High Prevalence of Polyclonal Plasmodium falciparum Infections and Association with Poor IgG Antibody Responses in a Hyper-Endemic Area in Cameroon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Study Design and Sample Collection
2.3. Detection and Quantification of Plasmodium Species
2.4. Plasmodium falciparum Genotyping Using msp2 and polyα Markers
2.5. Anti-P. falciparum IgG Antibody ELISA
2.6. Statistical Analysis
3. Results
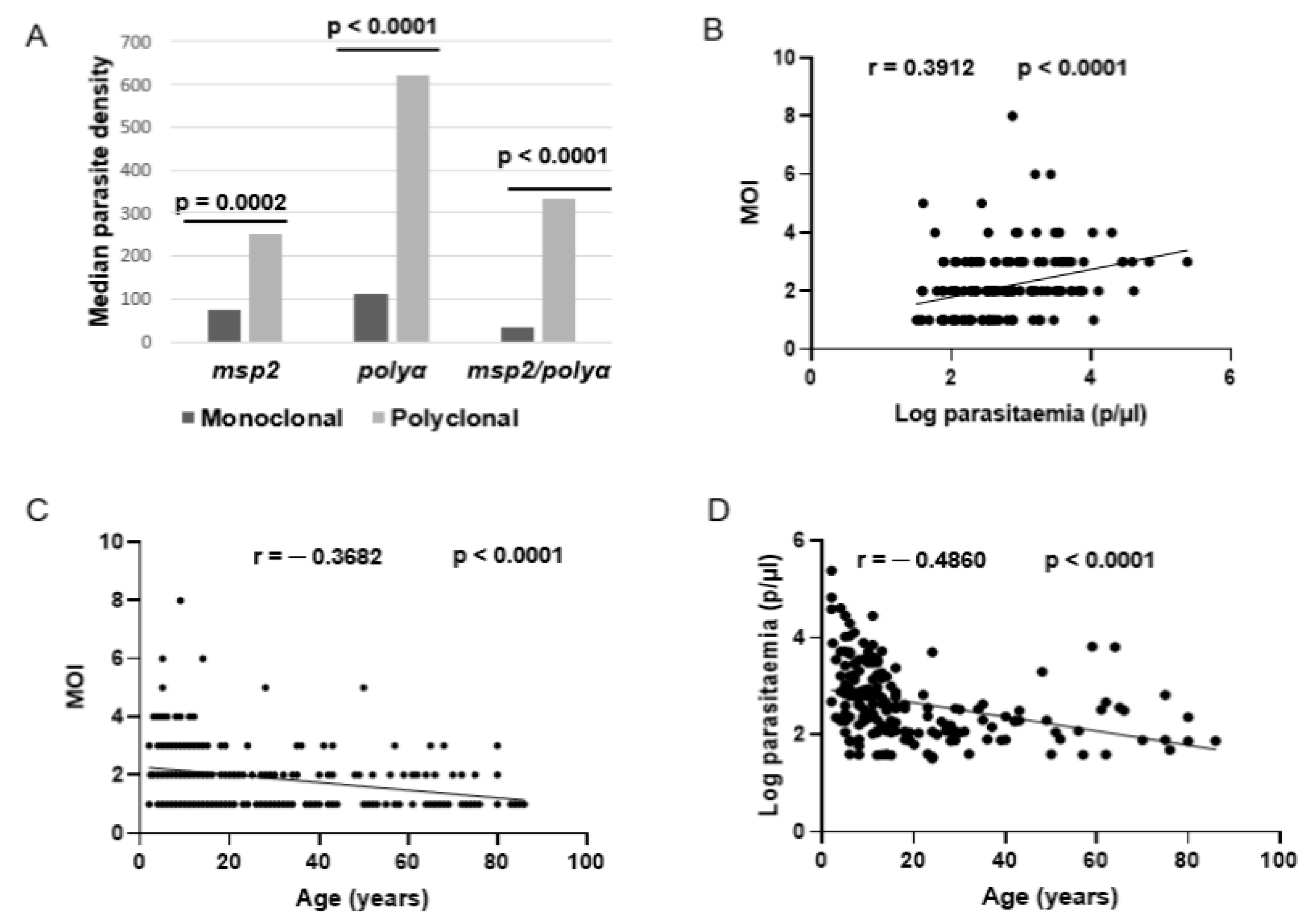
3.1. Frequency of Polyclonal P. falciparum Infection in the Study Population
3.2. Associated Factors of P. falciparum Polyclonal Infections in the Study Area
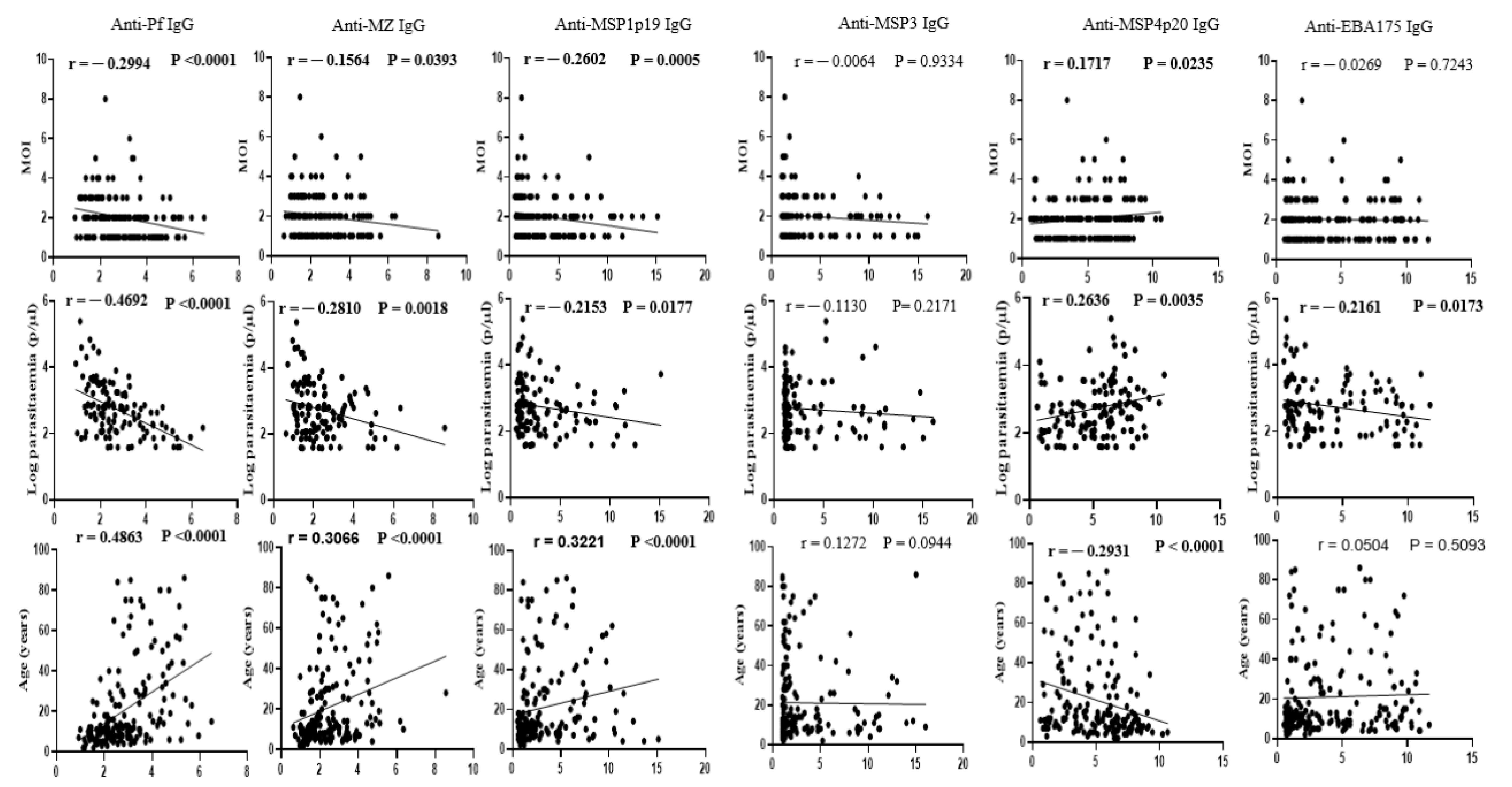
3.3. Association of P. falciparum Polyclonal Infection with Underlying Acquired Immunity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-156572-1. [Google Scholar]
- WHO. World Malaria Report 2020 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001579-1. [Google Scholar]
- WHO. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Dhorda, M.; Amaratunga, C.; Dondorp, A.M. Artemisinin and Multidrug-Resistant Plasmodium Falciparum—A Threat for Malaria Control and Elimination. Curr. Opin. Infect. Dis. 2021, 34, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cha, S.; Jacobs-Lorena, M. New Weapons to Fight Malaria Transmission: A Historical View. Entomol. Res. 2022, 52, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, R.; Kissoon, S.; Lakan, V.; Kheswa, N. Rolling Back Malaria in Africa—Challenges and Opportunities to Winning the Elimination Battle. S. Afr. Med. J. 2019, 109, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckee, C.O.; Gupta, S. Modelling Malaria Population Structure and Its Implications for Control. In Modelling Parasite Transmission and Control; Michael, E., Spear, R.C., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; Volume 673, pp. 112–126. ISBN 978-1-4419-6063-4. [Google Scholar]
