Nanoformulation-Based 1,2,3-Triazole Sulfonamides for Anti-Toxoplasma In Vitro Study
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemistry
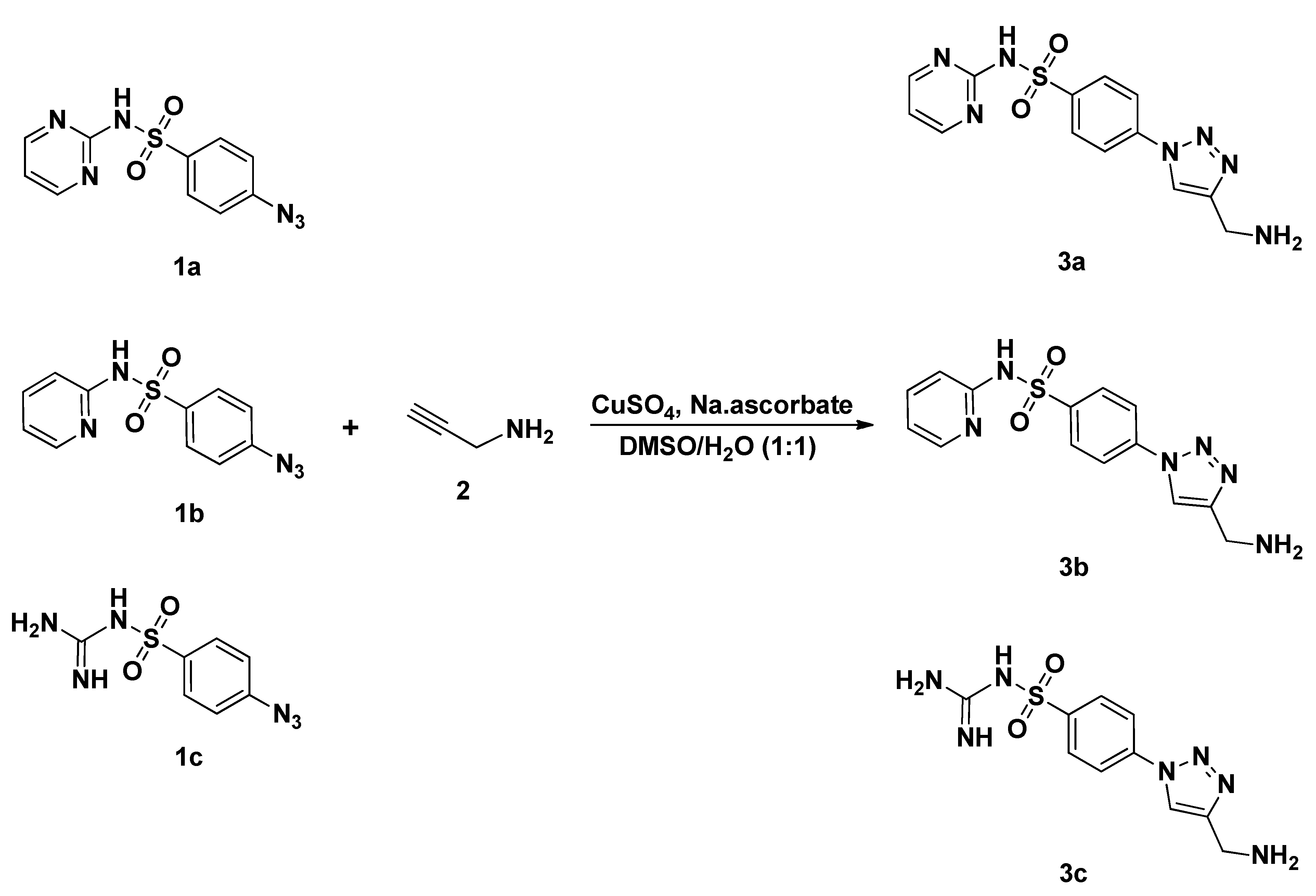
2.2. General Method for the Synthesis Sulfonamide-Based 1,2,3-Triazoles 3a–c
2.3. Preparation of Nanoformulated Sulfa Drugs
2.4. Characterization of Nanoformulae
2.5. Determination of Loading and Entrapment Efficiencies
2.6. Maintenance of Toxoplasma Strain
2.7. Vero Cell Line
2.8. Cytotoxicity Tests
2.9. In Vitro Growth Inhibition Assay
2.10. The Effect of Nanoformulae on Non-Infected and Infected Vero Cell Line under Light Microscopy
2.11. Scanning Electron Microscopy (SEM)
2.12. Transmission Electron Microscopy (TEM)
3. Results
3.1. Chemistry
3.2. Characterization of Nanoformulae
3.3. Cytotoxicity Tests
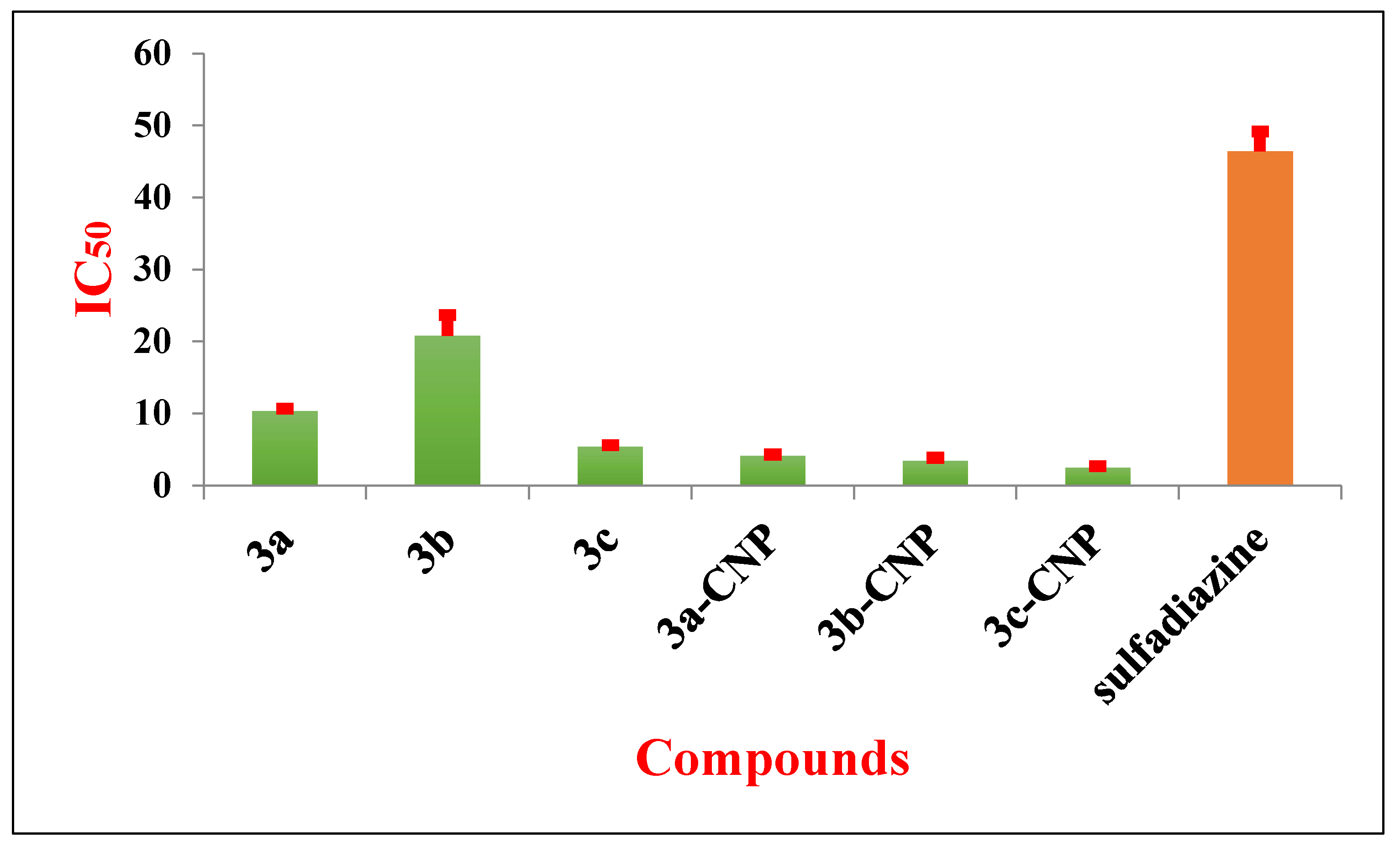
3.4. In Vitro Growth Inhibition Assay
3.5. The Effect of Nanoformulae on Non-Infected and Infected Vero Cell Line under Light Microscopy
3.6. Scanning Electron Microscopy (SEM)
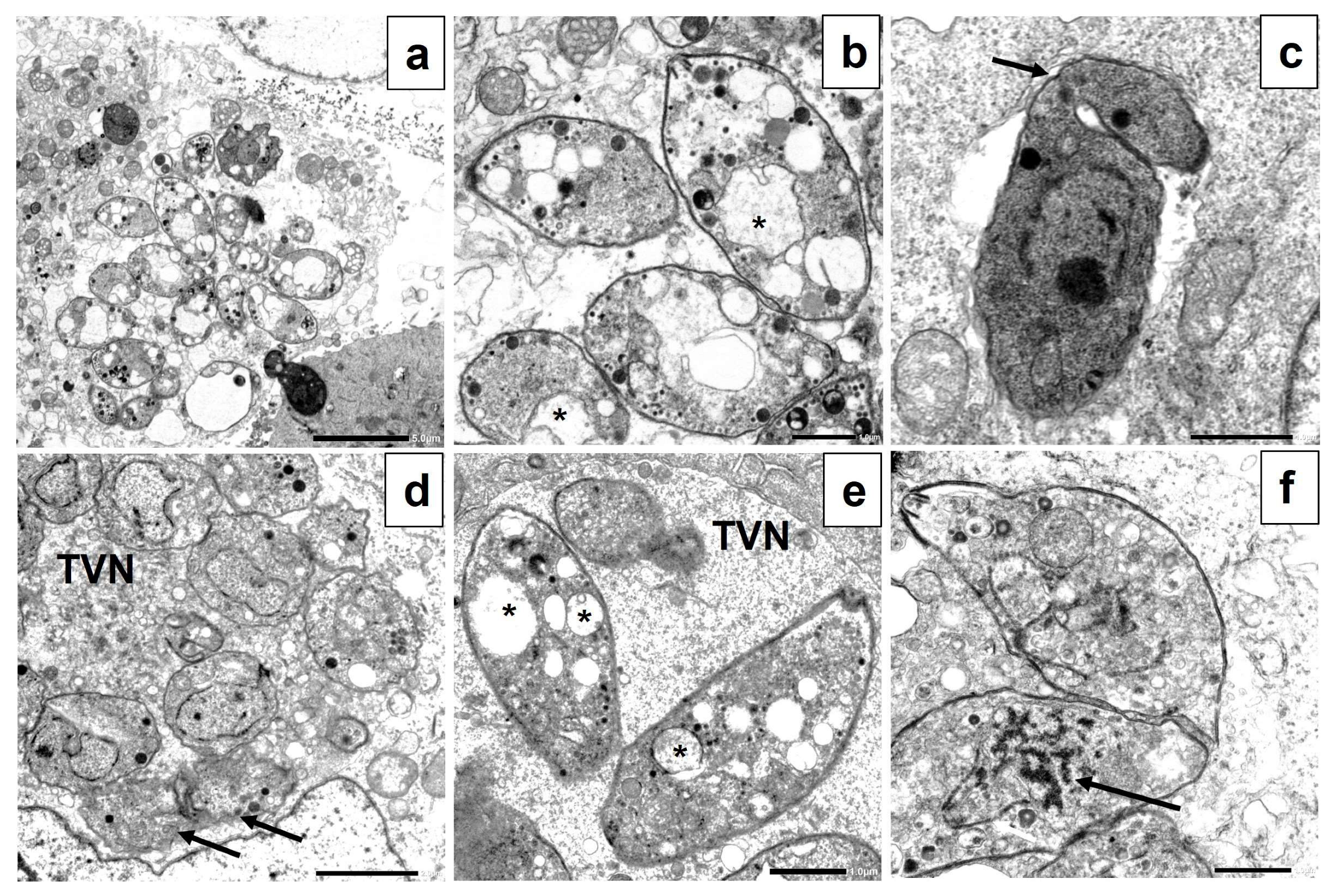
3.7. Transmission Electron Microscopy (TEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, J.; Lago, E.; Gennari, S.; Su, C.; Jones, J. Toxoplasmosis in humans and animals in Brazil: High prevalence, high burden of disease, and epidemiology. Parasitology 2012, 139, 1375–1424. [Google Scholar] [CrossRef] [Green Version]
- Schlundt, J.; Toyofuku, H.; Jansen, J.; Herbst, S. Emerging food–borne zoonoses. Rev. Sci. Et Tech.-Off. Int. Des. Epizoot. 2004, 23, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Esch, G.W. Toxoplasmosis of animals and humans. J. Parasitol. 2010, 96, 940. [Google Scholar] [CrossRef]
- Hermes, G.; Ajioka, J.W.; Kelly, K.A.; Mui, E.; Roberts, F.; Kasza, K.; Mayr, T.; Kirisits, M.J.; Wollmann, R.; Ferguson, D.J. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J. Neuroinflamm. 2008, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-H.; Han, J.-H.; Nam, H.-W. Clinical features of ocular toxoplasmosis in Korean patients. Korean J. Parasitol. 2011, 49, 167. [Google Scholar] [CrossRef]
- Petersen, E.; Schmidt, D.R. Sulfadiazine and pyrimethamine in the postnatal treatment of congenital toxoplasmosis: What are the options? Expert Rev. Anti-Infect. Ther. 2003, 1, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Morais, F.B.; Arantes, T.E.F.; Muccioli, C. Current practices in ocular toxoplasmosis: A survey of Brazilian uveitis specialists. Ocul. Immunol. Inflamm. 2018, 26, 317–323. [Google Scholar] [CrossRef]
