 75.5%) | 12 (12/34, 35.29%) | |
| Prophylaxis antibiotics | 0.99 | ||
| Carbapenem based regime | 19 (19/45, 42.22%) | 5 (5/19, 26.31%) | |
| Non-carbapenem based regime | 26 (26/45, 57.77%) | 9 (9/26, 34.61%) | |
| Alternative antibiotic | 0.99 | ||
| Carbapenem based regime | 20 (20/45, 44.44%) | 6 (6/20, 3%) | |
| Non-carbapenem based regime | 25 (25/45, 55.55%) | 8 (8/25, 32%) | |
| Chemotherapy drug | 0.03 b | ||
| Cyclophosphamide + Adriamycin + vincristine | 10 (10/45, 22.25%) | 1 (1/10, 10%) | |
| Cytosar | 8 (8/45, 17.7%) | 3 (3/8, 37.5%) | |
| GCSF | 7 (7/45, 15.5%) | 2 (2/7, 28.57%) | |
| Cyclophosphamide + vincristine | 7 (7/45, 15.5%) | 4 (4/7, 57.14%) | |
| Doxorubicin + cyclophosphamide + vincristine | 5 (5/45, 11.1%) | 1 (1/5, 20%) | |
| Fludarabin + cytocar | 4 (4/45, 8.8%) | 1 (1/4, 25%) | |
| 6MP | 4 (4/45, 8.8%) | 2 (2/14, 50%) | |
| Death | 0.02 b | ||
| Yes | 11 (11/45, 24.4%) | 7 (7/11, 63.63%) | |
| No | 34 (34/45, 75.5%) | 7 (7/34, 20.58%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasian Tehrani, N.; Azimi, L.; Armin, S.; Soleimani, N.; Fallah, F.; Karimi, A.; Shamsian, B.S.; Nazari, S.; Alebouyeh, M. Endogenous Bacteremia Caused by Intestinal Colonization of Carbapenem-Resistant Enterobacteriaceae (CRE) in Immunocompromised Children. Trop. Med. Infect. Dis. 2023, 8, 402. https://doi.org/10.3390/tropicalmed8080402
Almasian Tehrani N, Azimi L, Armin S, Soleimani N, Fallah F, Karimi A, Shamsian BS, Nazari S, Alebouyeh M. Endogenous Bacteremia Caused by Intestinal Colonization of Carbapenem-Resistant Enterobacteriaceae (CRE) in Immunocompromised Children. Tropical Medicine and Infectious Disease. 2023; 8(8):402. https://doi.org/10.3390/tropicalmed8080402
Chicago/Turabian StyleAlmasian Tehrani, Nasim, Leila Azimi, Shahnaz Armin, Neda Soleimani, Fatemeh Fallah, Abdollah Karimi, Bibi Shahin Shamsian, Shiva Nazari, and Masoud Alebouyeh. 2023. "Endogenous Bacteremia Caused by Intestinal Colonization of Carbapenem-Resistant Enterobacteriaceae (CRE) in Immunocompromised Children" Tropical Medicine and Infectious Disease 8, no. 8: 402. https://doi.org/10.3390/tropicalmed8080402
APA StyleAlmasian Tehrani, N., Azimi, L., Armin, S., Soleimani, N., Fallah, F., Karimi, A., Shamsian, B. S., Nazari, S., & Alebouyeh, M. (2023). Endogenous Bacteremia Caused by Intestinal Colonization of Carbapenem-Resistant Enterobacteriaceae (CRE) in Immunocompromised Children. Tropical Medicine and Infectious Disease, 8(8), 402. https://doi.org/10.3390/tropicalmed8080402