- Naung, M.T.; Martin, E.; Munro, J.; Mehra, S.; Guy, A.J.; Laman, M.; Harrison, G.L.A.; Tavul, L.; Hetzel, M.; Kwiatkowski, D.; et al. Global Diversity and Balancing Selection of 23 Leading Plasmodium Falciparum Candidate Vaccine Antigens. PLoS Comput. Biol. 2022, 18, e1009801. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, A.; Barry, A.E.; Dutta, S.; Remarque, E.J.; Beeson, J.G.; Plowe, C.V. Designing Malaria Vaccines to Circumvent Antigen Variability. Vaccine 2015, 33, 7506–7512. [Google Scholar] [CrossRef] [Green Version]
- Akpogheneta, O.J.; Duah, N.O.; Tetteh, K.K.A.; Dunyo, S.; Lanar, D.E.; Pinder, M.; Conway, D.J. Duration of Naturally Acquired Antibody Responses to Blood-Stage Plasmodium falciparum Is Age Dependent and Antigen Specific. Infect. Immun. 2008, 76, 1748–1755. [Google Scholar] [CrossRef] [Green Version]
- Day, K.P.; Marsh, K. Naturally Acquired immunity to Plasmodium falciparum. Parasitol. Today 1991, 7, 68–71. [Google Scholar] [CrossRef]
- Griffin, J.T.; Hollingsworth, T.D.; Reyburn, H.; Drakeley, C.J.; Riley, E.M.; Ghani, A.C. Gradual Acquisition of Immunity to Severe Malaria with Increasing Exposure. Proc. R. Soc. B 2015, 282, 20142657. [Google Scholar] [CrossRef]
- O’Flaherty, K.; Roe, M.; Fowkes, F.J. The Role of Naturally Acquired Antimalarial Antibodies in Subclinical Plasmodium spp. Infection. J. Leukoc. Biol. 2022, 111, 1097–1105. [Google Scholar] [CrossRef]