- Antczak, M.; Dzitko, K.; Długońska, H. Human toxoplasmosis–Searching for novel chemotherapeutics. Biomed. Pharmacother. 2016, 82, 677–684. [Google Scholar] [CrossRef]
- Sharma, A.; Sah, N.; Kannan, S.; Kannan, R.M. Targeted drug delivery for maternal and perinatal health: Challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 177, 113950. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Solubility advantage of sulfanilamide and sulfacetamide in natural deep eutectic systems: Experimental and theoretical investigations. Drug Dev. Ind. Pharm. 2019, 45, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Kodide, K.; Asadi, P.; Thati, J. Solubility and thermodynamic modeling of sulfanilamide in 12 mono solvents and 4 binary solvent mixtures from 278.15 to 318.15 K. J. Chem. Eng. Data 2019, 64, 5196–5209. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- Tittal, R.K.; Ghule, V.D.; Kumar, N.; Kumar, L.; Lal, K.; Kumar, A. Design, synthesis, biological activity, molecular docking and computational studies on novel 1,4–disubstituted–1,2,3–Triazole–Thiosemicarbazone hybrid molecules. J. Mol. Struct. 2020, 1209, 127951. [Google Scholar]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Aouad, M.R. Efficient eco–friendly solvent–free click synthesis and antimicrobial evaluation of new fluorinated 1,2, 3–triazoles and their conversion into Schiff Bases. J. Braz. Chem. Soc. 2015, 26, 2105–2115. [Google Scholar]
- Aouad, M.R. Synthesis and antimicrobial screening of novel thioglycosides and acyclonucleoside analogs carrying 1,2,3–triazole and 1,3,4–oxadiazole moieties. Nucleosides Nucleotides Nucleic Acids 2016, 35, 1–15. [Google Scholar] [CrossRef]
- Rezki, N.; Mayaba, M.M.; Al-Blewi, F.F.; Aouad, M.R.; El Ashry, E.S.H. Click 1,4–regioselective synthesis, characterization, and antimicrobial screening of novel 1,2,3–triazoles tethering fluorinated 1,2,4–triazole and lipophilic side chain. Res. Chem. Intermed. 2017, 43, 995–1011. [Google Scholar] [CrossRef]
- Aouad, M.R.; Almehmadi, M.A.; Albelwi, F.F.; Teleb, M.; Tageldin, G.N.; Abu-Serie, M.M.; Hagar, M.; Rezki, N. Targeting the interplay between MMP–2, CA II and VEGFR–2 via new sulfonamide–tethered isomeric triazole hybrids; Microwave–assisted synthesis, computational studies and evaluation. Bioorg. Chem. 2022, 124, 105816. [Google Scholar] [CrossRef]
