Evaluation of Laboratories Supporting Invasive Bacterial Vaccine-Preventable Disease (IB-VPD) Surveillance in the World Health Organization African Region, through the Performance of Coordinated External Quality Assessment
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
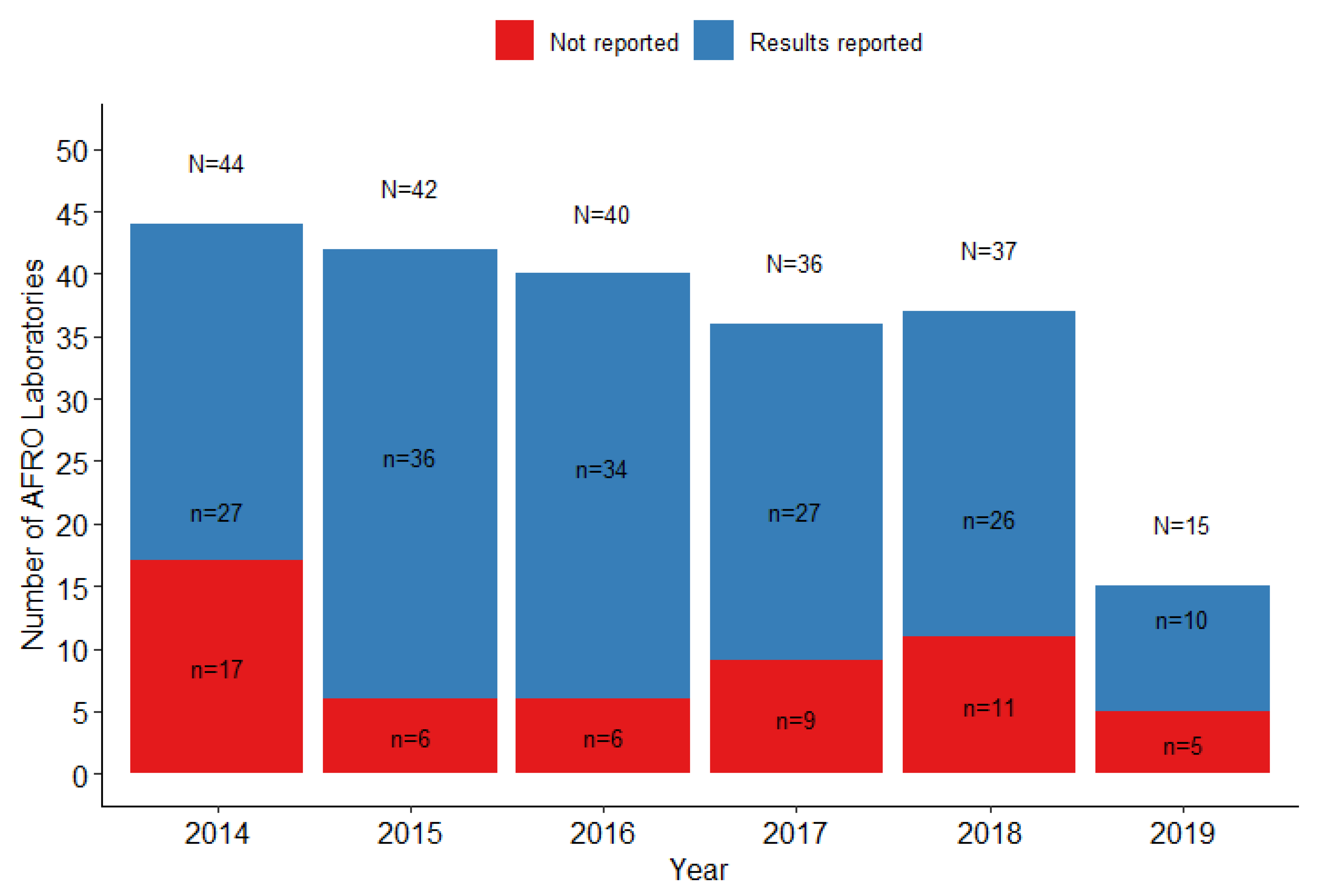
3.1. General Description
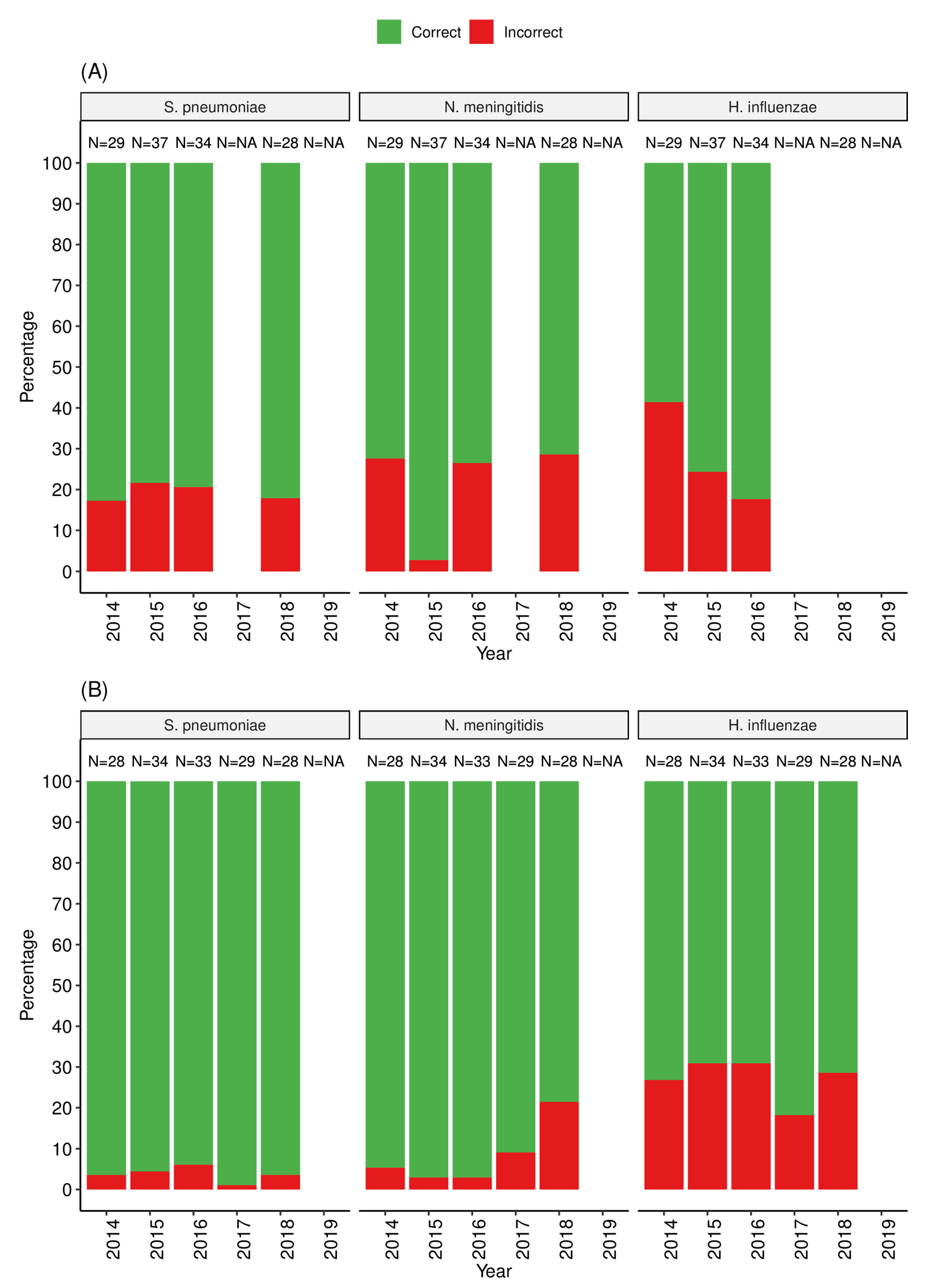
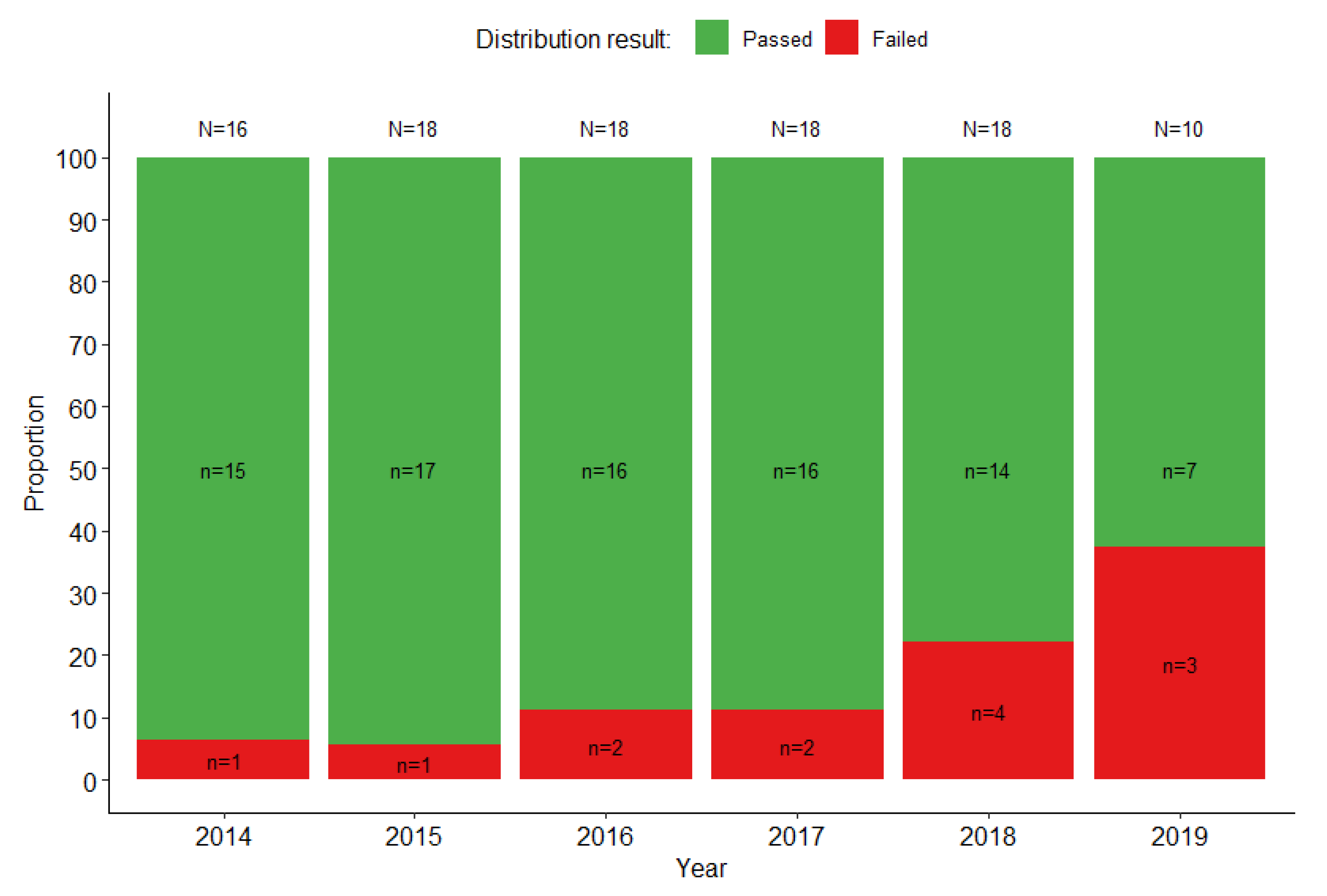
3.2. Performance of Laboratories That Tested the Partial Panel: SSLs and NLs
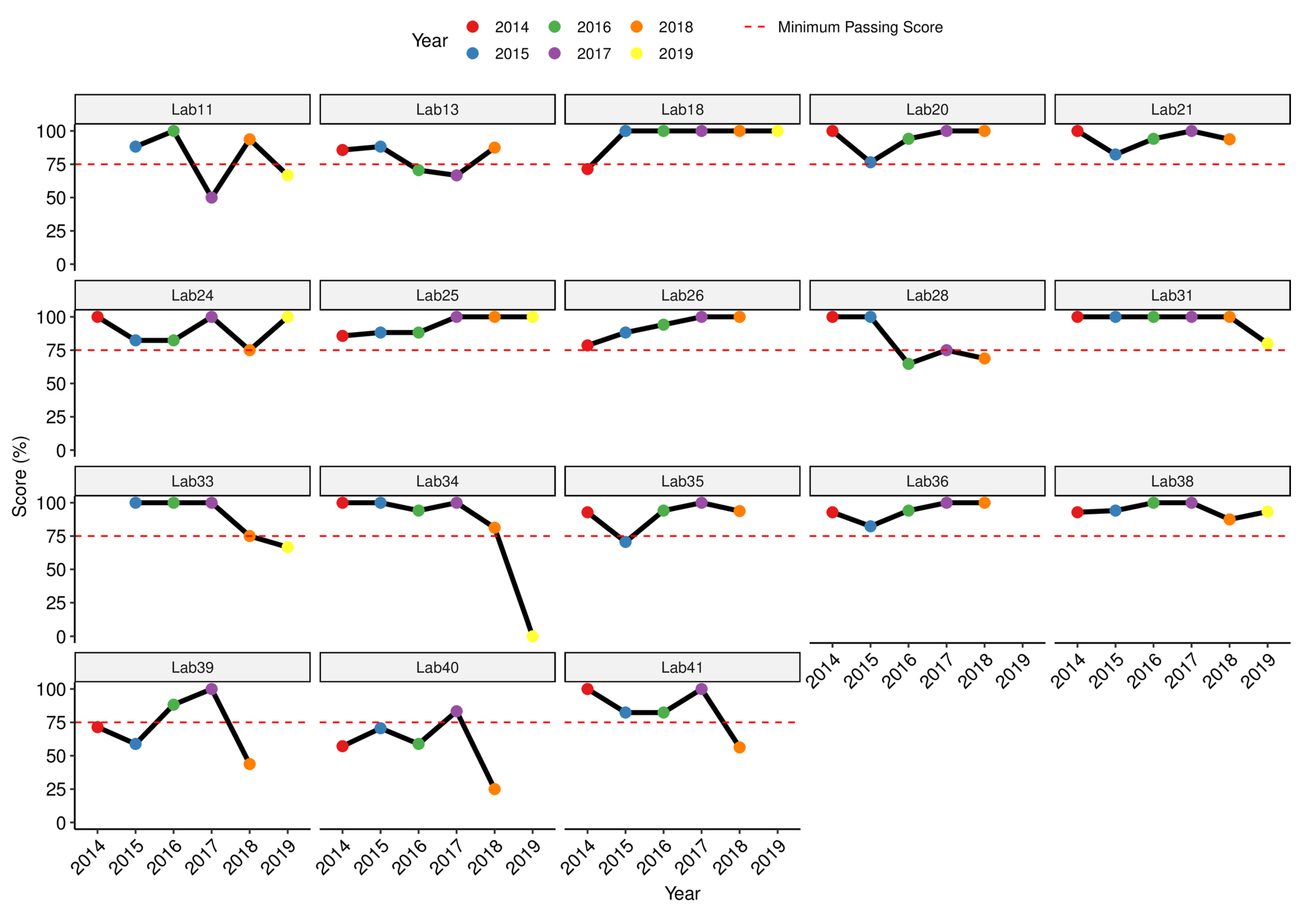
3.3. Performance of Laboratories That Tested the Full Panel: RRLs and Selected SSLs and NLs
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristina, E.R.; Sarah, C.J.; Kareha, M.A.; Katya, A.S.; Derrick, T.; Daniel, R.K.; Danny, V.C.; Kevin, S.I.; Niranjan, K.; Simon, F.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- GBD 2019 Under-5 Mortality Collaborators. Global, Regional, and National Progress towards Sustainable Development Goal 3.2 for Neonatal and Child Health: All-Cause and Cause-Specific Mortality Findings from the Global Burden of Disease Study 2019. Lancet 2021, 398, 870–905. [Google Scholar] [CrossRef]