- White, M.T.; Griffin, J.T.; Akpogheneta, O.; Conway, D.J.; Koram, K.A.; Riley, E.M.; Ghani, A.C. Dynamics of the Antibody Response to Plasmodium Falciparum Infection in African Children. J. Infect. Dis. 2014, 210, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Yman, V.; White, M.T.; Asghar, M.; Sundling, C.; Sondén, K.; Draper, S.J.; Osier, F.H.A.; Färnert, A. Antibody Responses to Merozoite Antigens after Natural Plasmodium Falciparum Infection: Kinetics and Longevity in Absence of Re-Exposure. BMC Med. 2019, 17, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoah, L.E.; Acquah, F.K.; Ayanful-Torgby, R.; Oppong, A.; Abankwa, J.; Obboh, E.K.; Singh, S.K.; Theisen, M. Dynamics of Anti-MSP3 and Pfs230 Antibody Responses and Multiplicity of Infection in Asymptomatic Children from Southern Ghana. Parasites Vectors 2018, 11, 13. [Google Scholar] [CrossRef]
- Bereczky, S.; Liljander, A.; Rooth, I.; Faraja, L.; Granath, F.; Montgomery, S.M.; Färnert, A. Multiclonal Asymptomatic Plasmodium Falciparum Infections Predict a Reduced Risk of Malaria Disease in a Tanzanian Population. Microbes Infect. 2007, 9, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rono, J.; Osier, F.H.A.; Olsson, D.; Montgomery, S.; Mhoja, L.; Rooth, I.; Marsh, K.; Färnert, A. Breadth of Anti-Merozoite Antibody Responses Is Associated With the Genetic Diversity of Asymptomatic Plasmodium Falciparum Infections and Protection Against Clinical Malaria. Clin. Infect. Dis. 2013, 57, 1409–1416. [Google Scholar] [CrossRef] [Green Version]