- Al-Humaidi, J.Y.; Shaaban, M.M.; Rezki, N.; Aouad, M.R.; Zakaria, M.; Jaremko, M.; Hagar, M.; Elwakil, B.H. 1,2,3–Triazole–Benzofused Molecular Conjugates as Potential Antiviral Agents against SARS-CoV-2 Virus Variants. Life 2022, 12, 1341. [Google Scholar] [CrossRef] [PubMed]
- Damej, M.; Molhi, A.; Lgaz, H.; Hsissou, R.; Aslam, J.; Benmessaoud, M.; Rezki, N.; Lee, H.; Lee, D. Performance and interaction mechanism of a new highly efficient benzimidazole–based epoxy resin for corrosion inhibition of carbon steel in HCl: A study based on experimental and first–principles DFTB simulations. J. Mol. Struct. 2023, 1273, 134232. [Google Scholar] [CrossRef]
- Albelwi, F.F.; Abdu Mansour, H.M.; Elshatanofy, M.M.; El Kilany, Y.; Kandeel, K.; Elwakil, B.H.; Hagar, M.; Aouad, M.R.; El Ashry, E.S.H.; Rezki, N. Design, Synthesis and Molecular Docking of Novel Acetophenone–1,2,3–Triazoles Containing Compounds as Potent Enoyl–Acyl Carrier Protein Reductase (InhA) Inhibitors. Pharmaceuticals 2022, 15, 799. [Google Scholar] [CrossRef] [PubMed]
- Al–Blewi, F.F.; Almehmadi, M.A.; Aouad, M.R.; Bardaweel, S.K.; Sahu, P.K.; Messali, M.; Rezki, N.; El Ashry, E.S.H. Design, synthesis, ADME prediction and pharmacological evaluation of novel benzimidazole–1,2,3–triazole–sulfonamide hybrids as antimicrobial and antiproliferative agents. Chem. Cent. J. 2018, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assolini, J.P.; Concato, V.M.; Gonçalves, M.D.; Carloto, A.C.M.; Conchon-Costa, I.; Pavanelli, W.R.; Melanda, F.N.; Costa, I.N. Nanomedicine advances in toxoplasmosis: Diagnostic, treatment, and vaccine applications. Parasitol. Res. 2017, 116, 1603–1615. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Said, D.; Elsamad, L.; Gohar, Y. Validity of silver, chitosan, and curcumin nanoparticles as anti–Giardia agents. Parasitol. Res. 2012, 111, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, A.; Azami, S.J.; Keshavarz, H.; Esmaeili, F.; Alimi, R.; Mavi, S.A.; Shojaee, S. Anti–Toxoplasma activity of various molecular weights and concentrations of chitosan nanoparticles on tachyzoites of RH strain. Int. J. Nanomed. 2018, 13, 1341. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Li, Y.; Wang, M. Cytotoxic activities of chitosan nanoparticles and copper–loaded nanoparticles. Bioorg. Med. Chem. Lett. 2005, 15, 1397–1399. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Rezki, N.; Aouad, M.R.; Hagar, M.; Bakr, B.A.; Shaaban, M.M.; Elwakil, B.H. Novel 1,2,3–Triazole–sulphadiazine–ZnO Hybrids as Potent Antimicrobial Agents against Carbapenem Resistant Bacteria. Antibiotics 2022, 11, 916. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Rezki, N.; Aouad, M.R.; Elwakil, B.H.; Hagar, M.; Sheta, E.; Hussein Mogahed, N.M.F.; Bardaweel, S.K.; Hagras, N.A.-E. Synthesis, Characterization and Nanoformulation of Novel Sulfonamide–1,2,3–triazole Molecular Conjugates as Potent Antiparasitic Agents. Int. J. Mol. Sci. 2022, 23, 4241. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.; Elwakil, B.H.; Elshewemi, S.S.; El-Naggar, M.Y.; Bekhit, A.A.; Olama, Z.A. Novel Siwa propolis and colistin–integrated chitosan nanoparticles: Elaboration; in vitro and in vivo appraisal. Nanomedicine 2020, 15, 1269–1284. [Google Scholar] [CrossRef]
- Conseil, V.; Soete, M.; Dubremetz, J. Serine protease inhibitors block invasion of host cells by Toxoplasma gondii. Antimicrob. Agents Chemother. 1999, 43, 1358–1361. [Google Scholar] [CrossRef] [Green Version]
- Cannella, V.; Altomare, R.; Chiaramonte, G.; Di Bella, S.; Mira, F.; Russotto, L.; Pisano, P.; Guercio, A. Cytotoxicity evaluation of endodontic pins on L929 cell line. BioMed Res. Int. 2019, 2019, 3469525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alomar, M.L.; Rasse-Suriani, F.A.; Ganuza, A.; Cóceres, V.M.; Cabrerizo, F.M.; Angel, S.O. In vitro evaluation of β–carboline alkaloids as potential anti–Toxoplasma agents. BMC Res. Notes 2013, 6, 193–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montazeri, M.; Mirzaee, F.; Daryani, A.; Naeimayi, R.; Karimabad, S.M.; Arjmandi, H.K.; Esmaealzadeh, N.; Shahani, S. Anti–Toxoplasma activities of the hydroalcoholic extract of some brassicaceae species. Adv. Biomed. Res. 2020, 9, 5. [Google Scholar]
- Chou, T.-C.; Talaly, P. A simple generalized equation for the analysis of multiple inhibitions of Michaelis–Menten kinetic systems. J. Biol. Chem. 1977, 252, 6438–6442. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-X.; Wu, L.; Jiang, X.-G.; Feng, Y.-Y.; Cao, J.-P. Anti–Toxoplasma gondii activity of GAS in vitro. J. Ethnopharmacol. 2008, 118, 503–507. [Google Scholar] [CrossRef]
- Khosravi, M.; Mohammad Rahimi, H.; Doroud, D.; Mirsamadi, E.S.; Mirjalali, H.; Zali, M.R. In vitro evaluation of mannosylated paromomycin–loaded solid lipid nanoparticles on acute toxoplasmosis. Front. Cell. Infect. Microbiol. 2020, 10, 33. [Google Scholar] [CrossRef]