- Peltola, H. Burden of Meningitis and Other Severe Bacterial Infections of Children in Africa: Implications for Prevention. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 32, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Mduma, E.; Halidou, T.; Kaboré, B.; Walongo, T.; Lompo, P.; Museveni, J.; Gidabayda, J.; Gratz, J.; Guga, G.; Kimathi, C.; et al. Etiology of Severe Invasive Infections in Young Infants in Rural Settings in Sub-Saharan Africa. PLoS ONE 2022, 17, e0264322. [Google Scholar] [CrossRef]
- Kumasaka, K. External quality assessment for clinical microbiology and good laboratory management. Rinsho byori. Jpn. J. Clin. Pathol. 1998, 46, 124–131. [Google Scholar]
- Frean, J.; Perovic, O.; Fensham, V.; McCarthy, K.; von Gottberg, A.; de Gouveia, L.; Poonsamy, B.; Dini, L.; Rossouw, J.; Keddy, K.; et al. External Quality Assessment of National Public Health Laboratories in Africa, 2002–2009. Bull. World Health Organ. 2012, 90, 191–199. [Google Scholar] [CrossRef]
- Boeras, D.I.; Peeling, R.W.; Onyebujoh, P.; Yahaya, A.A.; Gumede-Moeletsi, H.N.; Ndihokubwayo, J.B. The WHO AFRO External Quality Assessment Programme (EQAP): Linking Laboratory Networks through EQA Programmes. Afr. J. Lab. Med. 2016, 5, 560. [Google Scholar] [CrossRef] [PubMed]
- Perovic, O.; Yahaya, A.A.; Viljoen, C.; Ndihokubwayo, J.-B.; Smith, M.; Coulibaly, S.O.; De Gouveia, L.; Oxenford, C.J.; Cognat, S.; Ismail, H.; et al. External Quality Assessment of Bacterial Identification and Antimicrobial Susceptibility Testing in African National Public Health Laboratories, 2011–2016. Trop. Med. Infect. Dis. 2019, 4, 144. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. The Paediatric Bacterial Meningitis Surveillance Network in WHO’s African Region, 2001–2008. Releve Epidemiol. Hebd. 2009, 84, 179–185. [Google Scholar]
- Murray, J.; Agócs, M.; Serhan, F.; Singh, S.; Deloria-Knoll, M.; O’Brien, K.; Mwenda, J.M.; Mihigo, R.; Oliveira, L.; Teleb, N.; et al. Global Invasive Bacterial Vaccine-Preventable Diseases Surveillance—2008–2014. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 1159–1162. [Google Scholar] [PubMed]