- Sondén, K.; Doumbo, S.; Hammar, U.; Vafa Homann, M.; Ongoiba, A.; Traoré, B.; Bottai, M.; Crompton, P.D.; Färnert, A. Asymptomatic Multiclonal Plasmodium falciparum Infections Carried Through the Dry Season Predict Protection Against Subsequent Clinical Malaria. J. Infect. Dis. 2015, 212, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumner, K.M.; Freedman, E.; Mangeni, J.N.; Obala, A.A.; Abel, L.; Edwards, J.K.; Emch, M.; Meshnick, S.R.; Pence, B.W.; Prudhomme-O’Meara, W.; et al. Exposure to Diverse Plasmodium falciparum Genotypes Shapes the Risk of Symptomatic Malaria in Incident and Persistent Infections: A Longitudinal Molecular Epidemiologic Study in Kenya. Clin. Infect. Dis. 2021, 73, 1176–1184. [Google Scholar] [CrossRef]
- Chauhan, C.; Sinha, A. Plasmodium and Malaria: Adding to the Don’ts. Trends Parasitol. 2021, 37, 935–936. [Google Scholar] [CrossRef]
- Wong, W.; Volkman, S.; Daniels, R.; Schaffner, S.; Sy, M.; Ndiaye, Y.D.; Badiane, A.S.; Deme, A.B.; Diallo, M.A.; Gomis, J.; et al. R H: A Genetic Metric for Measuring Intrahost Plasmodium falciparum Relatedness and Distinguishing Cotransmission from Superinfection. PNAS Nexus 2022, 1, pgac187. [Google Scholar] [CrossRef]
- Lopez, L.; Koepfli, C. Systematic Review of Plasmodium Falciparum and Plasmodium Vivax Polyclonal Infections: Impact of Prevalence, Study Population Characteristics, and Laboratory Procedures. PLoS ONE 2021, 16, e0249382. [Google Scholar] [CrossRef]
- Antonio-Nkondjio, C.; Ndo, C.; Njiokou, F.; Bigoga, J.D.; Awono-Ambene, P.; Etang, J.; Ekobo, A.S.; Wondji, C.S. Review of Malaria Situation in Cameroon: Technical Viewpoint on Challenges and Prospects for Disease Elimination. Parasites Vectors 2019, 12, 501. [Google Scholar] [CrossRef] [Green Version]
- Esse Council. Esse Municipal Council Development Plan; Esse Council: Esse, Cameroon, 2013; 209p.