- Diab, M.; El–Bahy, M. Toxoplasma gondii: Virulence of tachyzoites in serum free media at different temperatures. Exp. Parasitol. 2008, 118, 75–79. [Google Scholar] [CrossRef]
- Carvalho, C.; De Melo, E. Anti–parasitic action and elimination of intracellular Toxoplasma gondii in the presence of novel thiosemicarbazone and its 4–thiazolidinone derivatives. Braz. J. Med. Biol. Res. 2010, 43, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Shaw, M.K.; Roos, D.S.; Tilney, L.G. Cysteine and serine protease inhibitors block intracellular development and disrupt the secretory pathway of Toxoplasma gondii. Microbes Infect. 2002, 4, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Winey, M.; Meehl, J.B.; O’Toole, E.T.; Giddings, T.H., Jr. Conventional transmission electron microscopy. Mol. Biol. Cell 2014, 25, 319–323. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Ryu, B.-Y.; Emrick, T. Bisphenol–1,2,3–triazole (BPT) epoxies and cyanate esters: Synthesis and self–catalyzed curing. Macromolecules 2011, 44, 5693–5700. [Google Scholar] [CrossRef]
- Singhi, P. Infectious causes of seizures and epilepsy in the developing world. Dev. Med. Child Neurol. 2011, 53, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Wohlfert, E.A.; Blader, I.J.; Wilson, E.H. Brains and brawn: Toxoplasma infections of the central nervous system and skeletal muscle. Trends Parasitol. 2017, 33, 519–531. [Google Scholar] [CrossRef]
- El-Zawawy, L.A.; El-Said, D.; Mossallam, S.F.; Ramadan, H.S.; Younis, S.S. Triclosan and triclosan–loaded liposomal nanoparticles in the treatment of acute experimental toxoplasmosis. Exp. Parasitol. 2015, 149, 54–64. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Githiori, J.; Kyriazakis, I. Medicinal plants for helminth parasite control: Facts and fiction. Animal 2007, 1, 1392–1400. [Google Scholar] [CrossRef] [Green Version]
- Peña–Espinoza, M.; Valente, A.H.; Thamsborg, S.M.; Simonsen, H.T.; Boas, U.; Enemark, H.L.; López–Muñoz, R.; Williams, A.R. Antiparasitic activity of chicory (Cichorium intybus) and its natural bioactive compounds in livestock: A review. Parasites Vectors 2018, 11, 475. [Google Scholar] [CrossRef] [Green Version]