- WHO|WHO/NICD Microbiology External Quality Assessment Programme in Africa Years 1 to 4 2002–2006. Available online: http://www.who.int/ihr/publications/policy_procedures_eqa/en/ (accessed on 31 January 2021).
- Centers for Disease Control and Prevention (CDC). Pediatric Bacterial Meningitis Surveillance–African Region, 2002–2008. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 493–497. [Google Scholar]
- Mwenda, J.M.; Soda, E.; Weldegebriel, G.; Katsande, R.; Biey, J.N.-M.; Traore, T.; de Gouveia, L.; du Plessis, M.; von Gottberg, A.; Antonio, M.; et al. Pediatric Bacterial Meningitis Surveillance in the World Health Organization African Region Using the Invasive Bacterial Vaccine-Preventable Disease Surveillance Network, 2011–2016. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, S49–S57. [Google Scholar] [CrossRef] [Green Version]
- Ndihokubwayo, J.-B.; Maruta, T.; Ndlovu, N.; Moyo, S.; Yahaya, A.A.; Coulibaly, S.O.; Kasolo, F.; Turgeon, D.; Abrol, A.P. Implementation of the World Health Organization Regional Office for Africa Stepwise Laboratory Quality Improvement Process Towards Accreditation. Afr. J. Lab. Med. 2016, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Maruta, T.; Motebang, D.; Mathabo, L.; Rotz, P.J.; Wanyoike, J.; Peter, T. Impact of Mentorship on WHO-AFRO Strengthening Laboratory Quality Improvement Process Towards Accreditation (SLIPTA). Afr. J. Lab. Med. 2012, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemnji, G.A.; Zeh, C.; Yao, K.; Fonjungo, P.N. Strengthening National Health Laboratories in Sub-Saharan Africa: A Decade of Remarkable Progress. Trop. Med. Int. Health 2014, 19, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Litt, D.; Slack, M.P.E.; Nakamura, T.; Gray, S.; Seaton, S.; Fagan, E.J.; Sheppard, C.; Mwenda, J.M.; Rey-Benito, G.; Ghoniem, A.; et al. Evaluation of the World Health Organization Global Invasive Bacterial Vaccine-Preventable Disease (IB-VPD) Surveillance Network’s Laboratory External Quality Assessment Programme, 2014–2019. J. Med. Microbiol. 2023, 72, 001644. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Prevention (U.S.). Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria Meningitidis, Streptococcus Pneumoniae, and Haemophilus Influenzae: WHO Manual; World Health Organization: Geneva, Switzerland, 2011.