- Essangui, E.; Eboumbou Moukoko, C.E.; Nguedia, N.; Tchokwansi, M.; Banlanjo, U.; Maloba, F.; Fogang, B.; Donkeu, C.; Biabi, M.; Cheteug, G.; et al. Demographical, Hematological and Serological Risk Factors for Plasmodium Falciparum Gametocyte Carriage in a High Stable Transmission Zone in Cameroon. PLoS ONE 2019, 14, e0216133. [Google Scholar] [CrossRef] [Green Version]
- Fogang, B.; Biabi, M.F.; Megnekou, R.; Maloba, F.M.; Essangui, E.; Donkeu, C.; Cheteug, G.; Kapen, M.; Keumoe, R.; Kemleu, S.; et al. High Prevalence of Asymptomatic Malarial Anemia and Association with Early Conversion from Asymptomatic to Symptomatic Infection in a Plasmodium Falciparum Hyperendemic Setting in Cameroon. Am. J. Trop. Med. Hyg. 2021, 106, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Plowe, C.V.; Djimde, A.; Bouare, M.; Doumbo, O.; Wellems, T.E. Pyrimethamine and Proguanil Resistance-Conferring Mutations in Plasmodium Falciparum Dihydrofolate Reductase: Polymerase Chain Reaction Methods for Surveillance in Africa. Am. J. Trop. Med. Hyg. 1995, 52, 565–568. [Google Scholar] [CrossRef]
- Snounou, G.; Viriyakosol, S.; Jarra, W.; Thaithong, S.; Brown, K.N. Identification of the Four Human Malaria Parasite Species in Field Samples by the Polymerase Chain Reaction and Detection of a High Prevalence of Mixed Infections. Mol. Biochem. Parasitol. 1993, 58, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Ajogbasile, F.V.; Kayode, A.T.; Oluniyi, P.E.; Akano, K.O.; Uwanibe, J.N.; Adegboyega, B.B.; Philip, C.; John, O.G.; Eromon, P.J.; Emechebe, G.; et al. Genetic Diversity and Population Structure of Plasmodium Falciparum in Nigeria: Insights from Microsatellite Loci Analysis. Malar. J. 2021, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.C.; Su, X.-Z.; Bockarie, M.; Lagog, M.; Day, K.P. Twelve Microsatellite Markers for Characterization of Plasmodium Falciparum from Finger-Prick Blood Samples. Parasitology 1999, 119, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Metoh, T.N.; Chen, J.-H.; Fon-Gah, P.; Zhou, X.; Moyou-Somo, R.; Zhou, X.-N. Genetic Diversity of Plasmodium Falciparum and Genetic Profile in Children Affected by Uncomplicated Malaria in Cameroon. Malar. J. 2020, 19, 115. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.; Kassa, M.; Mekete, K.; Assefa, A.; Taye, G.; Commons, R.J. Genetic Diversity of the Msp-1, Msp-2, and Glurp Genes of Plasmodium Falciparum Isolates in Northwest Ethiopia. Malar. J. 2018, 17, 386. [Google Scholar] [CrossRef] [Green Version]
- Ranford-Cartwright, L.C.; Taylor, J.; Umasunthar, T.; Taylor, L.H.; Babiker, H.A.; Lell, B.; Schmidt-Ott, J.R.; Lehman, L.G.; Walliker, D.; Kremsner, P.G. Molecular Analysis of Recrudescent Parasites in a Plasmodium Falciparum Drug Efficacy Trial in Gabon. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 719–724. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, A.; Israr, M.; Shah, M.; Shams, S.; Khan, W.; Shah, M.; Siraj, M.; Akbar, K.; Naz, T.; et al. Genomic Miscellany and Allelic Frequencies of Plasmodium Falciparum Msp-1, Msp-2 and Glurp in Parasite Isolates. PLoS ONE 2022, 17, e0264654. [Google Scholar] [CrossRef]