- Dzitko, K.; Paneth, A.; Plech, T.; Pawełczyk, J.; Węglińska, L.; Paneth, P. Triazole–based compound as a candidate to develop novel medicines to treat toxoplasmosis. Antimicrob. Agents Chemother. 2014, 58, 7583–7585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, K.; Ting, L.M.; Kim, K.; Roos, D.S. Toxoplasma gondii purine nucleoside phosphorylase biochemical characterization, inhibitor profiles, and comparison with the Plasmodium falciparum ortholog. J. Biol. Chem. 2006, 281, 25652–25658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, T.M.; Cassera, M.B.; Ho, M.-C.; Zhan, C.; Merino, E.F.; Evans, G.B.; Tyler, P.C.; Almo, S.C.; Schramm, V.L.; Kim, K. Inhibition and structure of Toxoplasma gondii purine nucleoside phosphorylase. Eukaryot. Cell 2014, 13, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]–doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Moneim, A.; El-Shahawy, A.; Yousef, A.I.; Abd El-Twab, S.M.; Elden, Z.E.; Taha, M. Novel polydatin–loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: In silico, in vitro and in vivo approaches. Int. J. Biol. Macromol. 2020, 154, 1496–1504. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.; Samarakoon, S.R.; Soysa, P.; Karunaratne, V.; Amaratunga, G.A.; Karunaratne, D. Chitosan–alginate nanoparticle system efficiently delivers doxorubicin to MCF–7 cells. J. Nanomater. 2016, 2016, 12. [Google Scholar] [CrossRef] [Green Version]
- de Mélo Silva, I.S.; do Amorim Costa Gaspar, L.M.; Rocha AM, O.; da Costa, L.P.; Tada, D.B.; Franceschi, E.; Padilha, F.F. Encapsulation of red propolis in polymer nanoparticles for the destruction of pathogenic biofilms. Aaps Pharmscitech 2020, 21, 49. [Google Scholar] [CrossRef]
- Hopper, A.T.; Brockman, A.; Wise, A.; Gould, J.; Barks, J.; Radke, J.B.; Sibley, L.D.; Zou, Y.; Thomas, S. Discovery of selective Toxoplasma gondii dihydrofolate reductase inhibitors for the treatment of toxoplasmosis. J. Med. Chem. 2019, 62, 1562–1576. [Google Scholar] [CrossRef] [Green Version]
- Devanthran, K.; Unyah, Z.; Majid, R.A.; Abdullah, W.O. In vitro activity of Piper sarmentosum ethanol leaf extract against Toxoplasma gondii tachyzoites. Trop. J. Pharm. Res. 2017, 16, 2667–2673. [Google Scholar] [CrossRef] [Green Version]
- Souto, X.M.; Barbosa, H.S.; Menna-Barreto, R.F.S. The morphological analysis of autophagy in primary skeletal muscle cells infected with Toxoplasma gondii. Parasitol. Res. 2016, 115, 2853–2861. [Google Scholar] [CrossRef]