- Pai, R.; Gertz, R.E.; Beall, B. Sequential Multiplex PCR Approach for Determining Capsular Serotypes of Streptococcus Pneumoniae Isolates. J. Clin. Microbiol. 2006, 44, 124–131. [Google Scholar] [CrossRef] [Green Version]
- da Gloria Carvalho, M.; Pimenta, F.C.; Jackson, D.; Roundtree, A.; Ahmad, Y.; Millar, E.V.; O’Brien, K.L.; Whitney, C.G.; Cohen, A.L.; Beall, B.W. Revisiting Pneumococcal Carriage by Use of Broth Enrichment and PCR Techniques for Enhanced Detection of Carriage and Serotypes. J. Clin. Microbiol. 2010, 48, 1611–1618. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, F.C.; Roundtree, A.; Soysal, A.; Bakir, M.; du Plessis, M.; Wolter, N.; von Gottberg, A.; McGee, L.; da Carvalho, M.G.; Beall, B. Sequential Triplex Real-Time PCR Assay for Detecting 21 Pneumococcal Capsular Serotypes That Account for a High Global Disease Burden. J. Clin. Microbiol. 2013, 51, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.A.; Teixeira, L.M.; da Carvalho, M.G.; Beall, B. Sequential Multiplex PCR for Determining Capsular Serotypes of Pneumococci Recovered from Brazilian Children. J. Med. Microbiol. 2007, 56, 1185–1188. [Google Scholar] [CrossRef]
- Zhang, H.L.; Omondi, M.W.; Musyoka, A.M.; Afwamba, I.A.; Swai, R.P.; Karia, F.P.; Muiruri, C.; Reddy, E.A.; Crump, J.A.; Rubach, M.P. Challenges of Maintaining Good Clinical Laboratory Practices in Low-Resource Settings: A Health Program Evaluation Framework Case Study From East Africa. Am. J. Clin. Pathol. 2016, 146, 199–206. [Google Scholar] [CrossRef]
- Bankole, O.T.; Ajayi, I.O. Evaluation of Diagnostic Microbiology Capacity and Barriers in Testing for HIV and TB at Peripheral Hospital-Based Laboratories in Oyo-State, Nigeria. Microbiol. Spectr. 2022, 10, e0045921. [Google Scholar] [CrossRef] [PubMed]
- Girma, M.; Desale, A.; Hassen, F.; Sisay, A.; Tsegaye, A. Survey-Defined and Interview-Elicited Challenges That Faced Ethiopian Government Hospital Laboratories as They Applied ISO 15189 Accreditation Standards in Resource-Constrained Settings in 2017. Am. J. Clin. Pathol. 2018, 150, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Akpavie, S.O.; Ikheloa, J.O. An Outbreak of Listeriosis in Cattle in Nigeria. Rev. Elev. Med. Vet. Pays Trop. 1992, 45, 263–264. [Google Scholar] [CrossRef]
- Smith, A.M.; Tau, N.P.; Smouse, S.L.; Allam, M.; Ismail, A.; Ramalwa, N.R.; Disenyeng, B.; Ngomane, M.; Thomas, J. Outbreak of Listeria Monocytogenes in South Africa, 2017–2018: Laboratory Activities and Experiences Associated with Whole-Genome Sequencing Analysis of Isolates. Foodborne Pathog. Dis. 2019, 16, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Ercibengoa, M.; Perez-Gavilan, A.; Alonso, M.; Azcue, N.; Cilla, G.; Marimón, J.M. Comparison of Four Methods for Streptococcus Pneumoniae Capsular Typing. Clin. Lab. 2021, 67, 1598–1602. [Google Scholar] [CrossRef]
- Kariuki, S.; Mbae, C.; Van Puyvelde, S.; Onsare, R.; Kavai, S.; Wairimu, C.; Ngetich, R.; Clemens, J.; Dougan, G. High Relatedness of Invasive Multi-Drug Resistant Non-Typhoidal Salmonella Genotypes among Patients and Asymptomatic Carriers in Endemic Informal Settlements in Kenya. PLoS Negl. Trop. Dis. 2020, 14, e0008440. [Google Scholar] [CrossRef] [PubMed]