- Abukari, Z.; Okonu, R.; Nyarko, S.B.; Lo, A.C.; Dieng, C.C.; Salifu, S.P.; Gyan, B.A.; Lo, E.; Amoah, L.E. The Diversity, Multiplicity of Infection and Population Structure of P. Falciparum Parasites Circulating in Asymptomatic Carriers Living in High and Low Malaria Transmission Settings of Ghana. Genes 2019, 10, 434. [Google Scholar] [CrossRef] [Green Version]
- Mbengue, B.; Fall, M.M.; Varela, M.-L.; Loucoubar, C.; Joos, C.; Fall, B.; Niang, M.S.; Niang, B.; Mbow, M.; Dieye, A.; et al. Analysis of Antibody Responses to Selected Plasmodium falciparum Merozoite Surface Antigens in Mild and Cerebral Malaria and Associations with Clinical Outcomes. Clin. Exp. Immunol. 2019, 196, 86–96. [Google Scholar] [CrossRef]
- Victor, Y.; James, T.; White, M.T.; Gathoni, K.; Kennedy, M.; Nelson, K.; Muhammad, A.; Christopher, S.; Klara, S.; Linda, M.; et al. Distinct Kinetics of Antibodies to 111 Plasmodium Falciparum Proteins Identifies Markers of Recent Malaria Exposure. Nat. Commun. 2022, 13, 331. [Google Scholar] [CrossRef]
- Bonnet, S.; Petres, S.; Holm, I.; Fontaine, T.; Rosario, S.; Roth, C.; Longacre, S. Soluble and Glyco-Lipid Modified Baculovirus Plasmodium Falciparum C-Terminal Merozoite Surface Protein 1, Two Forms of a Leading Malaria Vaccine Candidate. Vaccine 2006, 24, 5997–6008. [Google Scholar] [CrossRef] [PubMed]
- Oeuvray, C.; Bouharoun-Tayoun, H.; Gras-Masse, H.; Bottius, E.; Kaidoh, T.; Aikawa, M.; Filgueira, M.; Tartar, A.; Druilhe, P. Merozoite Surface Protein-3: A Malaria Protein Inducing Antibodies That Promote Plasmodium Falciparum Killing by Cooperation with Blood Monocytes. Blood 1994, 84, 1594–1602. [Google Scholar] [CrossRef] [Green Version]
- OMS (Organisation Mondiale de la Sante). Concentrations en Hémoglobine Permettant de Diagnostiquer l’anémie et d’en Évaluer La Sévérité; Organisation Mondiale de la Sante: Geneve, Switzerland, 2011; pp. 1–6. [Google Scholar]
- Bendixen, M.; Msangeni, H.A.; Pedersen, B.V.; Shayo, D.; Bedker, R. Diversity of Plasmodium Falciparum Populations and Complexity of Infections in Relation to Transmission Intensity and Host Age: A Study from the Usambara Mountains, Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 143–148. [Google Scholar] [CrossRef]
- Diouf, B.; Diop, F.; Dieye, Y.; Loucoubar, C.; Dia, I.; Faye, J.; Sembène, M.; Perraut, R.; Niang, M.; Toure-Balde, A. Association of High Plasmodium Falciparum Parasite Densities with Polyclonal Microscopic Infections in Asymptomatic Children from Toubacouta, Senegal. Malar. J. 2019, 18, 48. [Google Scholar] [CrossRef] [Green Version]
- Oyedeji, S.I.; Bassi, P.U.; Oyedeji, S.A.; Ojurongbe, O.; Awobode, H.O. Genetic Diversity and Complexity of Plasmodium Falciparum Infections in the Microenvironment among Siblings of the Same Household in North-Central Nigeria. Malar. J. 2020, 19, 338. [Google Scholar] [CrossRef] [PubMed]
- Rogier, C.; Trape, J.-F.; Bonnefoy, S.; Ntoumi, F.; Mercereau-Puijalon, O.; Contamin, H. Age-Dependent Carriage of Multiple Plasmodium Falciparum Merozoite Surface Antigen-2 Alleles in Asymptomatic Malaria Infections. Am. J. Trop. Med. Hyg. 1995, 52, 81–88. [Google Scholar] [CrossRef]
- Sondo, P.; Derra, K.; Rouamba, T.; Nakanabo Diallo, S.; Taconet, P.; Kazienga, A.; Ilboudo, H.; Tahita, M.C.; Valéa, I.; Sorgho, H.; et al. Determinants of Plasmodium Falciparum Multiplicity of Infection and Genetic Diversity in Burkina Faso. Parasites Vectors 2020, 13, 427. [Google Scholar] [CrossRef]