- Courret, N.; Darche, S.; Sonigo, P.; Milon, G.; Buzoni–Gâtel, D.; Tardieux, I. CD11c–and CD11b–expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 2006, 107, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkerdany, E.D.; Elnassery, S.M.; Arafa, F.M.; Zaki, S.A.-F.; Mady, R.F. In vitro effect of a novel protease inhibitor cocktail on Toxoplasma gondii tachyzoites. Exp. Parasitol. 2020, 219, 108010. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.C.; de Souza, G.; Borges, B.C.; de Araújo, T.E.; Rosini, A.M.; Aguila, F.A.; Ambrósio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Silva, M.J.B. Copaifera spp. oleoresins impair Toxoplasma gondii infection in both human trophoblastic cells and human placental explants. Sci. Rep. 2020, 10, 15158. [Google Scholar] [CrossRef]
- Derouin, F.; Chastang, C. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob. Agents Chemother. 1989, 33, 1753–1759. [Google Scholar] [CrossRef] [Green Version]
- Portes, J.-d.A.; Azeredo, N.F.; Siqueira, P.G.; de Souza, T.G.; Fernandes, C.; Horn, A.; Candela, D.R.; de Souza, W.; DaMatta, R.A.; Seabra, S.H. A new iron (III) complex–containing sulfadiazine inhibits the proliferation and induces cystogenesis of Toxoplasma gondii. Parasitol. Res. 2018, 117, 2795–2805. [Google Scholar] [CrossRef]
- Giovati, L.; Santinoli, C.; Mangia, C.; Vismarra, A.; Belletti, S.; D’Adda, T.; Fumarola, C.; Ciociola, T.; Bacci, C.; Magliani, W. Novel activity of a synthetic decapeptide against Toxoplasma gondii tachyzoites. Front. Microbiol. 2018, 9, 753. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Lv, K.; Li, B.; Yan, B.; Gai, L.; Shi, C.; Wang, X.; Si, H.; Zhang, J. Myrislignan Induces Redox Imbalance and Activates Autophagy in Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2021, 11, 730222. [Google Scholar] [CrossRef]
- Zhang, J.; Si, H.; Lv, K.; Qiu, Y.; Sun, J.; Bai, Y.; Li, B.; Zhou, X.; Zhang, J. Licarin–B exhibits activity against the Toxoplasma gondii RH strain by damaging mitochondria and activating autophagy. Front. Cell Dev. Biol. 2021, 9, 684393. [Google Scholar] [CrossRef]
- Menna-Barreto, R.F.; Salomão, K.; Dantas, A.P.; Santa-Rita, R.M.; Soares, M.J.; Barbosa, H.S.; de Castro, S.L. Different cell death pathways induced by drugs in Trypanosoma cruzi: An ultrastructural study. Micron 2009, 40, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Abou-El-Naga, I.F.; Mady, R.F.; Hussien Mogahed, N.M.F. In vitro effectivity of three approved drugs and their synergistic interaction against Leishmania infantum. Biomédica 2020, 40, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Portes, J.; Souza, T.; Dos Santos, T.; Da Silva, L.; Ribeiro, T.; Pereira, M.; Horn, A., Jr.; Fernandes, C.; DaMatta, R.; De Souza, W. Reduction of Toxoplasma gondii development due to inhibition of parasite antioxidant enzymes by a dinuclear iron (III) compound. Antimicrob. Agents Chemother. 