- Valian, S.K.; Mahmoudi, S.; Pourakbari, B.; Banar, M.; Ashtiani, M.T.H.; Mamishi, S. The Causative Organisms of Bacterial Meningitis and Their Antimicrobial Resistance Profiles in Iranian Children in 2011–2016. Infect. Disord. Drug Targets 2020, 20, 229–236. [Google Scholar] [CrossRef]

| Samples | Test Requested | Intended Result to be Reported (Partial Panel) | Intended Result to be Reported (Full Panel) |
|---|---|---|---|
| Slide smear | Gram staining | Cellular morphologies | |
| Viable culture | Culture and species identification | Phenotypic or genotypic identification (Serogrouping or serotyping optional and not scored) | Phenotypic or genotypic identification Serogrouping or serotyping * |
| Antimicrobial sensitivity testing | MIC results (if available) and S/I/R results, according to each participant’s local guidelines, for a predefined list of appropriate antibiotics (not scored) | ||
| Simulated cerebrospinal fluid (CSF) | PCR testing | (Not included in the partial panel) | Genotypic identification * Genogrouping or genotyping * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandomando, I.; Mwenda, J.M.; Nakamura, T.; de Gouveia, L.; von Gottberg, A.; Kwambana-Adams, B.A.; Antonio, M.; Messa, A., Jr.; Litt, D.; Seaton, S.; et al. Evaluation of Laboratories Supporting Invasive Bacterial Vaccine-Preventable Disease (IB-VPD) Surveillance in the World Health Organization African Region, through the Performance of Coordinated External Quality Assessment. Trop. Med. Infect. Dis. 2023, 8, 413. https://doi.org/10.3390/tropicalmed8080413
Mandomando I, Mwenda JM, Nakamura T, de Gouveia L, von Gottberg A, Kwambana-Adams BA, Antonio M, Messa A Jr., Litt D, Seaton S, et al. Evaluation of Laboratories Supporting Invasive Bacterial Vaccine-Preventable Disease (IB-VPD) Surveillance in the World Health Organization African Region, through the Performance of Coordinated External Quality Assessment. Tropical Medicine and Infectious Disease. 2023; 8(8):413. https://doi.org/10.3390/tropicalmed8080413
Chicago/Turabian StyleMandomando, Inacio, Jason M. Mwenda, Tomoka Nakamura, Linda de Gouveia, Anne von Gottberg, Brenda A. Kwambana-Adams, Martin Antonio, Augusto Messa, Jr., David Litt, Shila Seaton, and et al. 2023. "Evaluation of Laboratories Supporting Invasive Bacterial Vaccine-Preventable Disease (IB-VPD) Surveillance in the World Health Organization African Region, through the Performance of Coordinated External Quality Assessment" Tropical Medicine and Infectious Disease 8, no. 8: 413. https://doi.org/10.3390/tropicalmed8080413
APA StyleMandomando, I., Mwenda, J. M., Nakamura, T., de Gouveia, L., von Gottberg, A., Kwambana-Adams, B. A., Antonio, M., Messa, A., Jr., Litt, D., Seaton, S., Weldegebriel, G. G., Biey, J. N.-M., & Serhan, F. (2023). Evaluation of Laboratories Supporting Invasive Bacterial Vaccine-Preventable Disease (IB-VPD) Surveillance in the World Health Organization African Region, through the Performance of Coordinated External Quality Assessment. Tropical Medicine and Infectious Disease, 8(8), 413. https://doi.org/10.3390/tropicalmed8080413