- Engelbrecht, F.; Tögel, E.; Beck, H.-P.; Enwezor, F.; Oettli, A.; Felger, I. Analysis of Plasmodium Falciparum Infections in a Village Community in Northern Nigeria: Determination of Msp2 Genotypes and Parasite-Specific IgG Responses. Acta Trop. 2000, 74, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Koffi, D.; Touré, A.O.; Varela, M.-L.; Vigan-Womas, I.; Béourou, S.; Brou, S.; Ehouman, M.-F.; Gnamien, L.; Richard, V.; Djaman, J.A.; et al. Analysis of Antibody Profiles in Symptomatic Malaria in Three Sentinel Sites of Ivory Coast by Using Multiplex, Fluorescent, Magnetic, Bead-Based Serological Assay (MAGPIXTM). Malar. J. 2015, 14, 509. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Richie, T.L.; Stowers, A.; Nhan, D.H.; Coppel, R.L. Naturally Acquired Antibody Responses to Plasmodium falciparum Merozoite Surface Protein 4 in a Population Living in an Area of Endemicity in Vietnam. Infect. Immun. 2001, 69, 4390–4397. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Locus | Primer Sequence (5′-3′) | Amplification Conditions |
|---|---|---|
| Species identification | ||
| Outer | F: AGTGTGTATCAATCGAGTTTC | 95 °C for 15′, 43 cycles of 95 °C for 45″ and 55 °C for 1′30″, followed by 72 °C for 5′ |
| R: TAACTTTCTCGCTTGCGCG | ||
| Nested | F: AGTGTGTATCAATCGAGTTTC | 95 °C for 15′, 43 cycles of 95 °C for 45″ and 55 °C for 1′30″, followed by 72 °C for 5′ |
| R P.o: TCATTCCAATTACAAAACCATG | ||
| R P.m: CCAGACTTGCCCTCCAATTGCC | ||
| R P.f: GAAAAGCTAAAATAGTTCCCC | ||
| R P.v: GTAACAAGGACTTCCAAGC | ||
| R P.k: AAGGAAGCAATCTAAGAGTTC | ||
| msp 2 | ||
| Outer | F: GAAGGTAATTAAAACATTGTC | 95 °C for 15′, 35 cycles of 95 °C for 45″ and 45 °C for 1′30″, followed by 72 °C for 5′ |
| R: GAGGGATGTTGCTGCTCCACA | ||
| Nested | F: GAGTATAAGGAGAAGTATG | 95 °C for 15′, 35 cycles of 95 °C for 45″ and 55 °C for 1′ 30″, followed by 72 °C for 5′ |
| R: CTAGAACCATGCATATGTCC | ||
| polyα | ||
| Outer | F: AAAATATAGACGAACAGA | 95 °C for 15′, 25 cycles of 95 °C for 45″, 42 °C for 30″, 40 °C for 30″ and 72 °C for 30″, followed by 72 °C for 2′ |
| R: ATCAGATAATTGTTGGTA | ||
| Nested | F: AAAATATAGACGAACAGA | 95 °C for 15′, 27 cycles of 95 °C for 45″, 45 °C for 10″ and 72 °C for 30″, followed by 72 °C for 2′ |
| R: GAAATTATAACTCTACCA | ||
| Genotyped (N = 278) | Non-Genotyped (N = 38) | |
|---|---|---|
| Category | n (%) | n (%) |
| Age group (years) | ||
| <5 | 17 (6.12) | 2 (5.26) |
| 5–10 | 64 (23.02) | 8 (21.05) |
| 10–15 | 54 (19.42) | 3 (7.89) |
| ≥15 | 143 (51.44) | 25 (65.80) |
| Gender | ||
| Male | 130 (46.76) | 16 (42.10) |
| Female | 148 (53.24) | 22 (57.90) |
| Residence | ||
| Afanetouana | 40 (14.39) | 21 (55.26) |
| Koutou | 44 (15.83) | 2 (5.26) |
| Meboe | 77 (27.70) | 9 (23.69) |
| Ntouessong | 70 (25.18) | 4 (10.53) |
| Ondoundou | 47 (16.90) | 2 (5.26) |
| Parasitaemia | ||
| Microscopic | 192 (69.06) | 15 (39.47) |
| Submicroscopic | 86 (30.94) | 23 (60.53) |
| Clinical status | ||
| Asymptomatic | 222 (79.86) | 35 (92.11) |
| Symptomatic | 56 (20.14) | 3 (7.89) |
| Anemic | ||
| Yes | 65 (23.38) | 14 (36.84) |
| No | 112 (40.29) | 15 (39.47) |