2015, 59, 7374–7386. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Nano Formulae | PS (nm) | ζ Potential (mV) | PDI | Entrapment Efficiency % (EE%) | Loading Efficiency (LE%) |
|---|---|---|---|---|---|
| 3a.CNP | 87.5 ± 5.4 | +30.3 ± 1.0 | 0.23 ± 0.07 | 82.1 ± 0.8 | 27.23 ± 1.7 |
| 3b.CNP | 43.2 ± 8.1 | +36.4 ± 0.1 | 0.33 ± 0.13 | 81.3 ± 0.3 | 22.9 ± 1.0 |
| 3c.CNP | 56.8 ± 9.2 | +34.4 ± 0.3 | 0.41 ± 0.05 | 83.4 ± 1.1 | 29.4 ± 1.0 |
| Tested Drug | CC50 (µg/mL) | IC50 (µg/mL) | SI |
|---|---|---|---|
| 3a | 218.8 ± 1.104 | 10.35 ± 0.27 | 21 |
| 3b | 168.0 ± 9.300 | 20.78 ± 2.85 | 8.11 |
| 3c | 155.0 ± 3.355 | 5.39 ± 0.20 | 28.75 |
| 3a.CNP | 157.64 ± 1.332 | 6.14 ± 0.263 | 25.67 |
| 3b.CNP | 123.17 ± 2.99 | 4.93 ± 0.662 | 24.37 |
| 3c.CNP | 145.0 ± 5.900 | 3.64 ± 0.295 | 38.736 |
| CNP | >1000 | 14.2 ± 1.75 | ND |
| sulfadiazine | 361.45 ± 15.531 | 46.42 ± 2.77 | 7.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arafa, F.M.; Said, H.; Osman, D.; Rezki, N.; Aouad, M.R.; Hagar, M.; Osman, M.; Elwakil, B.H.; Jaremko, M.; Tolba, M.M. Nanoformulation-Based 1,2,3-Triazole Sulfonamides for Anti-Toxoplasma In Vitro Study. Trop. Med. Infect. Dis. 2023, 8, 401. https://doi.org/10.3390/tropicalmed8080401
Arafa FM, Said H, Osman D, Rezki N, Aouad MR, Hagar M, Osman M, Elwakil BH, Jaremko M, Tolba MM. Nanoformulation-Based 1,2,3-Triazole Sulfonamides for Anti-Toxoplasma In Vitro Study. Tropical Medicine and Infectious Disease. 2023; 8(8):401. https://doi.org/10.3390/tropicalmed8080401
Chicago/Turabian StyleArafa, Fadwa M., Heba Said, Doaa Osman, Nadjet Rezki, Mohamed R. Aouad, Mohamed Hagar, Mervat Osman, Bassma H. Elwakil, Mariusz Jaremko, and Mona Mohamed Tolba. 2023. "Nanoformulation-Based 1,2,3-Triazole Sulfonamides for Anti-Toxoplasma In Vitro Study" Tropical Medicine and Infectious Disease 8, no. 8: 401. https://doi.org/10.3390/tropicalmed8080401
APA StyleArafa, F. M., Said, H., Osman, D., Rezki, N., Aouad, M. R., Hagar, M., Osman, M., Elwakil, B. H., Jaremko, M., & Tolba, M. M. (2023). Nanoformulation-Based 1,2,3-Triazole Sulfonamides for Anti-Toxoplasma In Vitro Study. Tropical Medicine and Infectious Disease, 8(8), 401. https://doi.org/10.3390/tropicalmed8080401