| Not Determined | 101 (36.33) | 9 (23.69) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Parameters | Description (N) | % Polyclonal Infection (N) | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age group (years) | <15 (135) | 74.07 (100) | 3.241 (1.937–5.273) | <0.0001 | 1.835 (1.017–3.310) | 0.044 |
| ≥15 (143) | 46.85 (67) | 1 | ||||
| Gender | F (148) | 60.81 (90) | 1.068 (0.6583–1.73) | 0.7784 | ||
| M (130) | 59.23 (77) | 1 | ||||
| Village of residence | Afanetouana (40) | 65 (26) | 2.0334 (0.8445–4.691) | 0.1112 | 1.355 (1.120–1.639) | 0.002 |
| Koutou (44) | 47.73 (21) | 1 | ||||
| Meboe (77) | 49.35 (38) | 1.067 (0.5214–2.204) | 0.8636 | |||
| Ntouessong (70) | 74.29 (52) | 3.164 (1.410–6.869) | 0.0040 | |||
| Ondoundou (47) | 63.84 (30) | 1.933 (0.8616–4.548) | 0.1220 | |||
| Length of stay in the area (years) | <15 (191) | 63.35 (121) | 1.686 (0.9804–2.800) | 0.0504 | ||
| ≥15 (81) | 50.62 (41) | 1 | ||||
| Bednet and/or insecticide use | No (137) | 62.04 (85) | 1.176 (0.7278–1.911) | 0.5082 | ||
| Yes (141) | 58.16 (82) | 1 | ||||
| Clinical status | AS (222) | 60.36 (134) | 1.061 (0.5929–1.915) | 0.8450 | ||
| SY (56) | 58.93 (33) | 1 | ||||
| Parasitaemia (p/µL) * | Low (140) | 43.57 (61) | 1 | <0.0001 | 3.187 (1.762–5.766) | <0.0001 |
| High (138) | 76.81 (106) | 4.290 (2.515–7.163) | ||||
| Anemia | No (112) | 58.04 (65) | 1 | 0.1388 | ||
| Yes (65) | 69.23 (45) | 1.627 (0.8673–3.067) | ||||
| Time to previous fever episode | >2 months (207) | 58.45 (121) | 1 | 0.3470 | ||
| ≤2 months (71) | 64.79 (46) | 1.308 (0.7532–2.295) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biabi, M.F.A.B.; Fogang, B.; Essangui, E.; Maloba, F.; Donkeu, C.; Keumoe, R.; Cheteug, G.; Magoudjou, N.; Slam, C.; Kemleu, S.; et al. High Prevalence of Polyclonal Plasmodium falciparum Infections and Association with Poor IgG Antibody Responses in a Hyper-Endemic Area in Cameroon. Trop. Med. Infect. Dis. 2023, 8, 390. https://doi.org/10.3390/tropicalmed8080390
Biabi MFAB, Fogang B, Essangui E, Maloba F, Donkeu C, Keumoe R, Cheteug G, Magoudjou N, Slam C, Kemleu S, et al. High Prevalence of Polyclonal Plasmodium falciparum Infections and Association with Poor IgG Antibody Responses in a Hyper-Endemic Area in Cameroon. Tropical Medicine and Infectious Disease. 2023; 8(8):390. https://doi.org/10.3390/tropicalmed8080390
Chicago/Turabian StyleBiabi, Marie Florence A Bite, Balotin Fogang, Estelle Essangui, Franklin Maloba, Christiane Donkeu, Rodrigue Keumoe, Glwadys Cheteug, Nina Magoudjou, Celine Slam, Sylvie Kemleu, and et al. 2023. "High Prevalence of Polyclonal Plasmodium falciparum Infections and Association with Poor IgG Antibody Responses in a Hyper-Endemic Area in Cameroon" Tropical Medicine and Infectious Disease 8, no. 8: 390. https://doi.org/10.3390/tropicalmed8080390
APA StyleBiabi, M. F. A. B., Fogang, B., Essangui, E., Maloba, F., Donkeu, C., Keumoe, R., Cheteug, G., Magoudjou, N., Slam, C., Kemleu, S., Efange, N., Perraut, R., Nsango, S. E., Eboumbou Moukoko, C. E., Assam, J. P. A., Etoa, F.-X., Lamb, T., & Ayong, L. (2023). High Prevalence of Polyclonal Plasmodium falciparum Infections and Association with Poor IgG Antibody Responses in a Hyper-Endemic Area in Cameroon. Tropical Medicine and Infectious Disease, 8(8), 390. https://doi.org/10.3390/tropicalmed8080390





